- 1Department of Cardiology, University Hospital Basel, Basel, Switzerland
- 2School of Biomedical Engineering and Imaging Sciences, King’s College London, London, United Kingdom
- 3Department of Cardiology, Kantonsspital St. Gallen, St. Gallen, Switzerland
- 4Department of Cardiology, Erasmus Medical Center, Rotterdam, The Netherlands
- 5Department of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
Background: Some patients with cardiac resynchronisation therapy (CRT) experience super-response (LVEF improvements to ≥50%). At generator exchange (GE), downgrading (DG) from CRT-defibrillator (CRT-D) to CRT-pacemaker (CRT-P) could be an option for these patients on primary prevention ICD indication and no required ICD therapies. Long-term data on arrhythmic events in super-responders is scarce.
Methods: CRT-D patients with LVEF improvement to ≥50% at GE were identified in four large centres for retrospective analysis. Mortality, significant ventricular tachyarrhythmia and appropriate ICD-therapy were determined, and patient analysis was split into two groups (downgraded to CRT-P or not).
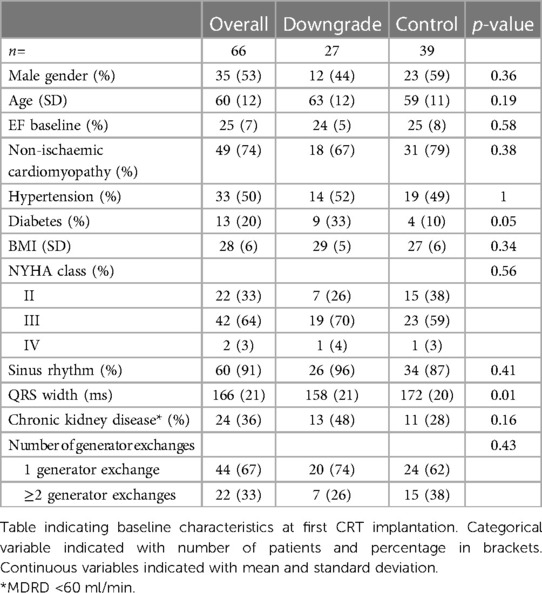
Results: Sixty-six patients (53% male, 26% coronary artery disease) on primary prevention were followed for a median of 129 months [IQR: 101–155] after implantation. 27 (41%) patients were downgraded to CRT-P at GE after a median of 68 [IQR: 58–98] months (LVEF 54% ± 4%). The other 39 (59%) continued with CRT-D therapy (LVEF 52% ± 6%). No cardiac death or significant arrhythmia occurred in the CRT-P group (median follow-up (FU) 38 months [IQR: 29–53]). Three appropriate ICD-therapies occurred in the CRT-D group [median FU 70 months (IQR: 39–97)]. Annualized event-rates after DG/GE were 1.5%/year and 1.0%/year in the CRT-D group and the whole cohort, respectively.
Conclusions: No significant tachyarrhythmia were detected in the patients downgraded to CRT-P during follow-up. However, three events were observed in the CRT-D group. Whilst downgrading CRT-D patients is an option, a small residual risk for arrhythmic events remains and decisions regarding downgrade should be made on a case-by-case basis.
Introduction
Cardiac resynchronization therapy (CRT) is an established treatment option in symptomatic patients with congestive heart failure (CHF) and left bundle branch block (LBBB), and has been shown to reduce both mortality and morbidity (1, 2). Because patients with an indication for CRT often also fulfil the indication for primary implantable cardioverter-defibrillator (ICD) implantation, most devices implanted are CRT defibrillators (CRT-D) (around 70%) (3).
A considerable subgroup of CRT patients demonstrate a “super-response”, i.e., an improvement of left ventricular ejection fraction (LVEF) to 50% or higher (1, 4–6). As different echocardiographic and clinical variables have been used in previous studies, a commonly accepted definition of super-response is not available, thus prevalence is difficult to estimate. If super-response is defined as an increase of LVEF of ≥50%, the prevalence ranges from 6% to 24% (5, 6).
Importantly, improvement of LVEF is associated with fewer arrhythmic events (1, 4, 5, 7–9). If improvement in LVEF is maintained to the first or second battery depletion, patients would not necessarily fulfil the indication for primary prevention implantable cardioverter defibrillator (ICD) at the time of generator exchange (GE). In patients with CRT-D devices, normalisation of LVEF and no previous requirement for significant ICD therapy downgrade to a CRT pacemaker (CRT-P) is becoming more common. However, in current practice less than 10% of patients are downgraded—with the most common reasons being life expectancy <1 year (61%), terminal severe heart failure (42%), and age >80 years (38%) (10). Frailty (28%) and prior inappropriate therapy (without the need for appropriate device therapy) (4%) were less frequent reasons (10).
Potential advantages of a downgrade to CRT-P include reduced risk of inappropriate ICD-shocks [∼20% (11)], decreased risk of infection (12), smaller pocket size, longer battery life and lower device costs. However, the protection against life-threatening ventricular arrhythmias is lost.
Downgrading devices with the nowadays mostly used DF-4 header requires the abandonment of the ICD lead and the additional implantation of a pace/sense lead, which complicates downgrading and involves some risks. Still, a significant number of patients with an active DF-1 system remain who need GE and in whom downgrading is a technically feasible option.
In the literature, data on long-term outcome of super-responders after GE is scarce and limited to small case series of downgraded patients (13, 14). In order to assess this further, we established a multi-centre retrospective cohort containing patients who experienced super-response by the time of GE, with a view to describing the clinical long-term outcome with respect to ventricular arrhythmias and death.
Materials and methods
In this retrospective study patients with a CRT-D system were screened for super-response at four different centres. LVEF ≥50% at the time of GE was used to identify patients defined as super-responders. Super-responder patients were included for this study if their device was implanted for primary prevention, no arrhythmia occurred after the censor period (see below), and they had consented for use of their health care data in research.
At GE, super-responders were offered downgrade to CRT-P by the treating physician: Patients were informed that as a persistent super-responders, they would not meet the ICD indication anymore and it is unclear from current knowledge whether to continue with ICD backup or not in this situation. Advantages (no inappropriate shocks, longer battery life, smaller device) and disadvantages (small residual risk of malignant arrhythmia) were discussed and the option for downgrade offered. The final decision to downgrade was made solely by the patient according to the his/her preference. No specific clinical parameters or financial considerations influenced this decision.
Significant arrhythmia were defined as occurrence of ventricular fibrillation (VF) or sustained ventricular tachycardia (VT) at least 12 months after initial CRT-D implantation. This includes any ventricular arrhythmia requiring appropriate ICD therapy [antitachycardia pacing (ATP) and/or cardioversion/defibrillation] or sustained ventricular arrhythmia in downgraded patients. Arrythmias detected within 12 months of initial CRT-D implantation were not regarded as event since patients were thought to be still in a stage of myocardial remodelling.
Final outcomes for patients were assessed from the last recorded follow-up visit prior to closure of the database, or at the point of death or significant arrhythmia. Whenever possible, cause of death was classified as either cardiac or non-cardiac. Cardiac death was defined as severe cardiac disease in the absence of another life-limiting disease.
Information including baseline demographics, LVEF both at the time of CRT-D implantation and during follow-up, mortality, hospitalization for arrhythmias and occurrence of ICD-therapies from device interrogation were extracted from medical records and analysed retrospectively. Patients were stratified according to underlying cardiomyopathy (ischaemic heart disease (IHD) vs. non-ischaemic cardiomyopathy (NICM)). IHD was defined as heart failure judged to be ischaemic in origin due to the presence of significant epicardial stenosis. If no significant coronary artery disease was present, underlying cardiomyopathy was classified as NICM.
Data were collected at each centre and pooled for statistical analysis. All patients consented for the use of their health care data and the study was carried out according to the principles of the Declaration of Helsinki from 1975.
Statistical analysis
Statistical analysis was performed with R (version 4.0.2) and SPSS™ (version 23). Continuous variables are expressed as mean values (± one standard deviation). Categorical variables are expressed as numbers (percentage). T-test and χ2 test were used where appropriate. Calculation of the cumulative event-free survival was performed with the Kaplan-Meier survival function and the Log Rank test was used to examine difference between groups. Annualized event-rate was calculated by dividing the number of events by patients-years (patients at risk multiplied by mean follow-up). No multivariable logistic regression model to explore for predictors of arrhythmic events could be performed since the event number was too low.
Results
Baseline characteristics
In the four participating centres, 66 patients with super-response were identified. Baseline characteristics of the patients are displayed in Table 1. First implantation of CRT-D was between 02/2000 and 04/2018. In downgraded patients, diabetes mellitus was more prevalent (33% vs. 10%, p = 0.05) and the QRS duration measured on 12-lead electrocardiogram (ECG) was significantly shorter (158 ms vs. 172 ms, p = 0.01). There were no other statistically significant differences between the groups.
Follow-up
Median overall follow-up after first CRT-D implantation was 129 months (IQR: 101–155). Median follow-up after downgrade or first GE was 53 (IQR: 30–82) months. First generator exchange was performed after median 64 (IQR: 51–71) months. 21 patients (32%) underwent a second GE after median 57 (IQR: 48–70) months. At first GE, mean baseline LVEF had improved from 25% ± 7% to 53% ± 6% (p < 0.001). 57 (86%) patients experienced super-response at first GE. 9 patients (14%) did not achieve super-response until the second GE, although it should be noted that mean LVEF in these patients was considerably higher compared to baseline at the time of first GE (43% ± 3% vs. 26% ± 5%, p < 0.001). In 12 patients (18%) LVEF was assessed at the last GE. In patients with available LVEF recently before file closure, 85% remained super-responders (46/54 patients).
Downgrades
During the follow-up period, 27 patients (41%) were downgraded from CRT-D to CRT-P. The other 39 patients (59%) had replacement CRT-D generators implanted at GE. The median time to downgrade to CRT-P was 68 months (IQR: 58–98) after implant. Mean LVEF at the time of downgrade was 54% ± 4% – this had improved significantly from baseline (24% ± 7%, p < 0.001). 21 patients (78% of those downgraded) were downgraded to CRT-P at first GE, whilst the other 6 patients (22%) were downgraded at second GE.
Events during censor period
Two arrhythmia events (3%) occurred in the first 12 months following initial CRT-D implant (censor period). This period following implantation is assumed to be the time of greatest myocardial remodelling (9).
• Monomorphic VT after one month in a female patient (60 y) with non-ischemic cardiomyopathy (NICM); LVEF 26% at baseline; terminated by ATP; treatment with amiodarone was started until 8 months (stopped for intolerance);
• Electrical storm with multiple VF episodes after 6 months in a male patient (71 y) with ischemic heart disease (IHD) including apical aneurysm; LVEF 28% at baseline; further improvement in LVEF to 50% after 65 months, but deterioration later and death of congestive heart failure after 97 months.
Importantly, although the events occurred in the censor period, both cases were subsequently not considered for downgrade to CRT-P by the treating physician.
Events after the censor period
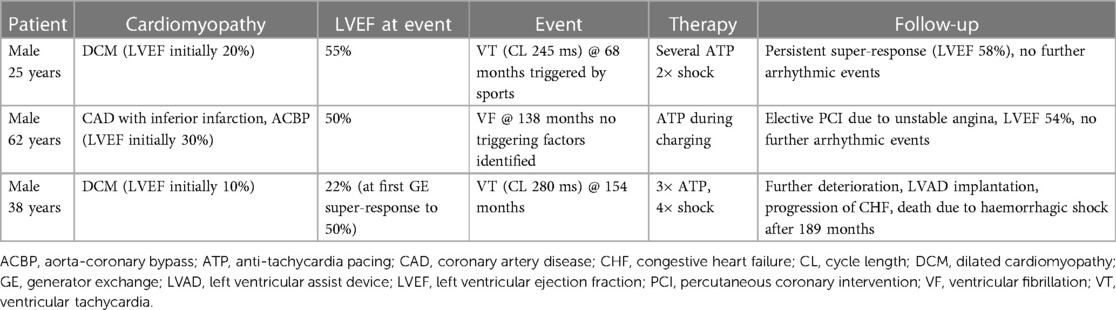
Out of those downgraded to CRT-P, no significant arrhythmia event occurred during a median follow-up of 38 (IQR: 29–53) months after DG as assessed by device interrogation. However, three events (8%, 3/39) were observed in the patients who had CRT-D replacement after the first GE over a median follow-up of 70 (IQR: 39–97) months (Table 2). Survival free of significant arrhythmia is depicted in Figure 1. The difference in event-free survival was not statistically significant (Log Rank p = 0.195). There was no difference in overall survival as shown in Figure 2 (Log Rank p = 0.868).
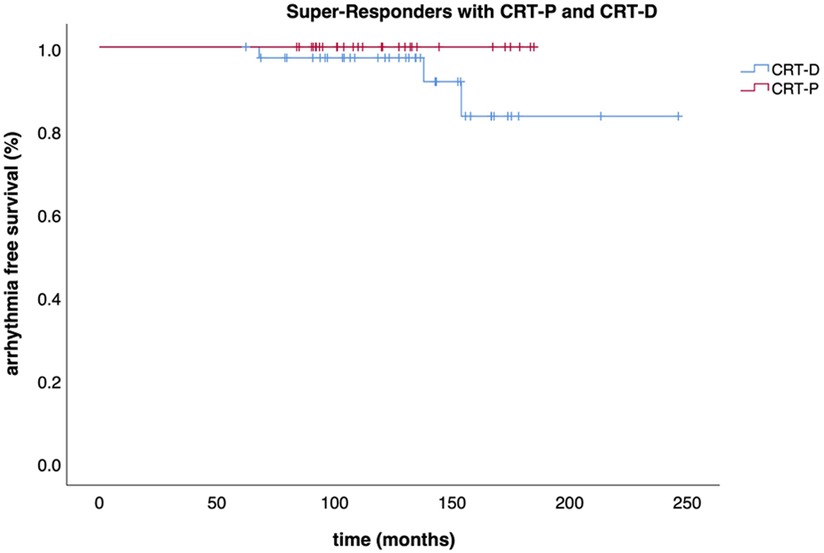
Figure 1. Survival free of significant arrhythmia. Kaplan-Meier curve displaying the event-free survival (significant arrhythmia) of CRT-P and CRT-D patients.
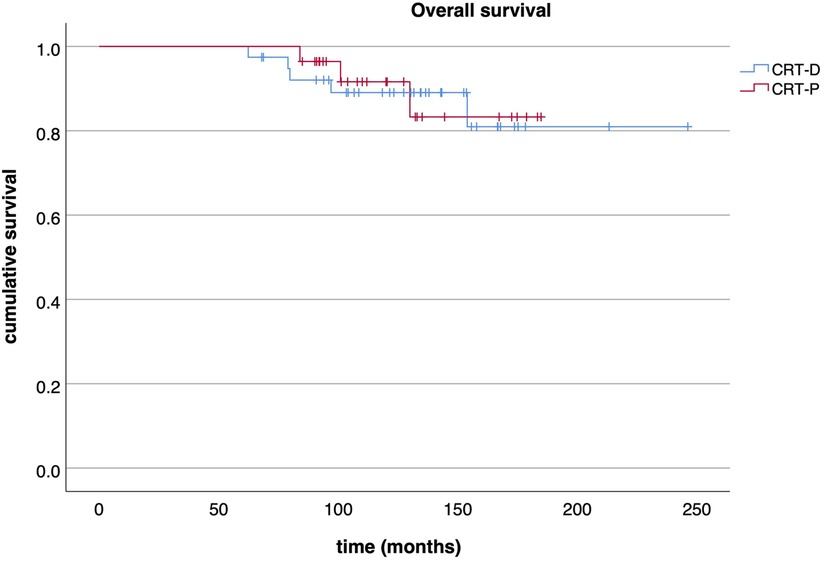
Figure 2. Overall survival. Kaplan-Meier curve displaying the overall survival of CRT-P and CRT-D patients.
Annualised event-rates after DG/GE amounted to 1.5%/year in the CRT-D group, reducing to 1.0%/year when considering the whole cohort. The annualised event-rates during total follow-up were 0.7%/year in the CRT-D group and 0.4%/year in the whole cohort.
As shown in Table 3, 8 (12%) deaths occurred after median 90 (IQR: 80–108) months, only two (25%) of these were cardiac. No cardiac death occurred in downgraded patients.
Discussion
To the best of our knowledge, this is the largest cohort assessment of CRT-D super-responders who have been downgraded to CRT-P. With a total follow-up of more than 10 years [median 129 (IQR: 101–155) months] and more than 4 years following downgrade/first GE [median 53 (IQR: 30–82) months], the observation period is very long.
The main finding of this study is that none of the 27 super-responder patients downgraded to CRT-P suffered from an arrhythmic event or cardiac death during follow-up. This suggests that super-responders without ICD therapies may be good candidates in whom to consider downgrade of their device at generator exchange. However, this special subgroup of all CRT-D patients reflects a minority of CRT-D patients only and generalizability to all CRT-P patients is limited. But, with further improvement of heart failure treatment (“fantastic four”) and a longer follow-up of these patients, the number of super-responders will increase, and consequently the question regarding downgrade. Yet, only small cohorts of CRT-D super-responders after generator exchange have been investigated (13–16). Therefore, this study adds significant data to this important research question.
Event rates in downgraded patients
Ogano et al. demonstrated the feasibility and mid-term safety of downgrading responders (defined as LVEF ≥45%, primary prevention, no VT/VF since implantation) (14). From this cohort of 49 consecutive patients, 7 (14%) were downgraded at GE and followed-up during an observation period of 40 ± 21 months. Similar to our cohort, downgraded patients experienced no arrhythmic events, although it should be noted that the number of downgraded patients in this study was relatively low. Additionally, the downgraded patients tended to have a lower all-cause mortality compared with those patients not deemed to be responders. This trend was not present in our cohort, which may be because all patients assessed in this study fulfilled the criteria for super-response.
Garcia and colleagues downgraded 14 patients to CRT-P following improvement of LVEF to >35% with no ICD therapies (13). Mean LVEF at GE was 49%. During a follow-up of 5.1 ± 1.3 years, there were 2 incidences of VT, but no sudden death. The research team concluded that downgrade was a safe and cost-effective treatment option. Comparability to our cohort is limited as the definitions of super-response differ significantly and the follow-up is twice as long.
Event rates in super-responders after GE
Event rates in our study were surprisingly low for such a long follow-up period, although low event rates in super-responders were also seen in the study by House et al. (15). In 30 ICD patients with super-response (LVEF ≥ 50%), no arrhythmias were seen during a mean follow-up of 25 ± 18 months after generator exchange. However, this study has several key differences compared with our study: the follow-up period was shorter and the sample size smaller, and the cohort included 16 CRT-D patients only.
Nesti et al. observed 103 CRT-D patients for 26 ± 10 months after GE (16). Responders were defined as decrease in left ventricular end-systolic volume of ≥15%. Four responders (4% of the cohort) experienced a first arrhythmia after GE. Unfortunately, it is not reported how many of these responders who suffered from arrhythmia events were super-responders with LVEF ≥50%, which would allow direct comparison with our cohort.
Event rates in super-responders
Arrhythmic events are rare in super-responders as shown by Ghani et al.—in this cohort of CRT-D patients followed until their first GE, none of those classified as super-responders (56/347, 16%) experienced an ICD therapy over a follow-up of 5.3 years (4).
Zecchini et al. showed that in the super-responder subgroup (24%, 62/259), only 7% had a first appropriate device therapy during a mean follow-up of 68 ± 30 months despite LVEF >50%, resulting in an annualized event rate of 1.2%/year (6). Using a similar definition of super-response, Killu and colleagues demonstrated a lower event rate of ICD therapy (0.4%/year, and a cumulative 5 year rate of 2.7%) (5). A recent meta-analysis revealed a rate of ventricular arrhythmia of 0.9%/year (17). Thus, all studies are in line with our findings (annualized event rate of 1.0%–1.5%/year after DG/GE, 0.4%–0.7%/year during total follow-up). With our study looking at a large cohort of downgraded super-responder patients over a longer follow-up period, this study adds to the growing body of evidence that downgrading to CRT-P appears safe and could be considered by clinicians.
Predictors of super-response and survival
Certain patient characteristics are associated with super-response, such as female gender, NICM, higher LVEF at baseline, LBBB morphology, wider QRS duration, BMI <30 kg/m2 and smaller baseline atrial size (4, 18).
Primary prevention defibrillator was not associated with a significant lower mortality compared to a combination of CRT-P and medical therapy in symptomatic patients with NICM (DANISH-trial) (12). The majority of patients (74%) in our cohort had NICM and therefore might represent patients similar to the DANISH-trial at their first GE. As ICD was superior in the subgroups of age <59 years and NT-proBNP <1,177 pg/ml only (12), these factors should influence choice of device at GE. Furthermore, survival advantage with ICD is less clear in older (≥75 years) and diabetic patients (EU-CERT-ICD) (19). In addition, extensive myocardial scaring on cardiac magnetic resonance is a known risk factor for the occurrence of arrhythmic events (HR 5.2) (20).
Although these predictors were established at the time of initial CRT-D implantation, knowledge of these is important to guide and counsel patients at the time of GE. A clinical score usable at GE incorporating baseline and follow-up factors (e.g., age, gender, frailty, malignant arrythmia, myocardial scar, comorbidities) would be desirable. Our sample size and event rate was too small for solid assessment of such factors. But further sub-studies from larger cohorts, meta-analyses or eventually data from the RESET-CRT trial could be useful (21).
Advantages of CRT downgrade and technical considerations
Potential advantages of a downgrade are no inappropriate ICD-shocks [which can be up to 20% (11)], lower infection rates (12) and smaller pocket size. Furthermore, the longer battery life, wider control interval and lower costs can be beneficial for certain healthcare systems. These factors gain more importance in patients with super-response and thus low risk for arrhythmic events. In a contemporary cohort study with CRT-D systems implanted after 2015, the percentage of inappropriate therapy and inappropriate shock were still 7.2% and 3.3%, respectively (22).
Different CRT-D header types exist which complicate the option of downgrading. In systems using DF-1 headers, high-voltage parts can simply be disconnected, and the pace-sense part be connected to a new CRT-P device. Despite being widely replaced by DF-4 leads, still a considerable number of patients have a functioning DF-1 lead and present themselves for GE. In patient with newer DF-4 leads, downgrade to CRT-P is not possible without implanting a new pace/sense lead and abandonment of the DF-4-ICD lead. This additional lead implantation could result in potential complications which might outweigh the benefits of a downgrade. Another possible, but manageable downside of a downgrade is that the CRT-system loses its MRI compatibility if the old ICD lead is not extracted.
CRT in the era of the “fantastic four”
Modern pharmacological treatment markedly improved mortality and morbidity of patients suffering from heart failure (23). With the addition of two potent agents (angiotensin receptor/neprilysin inhibitors (ARNIs) and sodium–glucose co-transporter 2 (SGLT-2)) many patients nowadays have the chance to profit from these “fantastic four”. All these agents improve cardiovascular outcomes, and even reduce arrhythmia and sudden cardiac death (24–27). Furthermore, accumulating data question the mortality benefit of ICDs in primary prevention, especially in patients with NICM (12, 19, 28). Hence, it can be anticipated that the clinical scenario with super-responders presenting for device replacement will occur more often, and consequently the question regarding downgrading to CRT-P. Additionally, with rising health care costs, also economical pressure might influence this decision in the future.
Limitations
Due to the retrospective design of this study, limitations associated with this study design are possible. Due to the lack of randomisation and a predefined protocol, this study represents real-world follow-up data with no predefined timepoints for device interrogation and echocardiography (no core lab available). But the study is based on clinical data which is also used for decision making in clinical routine.
Since not all study sites have a prospective registry, percentage of super-responders from the overall cohort could not be determined. Therefore, selection bias cannot be excluded.
The decision to downgrade was taken in consent between patient and treating physician and not in a randomised fashion. There is no data available for pharmacological heart failure treatment regarding intensity, duration and agents used. This limits the further analysis as optimal medical therapy itself was also shown to reduce mortality and prevent arrhythmia. Nevertheless, all patients were treated according to the current guidelines which includes optimal medical treatment.
To optimally analyse the debate about downgrade of super-responders, a randomized-controlled trial would be optimal. But such a study is very unlikely to ever be conducted. Hence despite the above-mentioned limitations, our study provides important long-term prognostic data on a special subgroup of CRT-patients. Since the majority of these patients had their device implanted before the novel DF-4 header was introduced, still a considerable number of these super-responder patients present with an active DF-1 lead. Knowledge of their long-term course can help clinicians in their daily decision making.
Although reverse remodelling with super-response seems to be persistent over time (29), there are still patients who can deteriorate during follow-up. In our cohort, 85% of patients remained super-responders.
Conclusion
No super-responder downgraded to CRT-P experienced an arrhythmia event during more than 4 years of follow-up. However, 3 (8%) significant arrhythmia events occurred in those who had reimplantation of CRT-D after generator exchange leading to an annualized event-rate of 1.5%/year over subsequent follow-up. Although risk of arrhythmia is relatively low, a residual lifetime risk for sudden cardiac death remains. To validate these findings, a prospective randomised controlled trial would be needed. Meanwhile, we suggest that the decision to downgrade has to be made on a “case-by-case” basis taking into consideration factors such as age, comorbidities, scar, inappropriate ICD therapies and patient's preference.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not provided for this study on human participants because Basel: The local ethics committee approved previously the use of health care data from this cohort (EKNZ 2018-329). St. Gallen: The local ethics committee approved previously the use of health care data from this cohort (2016-00551). Rotterdam: This retrospective study was not subjected to the Dutch Medical Research Involving Human Subjects Act and the need for written informed consent was waived. Jeddah: Informed consent was acquired according to local practise and law. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SF: data collection, statistical analysis, data interpretation, draft of manuscript, approval of final manuscript version. RB: data collection, critical revision and approval of final manuscript version. DT: data collection, critical revision and approval of final manuscript version NA: data collection, critical revision and approval of final manuscript version. RC: English language editing, critical revision and approval of final manuscript version. PA: critical revision and approval of final manuscript version. CS: critical revision and approval of final manuscript version. MK: critical revision and approval of final manuscript version. SO: critical revision and approval of final manuscript version. BS: senior author, concept and study design, data interpretation, critical revision and approval of final manuscript version. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ATP, anti-tachycardia pacing; ARNI, angiotensin receptor/neprilysin inhibitors; CRT, cardiac resynchronization therapy; CHF, congestive heart failure; GE, generator exchange; ICD, implantable cardioverter-defibrillator; IHD, ischemic heart disease; IQR, interquartile range; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NICM, non-ischemic cardiomyopathy; SGLT, sodium–glucose co-transporter; VF, ventricular fibrillation; VT, ventricular tachycardia.
References
1. Ruwald MH, Solomon SD, Foster E, Kutyifa V, Ruwald A-C, Sherazi S, et al. Left ventricular ejection fraction normalization in cardiac resynchronization therapy and risk of ventricular arrhythmias and clinical outcomes: results from the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy. Circulation. (2014) 130(25):2278–86. doi: 10.1161/CIRCULATIONAHA.114.011283
2. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. (2004) 350(21):2140–50. doi: 10.1056/NEJMoa032423
3. Dickstein K, Normand C, Auricchio A, Bogale N, Cleland JG, Gitt AK, et al. CRT Survey II: a European society of cardiology survey of cardiac resynchronisation therapy in 11 088 patients—who is doing what to whom and how? Eur J Heart Fail. (2018) 20(6):1039–51. doi: 10.1002/ejhf.1142
4. Ghani A, Delnoy PPHM, Adiyaman A, Ottervanger JP, Ramdat Misier AR, Smit JJJ, et al. Predictors and long-term outcome of super-responders to cardiac resynchronization therapy. Clin Cardiol. (2017) 40(5):292–9. doi: 10.1002/clc.22658
5. Killu AM, Mazo A, Grupper A, Madhavan M, Webster T, Brooke KL, et al. Super-response to cardiac resynchronization therapy reduces appropriate implantable cardioverter defibrillator therapy. Europace. (2018) 20(8):1303–11. doi: 10.1093/europace/eux235
6. Zecchin M, Proclemer A, Magnani S, Vitali-Serdoz L, Facchin D, Muser D, et al. Long-term outcome of “super-responder” patients to cardiac resynchronization therapy. Europace. (2014) 16(3):363–71. doi: 10.1093/europace/eut339
7. García-Lunar I, Castro-Urda V, Toquero-Ramos J, Mingo-Santos S, Moñivas-Palomero V, Daniela Mitroi C, et al. Ventricular arrhythmias in super-responders to cardiac resynchronization therapy. Rev Esp Cardiol (Engl Ed). (2014) 67(11):883–9. doi: 10.1016/j.rec.2014.01.016
8. Manne M, Rickard J, Varma N, Chung MK, Tchou P. Normalization of left ventricular ejection fraction after cardiac resynchronization therapy also normalizes survival. Pacing Clin Electrophysiol. (2013) 36(8):970–7. doi: 10.1111/pace.12174
9. Schaer BA, Osswald S, Di Valentino M, Soliman OI, Sticherling C, ten Cate FJ, et al. Close connection between improvement in left ventricular function by cardiac resynchronization therapy and the incidence of arrhythmias in cardiac resynchronization therapy-defibrillator patients. Eur J Heart Fail. (2010) 12(12):1325–32. doi: 10.1093/eurjhf/hfq171
10. Tilz R, Boveda S, Deharo J-C, Dobreanu D, Haugaa KH, Dagres N. Replacement of implantable cardioverter defibrillators and cardiac resynchronization therapy devices: results of the European heart rhythm association survey. Europace. (2016) 18(6):945–9. doi: 10.1093/europace/euw157
11. van Rees JB, Borleffs CJW, de Bie MK, Stijnen T, van Erven L, Bax JJ, et al. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. JACC. (2011) 57(5):556–62. doi: 10.1016/j.jacc.2010.06.059
12. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Eng J Med. (2016) 375(13):1221–30. doi: 10.1056/NEJMoa1608029
13. Garcia Quintana A, Blanco Nuez M, Ramirez Rodriguez R, Caballero Dorta E, Valeron Hernandez-Abad D, Diaz Escofet M, et al. Downgrade from CRT-D to CRT-P at the moment of device replacement, an opportunity for selective disinvestment. Eur Heart J. (2013) 34(suppl 1):P3207. doi: 10.1093/eurheartj/eht309.P3207
14. Ogano M, Iwasaki Y-K, Tsuboi I, Kawanaka H, Tajiri M, Takagi H, et al. Mid-term feasibility and safety of downgrade procedure from defibrillator to pacemaker with cardiac resynchronization therapy. Int J Cardiol Heart Vasc. (2019) 22:78–81. doi: 10.1016/j.ijcha.2018.12.012
15. House CM, Nguyen D, Thomas AJ, Nelson WB, Zhu DW. Normalization of left ventricular ejection fraction and incidence of appropriate antitachycardia therapy in patients with implantable cardioverter defibrillator for primary prevention of sudden death. J Card Fail. (2016) 22(2):125–32. doi: 10.1016/j.cardfail.2015.10.015
16. Nesti M, Riccciardi G, Pieragnoli P, Fumagalli S, Padeletti M, Paoletti Perini A, et al. Incidence of ventricular arrhythmias after biventricular defibrillator replacement: impact on safety of downgrading from CRT-D to CRT-P. Minerva Cardioangiol. (2020) 70(4):447–54. doi: 10.23736/S0026-4725.20.05352-9
17. Yuyun MF, Erqou SA, Peralta AO, Hoffmeister PS, Yarmohammadi H, Echouffo Tcheugui JB, et al. Risk of ventricular arrhythmia in cardiac resynchronization therapy responders and super-responders: a systematic review and meta-analysis. Europace. (2021) 23(8):1262–74. doi: 10.1093/europace/euaa414
18. Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. JACC. (2012) 59(25):2366–73. doi: 10.1016/j.jacc.2012.01.065
19. Zabel M, Willems R, Lubinski A, Bauer A, Brugada J, Conen D, et al. Clinical effectiveness of primary prevention implantable cardioverter-defibrillators: results of the EU-CERT-ICD controlled multicentre cohort study. Eur Heart J. (2020) 41(36):3437–47. doi: 10.1093/eurheartj/ehaa226
20. Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. JACC. (2012) 60(5):408–20. doi: 10.1016/j.jacc.2012.02.070
21. ClinicalTrials.gov. Re-evaluation of Optimal Re-synchronisation Therapy in Patients With Chronic Heart Failure (RESET-CRT). (2023). Available at: https://clinicaltrials.gov/ct2/show/NCT03494933
22. Gras D, Clémenty N, Ploux S, Guyomar Y, Legallois D, Segreti L, et al. CRT-D replacement strategy: results of the BioCONTINUE study. J Interv Card Electrophysiol. (2022). doi: 10.1007/s10840-022-01440-5
23. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
24. Jhuo SJ, Lin TH, Lin YH, Tsai WC, Liu IH, Wu BN, et al. Clinical observation of SGLT2 inhibitor therapy for cardiac arrhythmia and related cardiovascular disease in diabetic patients with controlled hypertension. J Pers Med. (2022) 12(2). doi: 10.3390/jpm12020271
25. Rohde LE, Chatterjee NA, Vaduganathan M, Claggett B, Packer M, Desai AS, et al. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter-defibrillator use and heart failure cause: a PARADIGM-HF analysis. JACC Heart Fail. (2020) 8(10):844–55. doi: 10.1016/j.jchf.2020.06.015
26. Rossello X, Ariti C, Pocock SJ, Ferreira JP, Girerd N, McMurray JJV, et al. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left-ventricular systolic dysfunction: an individual patient-level meta-analysis of three randomized-controlled trials. Clin Res Cardiol. (2019) 108(5):477–86. doi: 10.1007/s00392-018-1378-0
27. Ruwald AC, Gislason GH, Vinther M, Johansen JB, Nielsen JC, Philbert BT, et al. Importance of beta-blocker dose in prevention of ventricular tachyarrhythmias, heart failure hospitalizations, and death in primary prevention implantable cardioverter-defibrillator recipients: a danish nationwide cohort study. Europace. (2018) 20(FI2):f217–f24. doi: 10.1093/europace/euy077
28. Hadwiger M, Dagres N, Haug J, Wolf M, Marschall U, Tijssen J, et al. Survival of patients undergoing cardiac resynchronization therapy with or without defibrillator: the RESET-CRT project. Eur Heart J. (2022) 43(27):2591–9. doi: 10.1093/eurheartj/ehac053
29. Frey S, Sticherling C, Osswald S, Reichlin T, Kühne M, Schaer B. Persistent improvement of ejection fraction in patients with a cardiac resynchronisation therapy defibrillator correlates with fewer appropriate ICD interventions and lower mortality. Swiss Med Wkly. (2016) 146:w14300. doi: 10.4414/smw.2016.14300
Keywords: cardiac resynchronisation therapy (CRT), downgrade, super response, arrhythmic risk, appropriate ICD therapy, tachyarrhythmia
Citation: Frey SM, Brenner R, Theuns DA, Al-Shoaibi N, Crawley RJ, Ammann P, Sticherling C, Kühne M, Osswald S and Schaer B (2023) Follow-up of CRT-D patients downgraded to CRT-P at the time of generator exchange. Front. Cardiovasc. Med. 10:1217523. doi: 10.3389/fcvm.2023.1217523
Received: 5 May 2023; Accepted: 1 June 2023;
Published: 15 June 2023.
Edited by:
Alexander H. Maass, University Medical Center Groningen, NetherlandsReviewed by:
Helmut Ulrich Klein, University of Rochester, United StatesChristoph Edlinger, University Hospital Salzburg, Austria
© 2023 Frey, Brenner, Theuns, Al-Shoaibi, Crawley, Amman, Sticherling, Kühne, Osswald and Schaer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beat Schaer YmVhdC5zY2hhZXJAdXNiLmNo
 Simon Martin Frey
Simon Martin Frey Roman Brenner3
Roman Brenner3 Christian Sticherling
Christian Sticherling Michael Kühne
Michael Kühne Beat Schaer
Beat Schaer