
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 17 August 2023
Sec. Structural Interventional Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1218158
This article is part of the Research TopicFrom Interventional Pearls to Pioneering Technologies in Transcatheter Treatment of Congenital Heart DefectsView all 22 articles
Patent ductus arteriosus (PDA) is a common congenital heart disease affecting roughly one in every 2,000 term births. Although most of the patients are diagnosed and treated during childhood, few cases may persist into adulthood. We presented a 27-year-old male patient with a 20.2 mm diameter PDA who was referred to our hospital with progressive fatigue and exertional dyspnea. Given the potential complications, usual techniques such as coil occlusion and duct occluders were deemed inappropriate for this patient. Thoracic endovascular aortic repair (TEVAR) using a non-touch exclusion technique was successfully performed for this patient. The patient was discharged with no major post-surgical complications. TEVAR could be a new, safe, and effective alternative treatment to other transcatheter procedures for complicated PDAs in some patients.
Patent ductus arteriosus (PDA) is a prevalent congenital cardiovascular disorder that affects nearly one in every 2,000 term infants and accounts for 5%–10% of all cases of congenital heart disease (1). Although patients are usually identified and treated during childhood, few cases may remain intact until adulthood (2).
Currently, various surgical options are available that are effective and less invasive for closing PDA. Nonetheless, adult PDAs tend to be more complex in terms of size and shape, which can result in additional complications when using routine techniques. Consequently, selecting the most suitable cardiovascular repair technique for such patients has remained a matter of debate (3–5).
Using a non-touch exclusion technique in conjunction with thoracic endovascular aortic repair (TEVAR) may offer a viable alternative for treating PDA in high-risk complex patients (6). We present an adult patient with a large PDA treated successfully with TEVAR using a custom-made device.
A 27-year-old man complaining of exertional dyspnea functional class III, palpitation, and progressive fatigue was referred to our hospital for further evaluation. During the physical examination, a continuous murmur with systolic prominence was detected at the left second intercostal space and a loud pulmonary component of second heart sound was heard at the left sternal border second intercostal space, along with a blood pressure of 135/65 mm Hg and a heart rate of 85 beats/min. Additionally, oxygen saturation in both arms and legs was 98% and no cyanosis and clubbing were detected.
Transthoracic echocardiography (TTE) revealed severe left ventricular dilatation during diastole (73 mm) and an ejection fraction of 55%. Additionally, it showed that the patient has a bicuspid aortic valve with moderate aortic regurgitation (AR), as well as a mildly dilated ascending aorta (38 mm). Furthermore, the echocardiography indicated the presence of PDA, severe pulmonary valve regurgitation, and a significant enlargement of the pulmonary artery (73 mm). Following contrast-enhanced computed tomography (CT), it was found that the patient has a 20.2 mm PDA (Figure 1), which originates from the descending aorta, and 7 mm distal to the orifice of the left subclavian artery (LSA). According to the Krichenko classification, the PDA was classified as type B, which refers to a large duct with a short window-like structure at the aortic insertion (7).
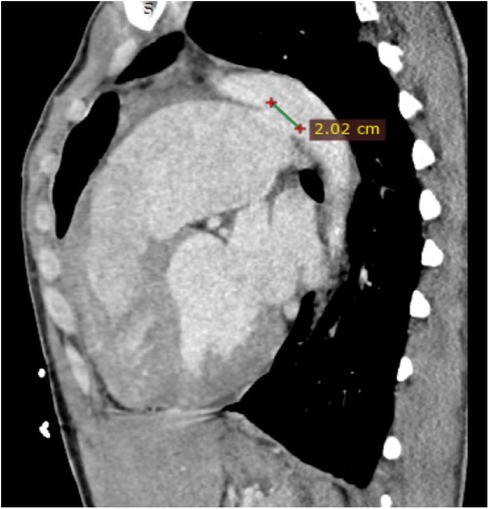
Figure 1. Cardiac CT in sagittal view shows a large PDA (20.2 mm) with a dilated pulmonary artery. CT, computed tomography; PDA, patent ductus arteriosus.
The patient had a mean pulmonary arterial pressure of 48 mmHg and a pulmonary vascular resistance of 2.1 WU during cardiac catheterization and hemodynamic assessment. Given the presenting symptoms, imaging results, and Qp/Qs ratio of 2.1, it was decided to perform a PDA closure procedure for this patient. Device closure is generally the preferred method for treating PDA, even in the presence of other concomitant cardiac lesions, due to its high success rate and low incidence of complications. However, due to the anatomical features of this patient's PDA, device closure may carry risks of potential complications such as device migration, dissection, and perforation, which renders this option inappropriate. After considering various available treatment options, the heart surgery team suggested a non-touch exclusion technique using TEVAR to reduce the risks associated with other endovascular procedures. Subsequently, we provided the patient with a comprehensive explanation of all the available options. Accordingly, the TEVAR procedure was chosen based on shared decision-making with the patient. The patient provided informed consent prior to the TEVAR procedure.
TEVAR was performed while the patient was under conscious sedation, using the pre-close technique with 2 Proglide devices. Custom-made Zenith Alpha Thoracic Endovascular Graft (44 26 128 mm, Cook Medical, Bloomington, IN, USA) was inserted through the right common iliac artery. The patient underwent a zone 2 deployment of a TEVAR graft with the coverage of the LSA. Echocardiography and aortography after TEVAR showed complete closure of PDA without any endoleaks (Figure 2). The post-surgical period was uneventful, and the patient was discharged two days after the procedure with no major complications, taking interpulmonary hypertensive drugs. One month after TEVAR, CT angiography was performed that revealed complete occlusion of PDA with no endoleaks (Figure 3). Due to residual pulmonary hypertension, echocardiography is being performed annually. A detailed, comprehensive timeline of the patient's clinical course is provided in Table 1.
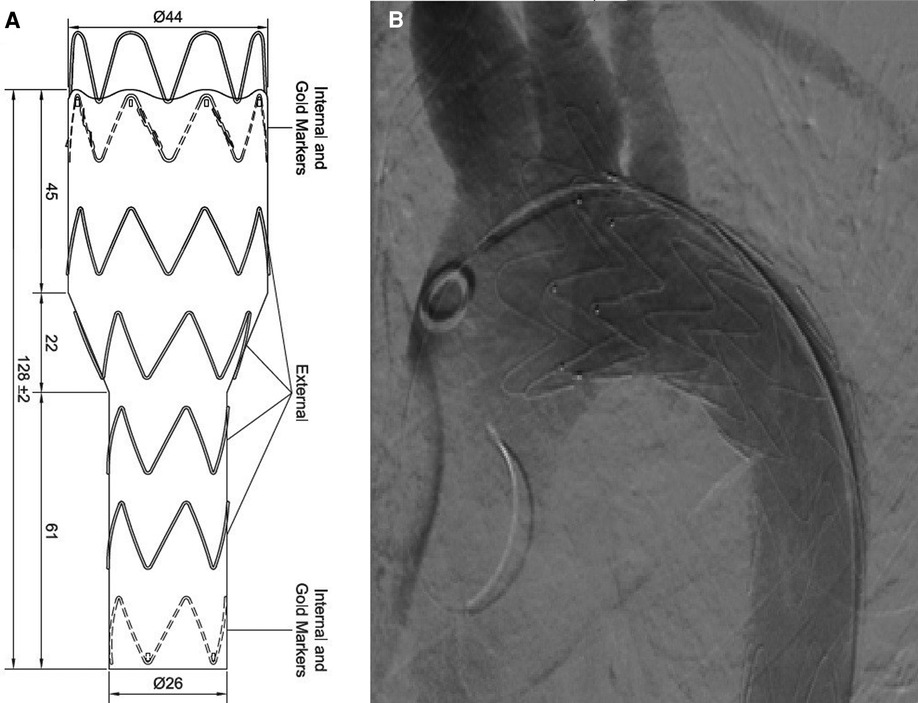
Figure 2. (A) Custom-made device. (B) Aortography after TEVAR revealed complete closure of PDA without any endoleaks. PDA, patent ductus arteriosus; TEVAR, thoracic endovascular aortic repair.
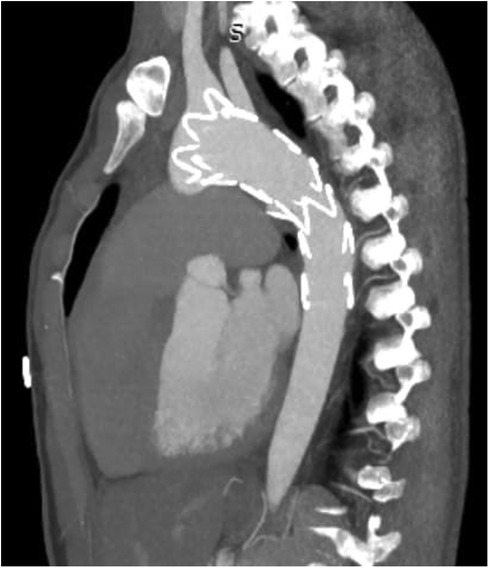
Figure 3. CT angiography after one month showed no endoleak with complete occlusion of PDA shunt. CT, computed tomography; PDA, patent ductus arteriosus.
We presented a 27-year-old patient with a large PDA complaining of exertional dyspnea and progressive fatigue who was treated successfully with TEVAR using a custom-made device.
PDA is a prevalent congenital heart disease with an estimated incidence rate of one in every 2,000 term births (1). While most patients are typically diagnosed and treated during childhood, some cases may persist into adulthood (2). Adult PDAs tend to be more complex in terms of their shape, size, and degree of calcification compared to those diagnosed in childhood. Therefore, routine surgical procedures utilized for children may not be suitable for adult patients and leads to a controversy regarding the optimal surgical approach in these patients (3–5).
Surgical closure and percutaneous catheterization are the two main treatment options for PDA. Generally, percutaneous catheterization technologies for PDA closure are considered the most commonly used method because they are more effective and less invasive. However, endovascular intervention may not be a feasible option for complex PDAs because of severe complications. As a result, surgical closure has remained the preferred method for adults with complicated PDAs until now (8).
Several cases of successful PDA closure using the Amplatzer septal occluder have been reported in adult patients. However, this alternative option has not been approved by the Food and Drug Administration for large PDAs, and results have not been satisfactory in some case series. Additionally, there are some potential disadvantages, such as residual shunting in PDAs with some anatomical features, which may make this option inappropriate for certain patients (9).
Non-touch exclusion technique with TEVAR presents a less invasive alternative to surgical closure and may be a viable option for treating PDA in high-risk complex patients (6). Few studies have been conducted on this alternative procedure. In a case series published by Lai et al. (10), four patients who had a large PDA along with pulmonary arterial hypertension were successfully treated with TEVAR in China. Follow-up was conducted for all participants for 3 to 18 months. A TTE performed one month after surgery showed a significant decrease in pulmonary arterial pressure and left ventricle end-diastolic diameter demonstrating the success of the surgery.
Yamabe et al. (11) presented a 52-year-old patient in Japan who was referred with a 7 mm PDA. Other transcatheter procedures were deemed unsuitable for this patient due to potential complications. Consequently, an endovascular aortic repair was performed successfully, closing the PDA with a talent stent prosthesis. The post-operative follow-up period was uneventful.
In a case presented by Kato et al. (12), an adult patient with a 9.7 mm PDA underwent TEVAR. PDA was closed, and the patient was discharged with no adverse events. Similarly, in another article by Orimoto et al. (13), a woman was admitted complaining of heart failure manifestations. Echocardiography showed a PDA with 14 mm diameter that TEVAR was performed for its closure. Completion angiography and post-TEVAR CT illustrated the closure of PDA with no endoleaks.
Endo et al. (14) presented a 66-year-old man who was admitted for the treatment of a large aneurysm and type B aortic dissection as evidenced by CT angiography. Echocardiography revealed a PDA, which was closed by TEVAR through the ascending aorta. The post-operative period was uneventful with no adverse events reported.
In another study by Kim et al. (15), an adult patient in South Korea complaining of exertional dyspnea and a continuous cardiac murmur found in her physical examination was admitted to the hospital. TTE demonstrated the existence of a PDA that was treated using a non-touch exclusion technique with TEVAR without any complication reported during the follow-up period. The post-operative angiography showed closure of PDA without endoleak.
Seguchi et al. (16) presented an adult patient in Japan with PDA, AR, and coarctation of the aorta (CoA) who underwent a two-stage surgery. The first stage involved the TEVAR procedure to treat CoA and PDA with the stent graft deployment at a diameter of 31 mm after balloon dilation. A few days later, an open surgery was performed for the treatment of AR, which involved aortic valve replacement through a median sternotomy. No adverse events were reported during discharge.
In another article by Freeman et al. (17), a 31-year-old man complaining of heart failure manifestations was diagnosed with a 2.7 cm PDA. Due to the complexity of the PDA and the inability of an occlusive device to close it, other treatment options were considered. Ultimately, a zone 2 deployment of a TEVAR graft was performed for this patient, who was discharged without experiencing adverse events.
TEVAR offers several advantages over other routine techniques; at first, there is no need for catheter manipulation during this procedure. This means that unfavorable procedure-related complications such as perforation and aortic dissection are greatly reduced. Furthermore, because TEVAR devices are mainly used for treating thoracic aortic aneurysms, TEVAR can be a safe alternative for PDA closure in cases with accompanying aortic aneurysm (18).
On the other hand, there are some disadvantages. Firstly, the TEVAR procedure has its own potential complications including endoleaks, endograft collapse, and vascular access-related adverse events such as arterial rupture, perforation or dissection which can lead to retroperitoneal hemorrhage and lower limb ischemia requiring the prompt implementation of necessary measures (19). Secondly, TEVAR necessitates a relatively wide access vessel due to the larger diameter of its delivery system compared to some transcatheter procedures (20).
In addition, during a TEVAR procedure, it might be necessary to cover the LSA. Since most chronic LSA occlusions are asymptomatic, surgeons typically adopt an expectant approach following endograft occlusion of the LSA during TEVAR. This approach may lead to rare but emergent ischemic or neurologic complications related to LSA coverage which may require considering elective revascularization (21).
Furthermore, there is a controversial debate between TEVAR and other alternative procedures in terms of financial aspects. Although several studies have shown that TEVARs generally have similar or lower in-hospital charges compared to open procedures, other studies have demonstrated higher costs for TEVARs in the long-term follow up due to the need for more frequent chest follow-up imaging tests and reintervention measures (22–26).
Adult PDAs that are recognized as more complex in terms of size, shape, and the presence of calcification can pose additional challenges when using routine surgical techniques. TEVAR may be a viable alternative to other transcatheter techniques, offering a safe and effective solution for treating complicated PDAs in some patients.
A 27-year-old male patient presented to our hospital with exertional dyspnea and progressive fatigue. After considering various available treatment options, we explained them to the patient who cooperated well and provided informed consent for the TEVAR procedure. The procedure was successfully performed with no adverse events, and the patient's symptoms were relieved. Follow-up CT angiography one month later showed complete occlusion of PDA without endoleak.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies involving humans because the review board of our institution, Tehran Heart Center, Tehran University of Medical Sciences, Tehran, Iran, doesn't need ethical approval for case reports. All of the authors declared that it has been conducted in accordance with the World Medical Association's Declaration of Helsinki. The study was conducted in accordance with the local legislation and institutional requirements. The participant provided his written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YJ, KH, ST and HG: idea making, supervision, and final revision. HSO: writing of the manuscript and critical revision. IA: critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
We thank all those who directly or indirectly helped us in conducting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mitchell S, Korones S, Berendes H. Congenital heart disease in 56,109 births incidence and natural history. Circulation. (1971) 43(3):323–32. doi: 10.1161/01.CIR.43.3.323
2. Campbell M. Natural history of persistent ductus arteriosus. Br Heart J. (1968) 30(1):4. doi: 10.1136/hrt.30.1.4
3. Moore JW, Levi DS, Moore SD, Schneider DJ, Berdjis F. Interventional treatment of patent ductus arteriosus in 2004. Catheter Cardiovasc Interv. (2005) 64(1):91–101. doi: 10.1002/ccd.20243
4. Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE. Multicenter USA amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol. (2004) 44(3):513–9. doi: 10.1016/j.jacc.2004.03.074
5. Tomita H, Takamuro M, Fuse S, Horita N, Hatakeyama K, Tsutsumi H, et al. Coil occlusion of patent ductus arteriosus impact of 0.052-inch gianturco coil without amplatzer duct occluder. Circ J. (2006) 70(1):28–30. doi: 10.1253/circj.70.28
6. Roques F, Hennequin J-L, Sanchez B, Ridarch A, Rousseau H. Aortic stent-graft for patent ductus arteriosus in adults: the aortic exclusion technique. Ann Thorac Surg. (2001) 71(5):1708–9. doi: 10.1016/S0003-4975(01)02456-0
7. Krichenko A, Benson LN, Burrows P, Möes C, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. (1989) 63(12):877–80. doi: 10.1016/0002-9149(89)90064-7
8. Djukanovic BP, Micovic S, Stojanovic I, Unic-Stojanovic D, Birovljev S, Vukovic PM. The current role of surgery in treating adult patients with patent ductus arteriosus. Congenit Heart Dis. (2014) 9(5):433–7. doi: 10.1111/chd.12164
9. Khajali Z, Firouzi A, Ghaderian H, Aliramezany M. Trans-catheter closure of large PDA in adult patients with amplatzer device: case series. Cardiol Young. (2022) 32(1):161–4. doi: 10.1017/S1047951121002602
10. Lai Y-Q, Xu S-D, Li Z-Z, Yang B-Z, Wang S, Li J-H, et al. Thoracic endovascular aortic repair of adult patent ductus arteriosus with pulmonary hypertension. J Thorac Cardiovasc Surg. (2008) 135(3):699–701. doi: 10.1016/j.jtcvs.2007.11.028
11. Yamabe K, Shimizu H, Nemoto A, Yozu R. Endovascular aortic repair of patent ductus arteriosus in an adult patient. Interact Cardiovasc Thorac Surg. (2012) 14(2):217–9. doi: 10.1093/icvts/ivr009
12. Kato G, Nakai M, Tokunaga N, Shimizu S, Okada M. Thoracic endovascular aortic repair for patent ductus arteriosus in an elderly patient with congestive heart failure. Gen Thorac Cardiovasc Surg. (2016) 64:280–2. doi: 10.1007/s11748-014-0457-z
13. Orimoto Y, Ishibashi H, Sugimoto I, Yamada T, Maruyama Y, Hagihara M, et al. A case of patent ductus arteriosus in an elderly patient treated by thoracic endovascular aortic repair. Ann Vasc Dis. (2016) 9(4):326–9. doi: 10.3400/avd.cr.16-00046
14. Endo Y, Irie Y, Nishida K, Fujimiya T, Katada Y. Total arch replacement with concomitant thoracic endovascular aortic repair via the ascending aorta for extended thoracic aneurysm; report of a case. Kyobu Geka the Jpn J Thorac Surg. (2018) 71(5):347–50.
15. Kim JH, Baek JH. Thoracic endovascular repair technique for the treatment of patent ductus arteriosus in an elderly patient: a case report. Medicine. (2018) 97(49):e13558. doi: 10.1097/MD.0000000000013558
16. Seguchi R, Horikawa T, Kiuchi R, Sanada J, Ohtake H, Watanabe G. Successful two-stage treatment for coarctation of the aorta-postductal type and aortic regurgitation with thoracic endovascular aortic repair and aortic valve replacement. Ann Vasc Dis. (2020) 13(4):414–7. doi: 10.3400/avd.cr.20-00040
17. Freeman KA, Arnaoutakis DJ, Martin TD, Fudge JC, Arnaoutakis GJ. Patent ductus arteriosus exclusion technique using thoracic endovascular aortic repair. Ann Thorac Surg. (2023) 115(2):e53–5. doi: 10.1016/j.athoracsur.2022.02.052
18. Fujii K, Saga T, Kitayama H, Nakamoto S, Kaneda T, Nishino T, et al. Successful closure of a patent ductus arteriosus using an aortic stent graft. Ann Thorac Cardiovasc Surg. (2012) 18(1):71–4. doi: 10.5761/atcs.cr.10.01656
19. Chen SW, Lee KB, Napolitano MA, Murillo-Berlioz AE, Sattah AP, Sarin S, et al. Complications and management of the thoracic endovascular aortic repair. Aorta. (2020) 8(03):049–58. doi: 10.1055/s-0040-1714089
20. Jim J, Rubin BG, Moon MR, Geraghty PJ, Sicard GA, Sanchez LA. Arterial access for thoracic endograft placement. Ann Vasc Surg. (2010) 24(5):640–5. doi: 10.1016/j.avsg.2010.02.032
21. Feezor RJ, Lee WA. Management of the left subclavian artery during TEVAR. In: Seminars in vascular surgery. WB Saunders (2009). p. 159–164.
22. Arnaoutakis GJ, Hundt JA, Shah AS, Cameron DE, Black JH III. Comparative analysis of hospital costs of open and endovascular thoracic aortic repair. Vasc Endovascular Surg. (2011) 45(1):39–45. doi: 10.1177/1538574410380471
23. Orandi BJ, Dimick JB, Deeb GM, Patel HJ, Upchurch GR Jr. A population-based analysis of endovascular versus open thoracic aortic aneurysm repair. J Vasc Surg. (2009) 49(5):1112–6. doi: 10.1016/j.jvs.2008.12.024
24. Glade G, Vahl A, Wisselink W, Linsen M, Balm R. Mid-term survival and costs of treatment of patients with descending thoracic aortic aneurysms; endovascular vs. Open repair: a case-control study. Eur J Vasc Endovasc Surg. (2005) 29(1):28–34. doi: 10.1016/j.ejvs.2004.10.003
25. Narayan P, Wong A, Davies I, Angelini GD, Bryan AJ, Wilde P, et al. Thoracic endovascular repair versus open surgical repair—which is the more cost-effective intervention for descending thoracic aortic pathologies? Eur J Cardiothorac Surg. (2011) 40(4):869–74. doi: 10.1016/j.ejcts.2011.01.010
Keywords: thoracic endovascular aortic repair, patent ductus arteriosus, elderly PDA, non-touch exclusion technique, custom-made device
Citation: Jenab Y, Salehi Omran H, Hosseini K, Tofighi S, Ghaderian H and Ates I (2023) Case report: Thoracic endovascular aortic repair using a non-touch exclusion technique with a custom-made device for the treatment of a large patent ductus arteriosus. Front. Cardiovasc. Med. 10:1218158. doi: 10.3389/fcvm.2023.1218158
Received: 6 May 2023; Accepted: 4 August 2023;
Published: 17 August 2023.
Edited by:
Francesco Sturla, IRCCS San Donato Polyclinic, ItalyReviewed by:
Ender Ödemiş, Koç University Hospital, Türkiye© 2023 Jenab, Salehi Omran, Hosseini, Tofighi, Ghaderian and Ates. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Homa Ghaderian aG9tYWdoYWRlcmlhbkBnbWFpbC5jb20=
Abbreviations AR, aortic regurgitation; CoA, coarctation of the aorta; CT, computed tomography; LSA, left subclavian artery; PDA, patent ductus arteriosus; TEVAR, thoracic endovascular aortic repair; TTE, transthoracic echocardiography.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.