
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 19 October 2023
Sec. Sex and Gender in Cardiovascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1217525
 Hilmi Alnsasra1,2*†
Hilmi Alnsasra1,2*† Gal Tsaban1,2,†
Gal Tsaban1,2,† Jean Marc Weinstein1,2
Jean Marc Weinstein1,2 Mhamad Nasasra1,2
Mhamad Nasasra1,2 Tal Ovdat3
Tal Ovdat3 Roy Beigel3
Roy Beigel3 Katia Orvin4
Katia Orvin4 Moti Haim1,2
Moti Haim1,2
Background: Acute myocardial infarction (AMI) complicated by tachyarrhythmias or high-grade atrioventricular block (HAVB) may lead to increased mortality.
Purpose: To evaluate the sex differences in patients with AMI complicated by tachyarrhythmias and HAVB and their associated outcomes.
Materials and methods: We analyzed the incidence rates of arrhythmias following AMI from the Acute Coronary Syndrome Israeli Survey database from 2000 to 2018. We assessed the differences in arrhythmias incidence and the associated mortality risk between men and women.
Results: This cohort of 14,280 consecutive patients included 3,159 (22.1%) women and 11,121 (77.9%) men. Women were less likely to experience early ventricular tachyarrhythmia (VTA), (1.6% vs. 2.3%, p = 0.034), but had similar rates of late VTA (2.3% vs. 2.2%, p = 0.62). Women were more likely to experience atrial fibrillation (AF) (8.6% vs. 5.0%, p < 0.001) and HAVB (3.7% vs. 2.3%, p < 0.001). The risk of early VTAs was similar in men and women [adjusted Odds Ratio (aOR) = 0.76, p = 0.09], but women had a higher risk of AF (aOR = 1.27, p = 0.004) and HAVB (aOR = 1.30, p = 0.03). Early [adjusted hazard ratio (aHR) = 2.84, p < 0.001] and late VTA (aHR =- 4.59, p < 0.001), AF (aHR = 1.52, p < 0.001) and HAVB (aHR = 2.83, p < 0.001) were associated with increased 30-day mortality. Only late VTA (aHR = 2.14, p < 0.001) and AF (aHR = 1.44, p = 0.002) remained significant in the post 30 days period.
Conclusions: During AMI women experienced more AF and HAVB but fewer early VTAs than men. Early and late VTAs, AF, and HAVB were associated with increased 30-day mortality. Only late VTA and AF were associated with increased post-30-day mortality.
Women with acute coronary syndrome (ACS) have longer hospitalizations, more in-hospital complications, and increased mortality as compared to men (1–6). Despite significant therapeutic advances over the last decades, acute myocardial infarction (AMI) is frequently complicated by supraventricular and ventricular tachyarrhythmia as well as conduction disturbances which are related to increased morbidity and mortality (7–14).
Early ventricular tachyarrhythmias (within 48 h after AMI) (VTAs) are the most frequent cause of death early after AMI (15, 16). Prior studies from the thrombolytic (17, 18) and percutaneous coronary intervention (PCI) era (19–22) showed a higher risk of long-term death associated with late VTAs (after 48 h from AMI) but not with early onset VTAs.
Atrial fibrillation (AF) often complicates AMI with an incidence between 6% and 21% (23–28). AF may cause further impairment of coronary circulation and left ventricular function and it remains a strong predictor of mortality (28, 29).
Despite the implementation of PCI and the decreasing occurrence of HAVB in patients hospitalized for AMI, the occurrence of HAVB in MI remains significant with reported incidence rates between 2.7% overall in AMI patients (30) and 3.5% in ST-elevation myocardial infarction (STEMI) patients treated with primary PCI (31). Moreover, this complication continues to have serious adverse prognostic implications also in the PCI era (30, 31).
Most of the studies regarding arrhythmias in the setting of AMI were conducted before the PCI era, and most of them did not specifically investigate differences between men and women in terms of incidence rates and outcomes. In the current study, we aimed to investigate the incidence and outcome of tachyarrhythmias and HAVB in women as compared to men in a large national registry of unselected consecutive patients with AMI.
We collected data from the Acute Coronary Syndrome Israeli Survey (ACSIS) conducted between 2000 and 2018. Briefly, the ACSIS Registry, a 2-month nationwide survey conducted biennially for more than 20 years, prospectively collects data from all consecutive ACS admissions in all 25 coronary care units in Israel. Patient management is left to the discretion of the attending physicians. Discharge diagnoses were recorded as determined by the attending physicians based on clinical, electrocardiographic, echocardiographic, and biomarker criteria. Dedicated study personnel recorded demographic, historical, and clinical data, including medical management, on prespecified study forms. The Central Data Coordinating Center was responsible for collecting all case report forms, and the Israel Heart Society was responsible for keeping the survey database. Thirty-day outcomes and 1-year mortality were ascertained by hospital chart review, telephone contact, and use of the Israeli National Population Registry. For the present study we used data solely from patients with STEMI or non-ST-elevation myocardial infarction (NSTEMI) This register-based analysis of pre-existing data was conducted according to the principles expressed in the Declaration of Helsinki and ethics committees approved the ACSIS in each of the participating centers. All patients provided written informed consent for data collection and subsequent analysis. Endpoints were prespecified by the ACSIS steering committee. The attending physician made the diagnosis of AMI using all available data based on the Universal Definition of Myocardial Infarction (32–34).
We compared the specific incidence rates of in-hospital arrhythmias between men and women. The arrhythmias of interest included: new-onset AF, HAVB, or sustained VTAs [sustained ventricular tachycardia (VT)/ventricular fibrillation (VF)].
New onset AF was defined as the occurrence of AF as an in-hospital complication in the absence of known previous AF at baseline. HAVB was defined if either complete AV block or Mobitz type II second grade AV block occurred during the index hospitalization.
Sustained VT during the index hospitalization was defined as either lasting more than 30 s or requiring termination earlier due to instability. Sustained ventricular arrhythmia (VT/VF) was further categorized as early (within 48 h from MI) or late (more than 48 h). For every subtype of these arrhythmias (AF, VT/VF, HAVB), the outcomes of patients were compared between those who experienced the specific arrhythmia during the index hospitalization compared to those without it in men and women.
Baseline demographic and clinical characteristics are presented as numbers and percentages for categorical variables and median values and interquartile ranges or mean (SD) values for continuous variables. Categorical variables were compared using the χ2 test, and continuous variables were compared using the Wilcoxon rank sum test or the t-test as appropriate. To obtain odds ratios (ORs) with a 95% confidence interval (CI) for the occurrence of every specific arrhythmia (AF, VTAs, and HAVB) in women vs. men (reference), univariate and multivariable logistic regression models were performed. Covariate adjustment with the propensity score was performed.; the propensity score was built using logistic regression model (one for each of the dependent variables: AF, VTAs, HAVB) and evaluates the probability for the dependent variable, including the following prespecified covariates: age, chronic renal failure at baseline, prior myocardial infarction (MI), congestive heart failure (CHF) at baseline, beta-blockers (BB) treatment at baseline, STEMI diagnosis, left ventricular ejection fraction (LVEF), Killip class at presentation, hypertension, diabetes and peak CK. Multiple imputation was used for missing values in the included covariates. Survival curves were presented to assess the relationship between gender and 1-year mortality, and the Kaplan-Meier pairwise log-rank tests with Holm's p-value adjustment were used. The associations between the occurrence of every specific in-hospital arrhythmia (AF, VTAs, HAVB) and all-cause mortality (30-day mortality, 1-year mortality, 30-day to 1-year mortality) were evaluated in the total cohort and separately among men and women using multivariable Cox proportional hazards models, adjusted for propensity score as described above. Interaction between gender and each of the in-hospital arrhythmias was assessed. All tests were conducted at a two-sided overall 5% significance level (p = 0.05). All analyses were performed using R statistical software (R-studio, V.4.0.3, Vienna, Austria).
Among 14,280 consecutive patients with AMI (93% had type 1 MI), 3,159 were (22.1%) women and 11,121 (77.9%) men. Women were older (72 vs. 61 years, p < 0.001) and were more likely to have diabetes, hypertension dyslipidemia, CHF, and a history of cerebrovascular disease. However, women were less likely to have prior MI, previous PCI or coronary artery bypass graft (CABG). Moreover, women were more likely to be treated with BB, calcium channels blockers (CCB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptors blockers (ARB), statins and diuretics. There were more women with LVEF below 40% as compared to men. Women were less likely to present with STEMI but were more likely to present with pulmonary edema and cardiogenic shock (Table 1).
Overall, 306 (2.1%) patients presented with early VTAs, and 316 (2.2%) patients experienced late VTAs (Table 2). Men were more likely to develop early VTA (2.1% vs. 1.6%, p = 0.034) but the rate of late VTA was similar in men and women (2.3% vs. 2.2%, p = 0.62). After multivariate adjustment, the risk of early VTA [adjusted OR (aOR) 0.76, CI (0.56, 1.03), p = 0.09] and late VTA [aOR 1.0, CI (0.76, 1.30), p = 0.99] was similar in men and women (Table 2). The aOR of the propensity score is 3.30, 95% CI (2.69, 4.09), p < 0.001 for early VTA and 3.84, 95% CI (2.88, 5.08), p < 0.001 for late VTA.
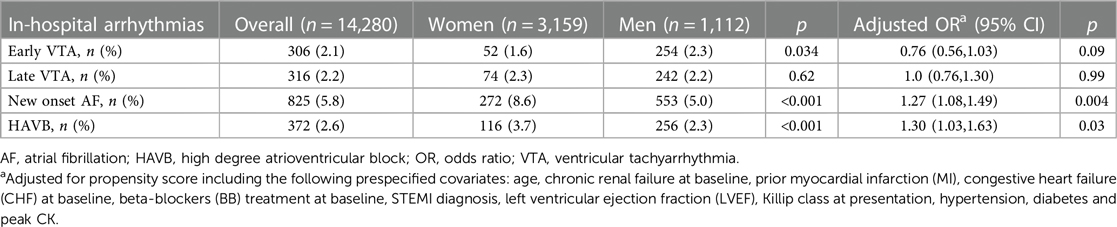
Table 2. Crude rates and multivariable-adjusted odds ratios of in-hospital arrhythmias in women as compared to men.
Overall, 825 (5.8%) patients had new-onset AF during the index hospitalization. The rate of new-onset AF was higher in women than in men (8.6% vs. 5%, p < 0.001). Compared to men, women were at higher risk of incident AF after multivariate analysis [aOR 1.27, CI (1.08, 1.49), p = 0.004] (Table 2). The aOR of the propensity score is 2.60, 95% CI (2.39, 2.82), p < 0.001.
HAVB was observed in 327 patients (2.6%), and women were more likely to experience HAVB during the index hospitalization as compared to men (3.7% vs. 2.3%, p < 0.001). Women were at higher risk to experience HAVB after multivariate analysis (aOR 1.30, CI (1.03, 1.63, p = 0.03) (Table 2). The aOR of the propensity score is 8.34, 95% CI (6.15, 11.24), p < 0.001.
One year after AMI, 1,472 (10.6%) deaths occurred. The mortality rate was 8.9% in men and 16% in women, p < 0.001 (Table 3).
Early VTAs were associated with increased 1-year mortality in the total cohort [adjusted HR (aHR) 2.19, CI (1.71, 2.80) p < 0.001]. However, this was driven by increased mortality risk at 30-days [aHR 2.84, CI (2.14, 3.77), p < 0.001] with no increased risk of death in the post 30 days period after AMI [aHR 1.24, CI (0.68, 2.26), p = 0.50]. This association was similar in men and women, as outlined in Table 3.
Overall, the occurrence of late VTA was found to be associated with increased mortality risk at 30 days [aHR 4.59, CI (3.70, 5.70), p < 0.001] and at 1 year [aHR 3.73, CI (3.11, 4.48), p < 0.01] including the post 30 days period after MI [aHR 2.14, CI (1.48, 3.09), p < 0.001]. The association between late VTAs and mortality was similar and significant in both men and women (Table 3, Figure 1A).
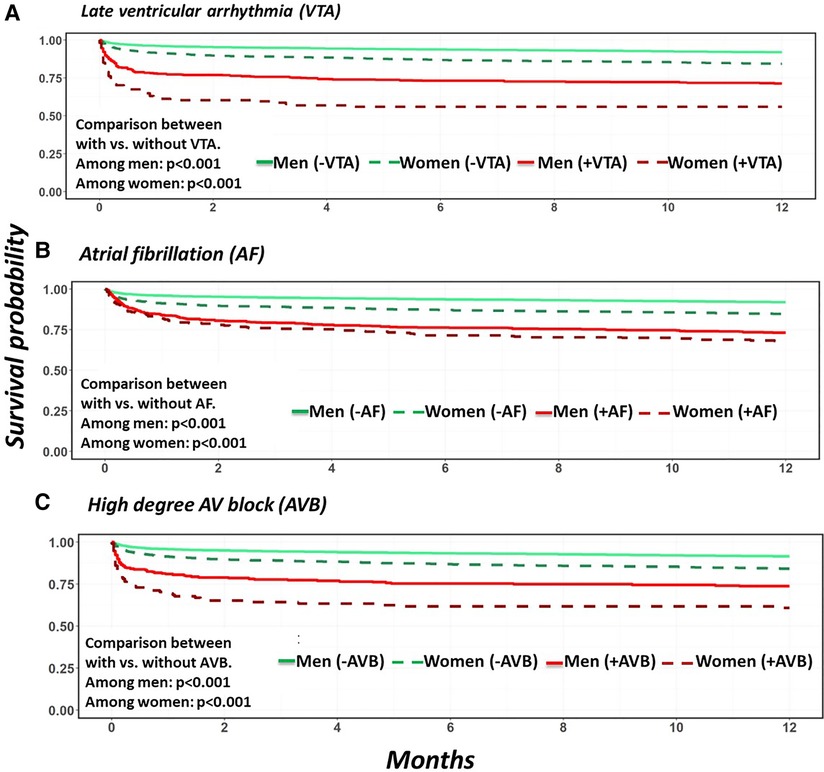
Figure 1. Unadjusted patients’ survival probabilities comparing patients with and without arrhythmia stratified by sex. (A) Late sustained ventricular arrhythmias. (B) Atrial fibrillation. (C) High degree AV block.
New onset AF was found to be associated with increased mortality risk at 30 days, [aHR 1.52, CI (1.25, 1.86), p < 0.001], 1 year [aHR 1.49, CI (1.28, 1.73 p < 0.01] and in the post 30 days period after AMI [aHR 1.44, CI (1.15, 1.82), p = 0.002]. Interestingly, AF was found to be associated with increased 30-day mortality risk in men [aHR 1.83, CI (1.42, 2.34), p < 0.001] but not in women [aHR 1.17, CI (0.85, 1.62), p = 0.35]. However, the post 30-day mortality risk and therefore the 1-year mortality risks were increased in both men [aHR 1.64, CI (1.36, 1.98), p < 0.001] and women [aHR 1.34, CI (1.05, 1.71), p = 0.02] who developed AF during their index hospitalization, with a higher relative risk of 1-year mortality in men than women (p for interaction <0.001) (Table 3, Figure 1B).
Notably, Patients with AF were more likely to have cardiovascular comorbidities, a history of MI, cerebrovascular disease, and peripheral vascular disease. Patients with AF were more likely to present with multivessel disease, severe left ventricular dysfunction, pulmonary edema, and cardiogenic shock. Bleeding events were higher in patients with AF, and they were less likely to undergo coronary revascularization compared to their counterparts. Patients with AF had a higher incidence of stroke during the index hospitalization and only 22.5% of them were discharged with oral anticoagulation (Supplementary Table S5 in the Supplementary Material).
The occurrence of HAVB was found to be associated with increased 1-year mortality in the total cohort [aHR 2.29, CI (1.88, 2.79), p < 0.01]. This was mainly driven by an increased risk of mortality at 30 days [aHR 2.83, CI (2.24, 3.58), p < 0.001]. However, the association between HAVB and mortality risk in the post 30 days period after MI did not reach statistical significance [aHR 1.47, CI (1.00, 2.15), p = 0.05] (Table 3, Figure 1C).
Analysis for patients with STEMI and non-STEMI are provided in the Supplementary Tables S1–S4.
The main findings of this real-world study of patients with AMI are: (1) Men were more likely than women to experience sustained early VTAs but the incidence of late VTAs was similar in men and women. (2) Women were more likely to experience AF and HAVB during their index hospitalization. (3) Early and late VTAs, AF, and HAVB were all associated with increased 30-day mortality whereas only late VTA and AF were associated with increased mortality in the post 30-day period. Early VTAs and HAVB were not associated with excess risk of death after hospital stay.
In the current study, VTAs occurred in 4.3% (2.1% early VTAs and 2.2% late VTAs) of patients, with early onset (<48 h) VTA less prevalent in women. Both early and late VTA were associated with short-term mortality whereas only late VTAs were associated with long-term mortality. These findings are similar in men and women. Earlier studies have reported incidence rates of VTAs between 6% in patients with AMI undergoing PCI and 10% in patients who received thrombolytic therapy (17, 19). However, these studies included mainly patients presenting with STEMI. In a previous report from our group, we demonstrated that VTAs occurred in 3.8% of patients [2.1% early (≤48 h) and 1.7% late (>48 h) VTA], similar to the present but somewhat later cohort (20). Prior studies from the thrombolytic era showed that women and men appear to be at similar risk for developing VTAs after AMI (17, 21). The impact of VTAs on the short and long-term prognosis of patients with AMI has been debated over the years. Sustained VTAs >48 h after index MI have been associated with increased mortality risk (35). However, the relationship between early VTAs and mortality remains controversial. Some studies reported that both early and late VTAs were found to be associated with increased late mortality, with late arrhythmias carrying a worse prognosis (17, 19). Others have suggested that the early post-MI sustained VTAs may be associated with increased short-term mortality but without increased risk over the long term (20, 22). In a very recent data analysis from the FAST-MI (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) program, 2.5% of patients developed VF. Similarly, women had a lower risk of developing VF during AMI compared with men, and the risk of death associated with VF was similar in men and women (36). However, the aforementioned study included only STEMI patients and the arrhythmia of interest was VF compared to combined VF or sustained VT in our study.
We found that 5.8% of the patients had new-onset AF with women's predominance. AF was found to be associated with decreased survival which was more pronounced in men as compared to women. In the APEX-AMI trial of 5,745 STEMI patients treated with primary PCI, 6.3% of patients developed new-onset AF, and it was associated with heart failure, cardiogenic shock, stroke, and increased 90-day mortality (28). Similarly, we found that patients with AF were more likely to present with multivessel disease, severe left ventricular dysfunction, pulmonary edema, and cardiogenic shock as well as a higher incidence of stroke during the index hospitalization. However, our study included patients with STEMI and NSTEMI, and the rate of new-onset AF was similar to APEX-AMI. In a recent study of 6,228 patients with AMI who underwent PCI, the rate of newly diagnosed AF was slightly higher (7.9%) and it was associated with an increased risk of death (37). Interestingly, new-onset AF was found to be associated with increased 1-year mortality risk in both men and women in the present study. Meta-analysis of 30 cohort studies that reported sex specific associations between AF (not MI-related) and all-cause mortality showed that AF is a stronger marker for death in women compared with men (38). However, data on specific sex related differences in the incidence and outcomes of AF complicating AMI are scarce. Thus, our findings prompt the need for further studies assessing the prognostic importance of AF and AF management in men as compared to women.
In our study, the overall incidence rate of HAVB was 2.6%. The occurrence of HAVB was higher in women and was associated with increased risk of death similarly in men and women. Data from the thrombolytic era suggest that 6.9% of patients hospitalized with STEMI develop HAVB, and it is associated with increased 30-day and 1-year mortality. Moreover, women were more likely to develop HAVB than men (39). Later report from the PCI era showed that the incidence of HAVB among STEMI patients has decreased (3.2%) with the implementation of primary PCI. Despite this, it remained a severe prognostic factor also in the PCI era. Notably, like in the present study, female gender was found to be predictive of developing HAVB in STEMI patients (31). More recent analysis from our group that included STEMI and NSTEMI patients suggests a similar rate (2.7%) of HAVB that decreases over time and is associated with increased 30-day and 1-year mortality (30). To the best of our knowledge, no previous studies specifically investigated differences in short and long-term outcomes of HAVB between men and women. Interestingly, a recent cohort study of 443 patients with unexplained syncope and bundle branch block showed that, compared to men, women have a lower risk of AV block and need for cardiac pacing. However, only 21% of patients had ischemic heart disease and no patients with AMI were included (40).
Our study has several limitations inherent to the observational, retrospective, nonrandomized design of this study. As in any observational study, we could not exclude residual confounding and associations despite the adjustment for the most clinically relevant variables. Our study focused on in-hospital arrhythmias, and arrhythmias occurring out of the hospital might be under-reported. Moreover, data on temporary or permanent pacing and antiarrhythmic medications were not systematically collected. The main strengths of our study are the large sample size with prospectively collected data in a uniform case report form and standard definitions used in all centers and endpoints that were centrally adjudicated.
In conclusion, in this contemporary cohort of patients with AMI, the incidence rate of early VTA was higher among men whereas the rate of late VTA was similar in men and women. Women experienced more AF and HAVB than men. Early VTA, late VTAs, AF, and HAVB were associated with increased short-term mortality risk whereas only late VTA and AF were associated with increased long-term mortality.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
HA: writing and conceptualizing. GT: statistical analysis. JW: editing. MN: organizing data and editing. MH: conceptualizing and editing. TO: statistical analysis. RB: reviewing and editing. KO: reviewing and editing. All authors contributed to the article and approved the submitted version.
MH received an unrestricted educational grant from the Michel Mirowski International Fund.
All mentioned authors contributed significantly to this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1217525/full#supplementary-material
ACE-I, angiotensin-converting enzymes inhibitors; AF, atrial fibrillation; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCB, calcium channels blockers; CHF, congestive heart failure; CI, confidence interval; CVA, cerebrovascular accident; HAVB, high degree atrioventricular block; HR, hazard ratio; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; OR, odds ratio; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SD, standard deviation; STEMI, ST-elevation myocardial infarction; TIA, transient ischemic attack; VF, ventricular fibrillation; VSR, ventricular septal rupture; VT, ventricular tachycardia; VTA, ventricular tachyarrhythmia.
1. Gholizadeh L, Davidson P. More similarities than differences: an international comparison of CVD mortality and risk factors in women. Health Care Women Int. (2008) 29:3–22. doi: 10.1080/07399330701723756
2. Dolor RJ, Melloni C, Chatterjee R, Allen LaPointe NM, Williams JB Jr, Coeytaux RR, et al. Treatment strategies for women with coronary artery disease. Report no.: 12-EHC070-EF. Rockville (MD): Agency for Healthcare Research and Quality (US) (2012).
3. Anderson ML, Peterson ED, Brennan JM, Rao SV, Dai D, Anstrom KJ, et al. Short- and long-term outcomes of coronary stenting in women versus men: results from the national cardiovascular data registry centers for medicare & medicaid services cohort. Circulation. (2012) 126:2190–9. doi: 10.1161/CIRCULATIONAHA.112.111369
4. Ahmed B, Dauerman HL. Women, bleeding, and coronary intervention. Circulation. (2013) 127:641–9. doi: 10.1161/CIRCULATIONAHA.112.108290
5. Poon S, Goodman SG, Yan RT, Bugiardini R, Bierman AS, Eagle KA, et al. Bridging the gender gap: insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am Heart J. (2012) 163:66–73. doi: 10.1016/j.ahj.2011.09.025
6. Wasfy JH, Rosenfield K, Zelevinsky K, Sakhuja R, Lovett A, Spertus JA, et al. A prediction model to identify patients at high risk for 30-day readmission after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes. (2013) 6:429–35. doi: 10.1161/CIRCOUTCOMES.111.000093
7. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC)endorsed by: association for European paediatric and congenital cardiology (AEPC). Europace. (2015) 17:1601–87. doi: 10.1093/europace/euv319
8. Solomon SD, Zelenkofske S, McMurray JJ, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med. (2005) 352:2581–8. doi: 10.1056/NEJMoa043938
9. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. (2002) 346:877–83. doi: 10.1056/NEJMoa013474
10. Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. (2004) 351:2481–8. doi: 10.1056/NEJMoa041489
11. Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, Wojciechowski D, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med. (2009) 361:1427–36. doi: 10.1056/NEJMoa0901889
12. Luo J, Li H, Qin X, Liu B, Zhao J, Maihe G, et al. Increased risk of ischemic stroke associated with new-onset atrial fibrillation complicating acute coronary syndrome: a systematic review and meta-analysis. Int J Cardiol. (2018) 265:125–31. doi: 10.1016/j.ijcard.2018.04.096
13. Biasco L, Radovanovic D, Moccetti M, Rickli H, Roffi M, Eberli F, et al. New-onset or pre-existing atrial fibrillation in acute coronary syndromes: two distinct phenomena with a similar prognosis. Rev Esp Cardiol. (2019) 72:383–91. doi: 10.1016/j.rec.2018.03.002
14. Zeymer U, Annemans L, Danchin N, Pocock S, Newsome S, Van de Werf F, et al. Impact of known or new-onset atrial fibrillation on 2-year cardiovascular event rate in patients with acute coronary syndromes: results from the prospective EPICOR registry. Eur Heart J Acute Cardiovasc Care. (2019) 8:121–9. doi: 10.1177/2048872618769057
15. Goldberg RJ, Gurwitz JH, Gore JM. Duration of, and temporal trends (1994-1997) in, prehospital delay in patients with acute myocardial infarction: the second national registry of myocardial infarction. Arch Intern Med. (1999) 159:2141–7. doi: 10.1001/archinte.159.18.2141
16. Liang JJ, Hodge DO, Mehta RA, Russo AM, Prasad A, Cha YM. Outcomes in patients with sustained ventricular tachyarrhythmias occurring within 48 h of acute myocardial infarction: when is ICD appropriate? Europace. (2014) 16:1759–66. doi: 10.1093/europace/euu138
17. Newby KH, Thompson T, Stebbins A, Topol EJ, Califf RM, Natale A. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO investigators. Circulation. (1998) 98:2567–73. doi: 10.1161/01.cir.98.23.2567
18. Volpi A, Cavalli A, Santoro E, Tognoni G. Incidence and prognosis of secondary ventricular fibrillation in acute myocardial infarction. Evidence for a protective effect of thrombolytic therapy. GISSI investigators. Circulation. (1990) 82:1279–88. doi: 10.1161/01.cir.82.4.1279
19. Mehta RH, Starr AZ, Lopes RD, Hochman JS, Widimsky P, Pieper KS, et al. Incidence of and outcomes associated with ventricular tachycardia or fibrillation in patients undergoing primary percutaneous coronary intervention. J Am Med Assoc. (2009) 301:1779–89. doi: 10.1001/jama.2009.600
20. Orvin K, Eisen A, Goldenberg I, Gottlieb S, Kornowski R, Matetzky S, et al. Outcome of contemporary acute coronary syndrome complicated by ventricular tachyarrhythmias. Europace. (2016) 18:219–26. doi: 10.1093/europace/euv027
21. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American heart association. Circulation. (2016) 133:916–47. doi: 10.1161/CIR.0000000000000351
22. Demidova MM, Smith JG, Höijer CJ, Holmqvist F, Erlinge D, Platonov PG. Prognostic impact of early ventricular fibrillation in patients with ST-elevation myocardial infarction treated with primary PCI. Eur Heart J Acute Cardiovasc Care. (2012) 1:302–11. doi: 10.1177/2048872612463553
23. Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. (2000) 101:969–74. doi: 10.1161/01.cir.101.9.969
24. Wong CK, White HD, Wilcox RG, Criger DA, Califf RM, Topol EJ, et al. New atrial fibrillation after acute myocardial infarction independently predicts death: the GUSTO-III experience. Am Heart J. (2000) 140:878–85. doi: 10.1067/mhj.2000.111108
25. Eldar M, Canetti M, Rotstein Z, Boyko V, Gottlieb S, Kaplinsky E, et al. Significance of paroxysmal atrial fibrillation complicating acute myocardial infarction in the thrombolytic era. SPRINT and thrombolytic survey groups. Circulation. (1998) 97:965–70. doi: 10.1161/01.cir.97.10.965
26. Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. Global utilization of streptokinase and TPA for occluded coronary arteries. J Am Coll Cardiol. (1997) 30:406–13. doi: 10.1016/s0735-1097(97)00194-0
27. Behar S, Zahavi Z, Goldbourt U, Reicher-Reiss H. Long-term prognosis of patients with paroxysmal atrial fibrillation complicating acute myocardial infarction. SPRINT study group. Eur Heart J. (1992) 13:45–50. doi: 10.1093/oxfordjournals.eurheartj.a060046
28. Lopes RD, Elliott LE, White HD, Hochman JS, Van de Werf F, Ardissino D, et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J. (2009) 30:2019–28. doi: 10.1093/eurheartj/ehp213
29. Lewis EF, Velazquez EJ, Solomon SD, Hellkamp AS, McMurray JJ, Mathias J, et al. Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J. (2008) 29:748–56. doi: 10.1093/eurheartj/ehn062
30. Alnsasra H, Ben-Avraham B, Gottlieb S, Ben-Avraham M, Kronowski R, Iakobishvili Z, et al. High-grade atrioventricular block in patients with acute myocardial infarction. Insights from a contemporary multi-center survey. J Electrocardiol. (2018) 51:386–91. doi: 10.1016/j.jelectrocard.2018.03.003
31. Gang UJ, Hvelplund A, Pedersen S, Iversen A, Jøns C, Abildstrøm SZ, et al. High-degree atrioventricular block complicating ST-segment elevation myocardial infarction in the era of primary percutaneous coronary intervention. Europace. (2012) 14:1639–45. doi: 10.1093/europace/eus161
32. Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined–a consensus document of the joint European society of cardiology/American college of cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol. (2000) 36:959–69. doi: 10.1016/s0735-1097(00)00804-4
33. Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. (2007) 28:2525–38. doi: 10.1093/eurheartj/ehm355
34. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Circulation. (2012) 126:2020–35. doi: 10.1161/CIR.0b013e31826e1058
35. Bhar-Amato J, Davies W, Agarwal S. Ventricular arrhythmia after acute myocardial infarction: ‘the perfect storm’. Arrhythm Electrophysiol Rev. (2017) 6:134–9. doi: 10.15420/aer.2017.24.1
36. Weizman O, Marijon E, Narayanan K, Boveda S, Defaye P, Martins R, et al. Incidence, characteristics, and outcomes of ventricular fibrillation complicating acute myocardial infarction in women admitted alive in the hospital. J Am Heart Assoc. (2022) 11:e025959. doi: 10.1161/JAHA.122.025959
37. Obayashi Y, Shiomi H, Morimoto T, Tamaki Y, Inoko M, Yamamoto K, et al. Newly diagnosed atrial fibrillation in acute myocardial infarction. J Am Heart Assoc. (2021) 10:e021417. doi: 10.1161/JAHA.121.021417
38. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, et al. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. Br Med J. (2016) 532:h7013. doi: 10.1136/bmj.h7013
39. Meine TJ, Al-Khatib SM, Alexander JH, Granger CB, White HD, Kilaru R, et al. Incidence, predictors, and outcomes of high-degree atrioventricular block complicating acute myocardial infarction treated with thrombolytic therapy. Am Heart J. (2005) 149(4):670–4. doi: 10.1016/j.ahj.2004.07.035
40. Francisco-Pascual J, Rivas-Gándara N, Bach-Oller M, Badia-Molins C, Maymi-Ballesteros M, Benito B, et al. Sex-related differences in patients with unexplained syncope and bundle branch block: lower risk of AV block and lesser need for cardiac pacing in women. Front Cardiovasc Med. (2022) 9:838473. doi: 10.3389/fcvm.2022.838473
Keywords: ventricular arrhythmia, atrial fibrillation, atrioventricular block, acute myocardial infarction, women
Citation: Alnsasra H, Tsaban G, Weinstein JM, Nasasra M, Ovdat T, Beigel R, Orvin K and Haim M (2023) Sex differences in ventricular arrhythmia, atrial fibrillation and atrioventricular block complicating acute myocardial infarction. Front. Cardiovasc. Med. 10:1217525. doi: 10.3389/fcvm.2023.1217525
Received: 5 May 2023; Accepted: 22 August 2023;
Published: 19 October 2023.
Edited by:
Emma Louise Robinson, University of Colorado, United StatesReviewed by:
Harsh Patel, Southern Illinois University Carbondale, United States© 2023 Alnsasra, Tsaban, Weinstein, Nasasra, Ovdat, Beigel, Orvin and Haim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hilmi Alnsasra aC5hbG5zYXNyYUBnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.