
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 12 October 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1213557
This article is part of the Research Topic Methods in Diagnosing Heart Failure View all 5 articles
Background: The relative importance of left atrial reservoir strain (LASr) regarding the Heart Failure Association Pre-test assessment, Echocardiography and natriuretic peptide, Functional testing, Final etiology (HFA-PEFF) score, a diagnostic tool for patients with heart failure with preserved ejection fraction (HFpEF), remains unclear. We aimed to identify the relative importance of LASr compared with variables associated with HFpEF and HFA-PEFF scores.
Methods: Between August 2021 and July 2022, we obtained retrospective data from the participants visiting a single cardiovascular center with subjective symptoms of heart failure, such as dyspnea or chest discomfort. In total, 2,712 participants with sinus rhythm and ejection fraction of more than 50% were enrolled. Multivariable logistic regression analysis, random forest analysis, and supervised machine learning algorithms were performed to identify the relative importance of LASr to the HFA-PEFF score.
Results: The average HFA-PEFF score was 2.4 ± 1.6 points. Two hundred and thirty-eight participants had 5 or 6 points. LASr showed a moderate correlation with the HFA-PEFF score (r = −0.50, p < 0.001). Impaired LASr < 25.2% was an independent variable affecting a high HFA-PEFF score with traditional diastolic function parameters and components of the HFA-PEFF diagnostic algorithm. The odds ratio (OR) [1.74, 95% confidence interval (CI) 1.23–2.47] for LASr was higher compared to that of left ventricular global longitudinal strain (OR 1.59, 95% CI 1.14–2.21), septal E/e’ (OR 1.23, 95% CI 0.85–1.77), and relative wall thickness (OR 1.20, 95% CI 0.76–1.89). LASr was also a relatively more important variable in estimating a high HFA-PEFF score than TR-Vmax, septal E/e’, septal e’, left ventricular mass index, and relative wall thickness, the major echocardiographic components of the HFA-PEFF score.
Conclusions: LASr is an important factor with components of the HFA-PEFF score and is a useful tool to assess patients with HFpEF.
Clinical Trial Registration: URL: https://clinicaltrials.org. Unique identifiers: NCT05638230.
Heart failure with preserved ejection fraction (HFpEF) accounts for nearly half of all heart failure patients, and as society ages, the incidence of this condition is increasing (1). The causes of HFpEF include dysfunction within the heart, such as cardiovascular disease, and various non-cardiac factors, such as obesity, renal impairment, diabetes mellitus, increased arterial stiffness, systemic inflammation, and frailty (2). Since assessment of diastolic dysfunction is complex and difficult, the 2016 diastolic function guidelines updated by the American Society of Echocardiography and the European Association of Cardiovascular Imaging (ASE/EACVI) stated the possibility of predicting left ventricular diastolic dysfunction using only four echocardiographic parameters (3). Recently, diagnostic algorithms combining echocardiographic parameters, clinical variables, and biomarkers have been proposed for diagnosing HFpEF (4, 5). These algorithms are complex and difficult to apply in real clinical practice; however, as measuring the left ventricular end-diastolic pressure in all patients is not feasible, efforts to validate and utilize these diagnostic algorithms in clinics continue (6).
Left atrial (LA) longitudinal strain, which measures the systolic and diastolic function of the left atrium during the entire cardiac cycle via speckle-tracking echocardiography, has less angle and load dependence and reflects the physiological characteristics according to the cardiac cycle of the left atrium more than conventional echocardiography parameters (7). LA strain (LAS), mainly LA reservoir strain (LASr), is useful for predicting the prognosis and treatment effects in various disease groups, including myocardial disease, heart failure, ventricular tachycardia, and stroke (8–12). In recent studies, LASr also provided additional benefits in diagnosing HFpEF (13, 14). However, there is no study regarding the relative importance and correlation of LASr with traditional cardiovascular risk factors and established echocardiographic parameters. We aimed to understand and utilize LASr more effectively in clinical practice by determining its relative importance compared to components of HFpEF diagnostic algorithms that are not yet used in guidelines.
Between August 2021 and July 2022, we retrospectively analyzed data from 3,183 patients aged ≥20 years who visited our cardiovascular center with subjective symptoms of heart failure, such as dyspnea or chest discomfort. Participants underwent transthoracic echocardiography (TTE) and had an International Classification of Disease-10 code of 150 (heart failure) (URL: https://clinicaltrials.org. Unique identifiers: NCT05638230). Patients were excluded based on the following parameters: supraventricular arrhythmia such as atrial fibrillation/flutter or atrial tachycardia (n = 177); left ventricular ejection fraction < 50% (n = 212); permanent pacemaker or implantable cardioverter defibrillator procedure (n = 12); and unable to complete strain analysis because of poor imaging (n = 70). In total, 2,712 participants were enrolled (Figure 1). This study was conducted based on the revised Helsinki Declaration of 2013 and approved by the Institutional Review Board of our hospital (IRB number: 9-2022-0101). The requirement for informed consent was waived because it was a retrospective study.
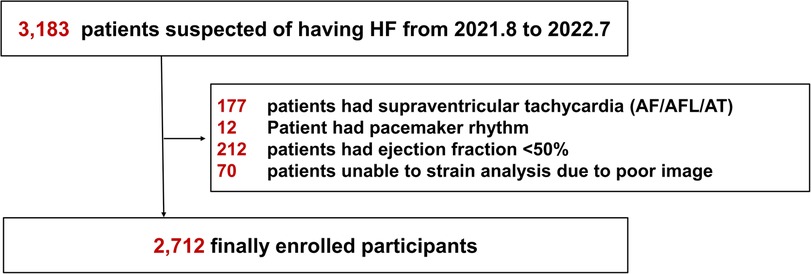
Figure 1. Flowchart of the study. HF, heart failure; AF, atrial fibrillation; AFL, atrial flutter; AT, atrial tachycardia.
We obtained participant information using the clinical data server analysis system, Severance Clinical Research Analysis Portal. Medical history, including hypertension, diabetes mellitus, chronic kidney disease, previous coronary revascularization, and previous stroke, was also obtained using the same analysis system. The operational definitions are summarized in Supplementary Table S1. As laboratory findings, we collected hemoglobin, glucose, glycated hemoglobin, creatinine, NT-proBNP, and total cholesterol levels and calculated the glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration equation (15). The TTE examination performed on participants using the commercially available echocardiographic vendor (Vivid E9/E95, GE HealthCare, Horton, Norway). The traditional echocardiographic parameters and left ventricular global longitudinal strain (LVGLS) were collected based on the guidelines (3, 16). The ejection fraction of the left ventricle (LV) was evaluated using Simpson's biplane method.
LAS was measured using commercially available software (EchoPAC version 204, AFI LA 3.0, GE HealthCare, Horton, Norway) through speckle-tracking and semiautomatic analysis methods per the guidelines (17). An experienced sonographer blinded to clinical information measured LAS. LAS was calculated from the non-foreshortened apical four-chamber view. The start point of the R-wave was used as the time reference; when this was not clear, the nadir of the LAS curve was defined as end-diastole (17). The region of interest (ROI) was automatically traced by selecting the septal and lateral parts of the proximal portion of the mitral annulus and the LA roof and adjusted to match the endocardial border of the LA as needed. The ROI was measured considering anatomical LA wall thickness by setting it as thin as possible (maximal thickness of 3 mm) to avoid including the pericardium. Care was taken to measure it without including the pulmonary vein and LA appendage (Supplementary Figure S1). The LAS curve was measured by dividing it into three phases: LASr, which is the peak value from the nadir of the LA strain curve; LA conduit strain (LAScd), calculated by subtracting the value at the time of start of atrial contraction from the value at mitral valve opening; and LA contraction strain (LASct), the difference between the end-diastolic strain of the ventricle and the value at the start of atrial contraction. We used the LAS obtained from the non-foreshortened apical four-chamber view. All LAS values are presented as absolute values throughout the manuscript for comparison convenience.
The endpoint was the relative importance of the LAS in predicting a high Heart Failure Association Pre-test assessment, Echocardiography and natriuretic peptide, Functional testing, Final etiology (HFA-PEFF) diagnostic algorithm score of 5 or 6 compared to components of the 2016 guideline for diastolic function and the HFA-PEFF diagnostic algorithm.
The values of each variable were presented as mean ± standard deviation or median (interquartile range) based on the fulfillment of normality for continuous variables and as numbers and percentages for categorical variables. The continuous variables were compared using Student's t-test, while the categorical variables were compared using the chi-squared test. Missing data were evaluated using the MssForest algorithm (18). The best cutoff value of LASr for predicting a high HFA-PEFF score was calculated using the Youden index (19). We used multivariable binary logistic regression to identify the factors predicting a high HFA-PEFF score by comparing it to the components of the ASE/EACVI 2016 diastolic function evaluation guideline and the HFA-PEFF diagnostic algorithm. Random forest analysis was used to evaluate the relative importance of LASr in contributing to a high HFA-PEFF score among demographic, clinical, and laboratory covariates. The group was divided into derivation (65%) and validation (35%) cohorts. This was done to evaluate the performance of the optimized model created from the derivation cohort using the receiver operating characteristic analysis, which was measured by the area under the curve. To determine the significance of each variable, the random forest trees were analyzed based on classification error, and the impact of each predictor variable was assessed by permuting it and measuring the resulting error. To observe changes in diagnostic performance upon substituting LASr for some variables in the existing models, we performed analyses of the net reclassification index (NRI) and integrated discrimination improvement (IDI). Two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.1.2 software (R Development Core Team, Vienna, Austria).
The baseline characteristics of 2,712 participants are described in Table 1. The mean age was 62.1 ± 16.5, and 53.0% were women. Participants tended to be obese (average body mass index: 25.0 ± 4.0 kg/m2), half of them had a history of hypertension, and 28.0% had a history of diabetes mellitus, which are risk factors for HFpEF (Table 1). The prevalence rates of chronic kidney disease, coronary artery disease needing revascularization, and previous stroke were 11.7%, 7.7%, and 7.2%, respectively. The mean NT-proBNP was higher than the reference value of 125 pg/ml (953.2 ± 4779.4 pg/ml).
The results of traditional echocardiographic and strain parameters are presented in Table 2. The mean value of the LV ejection fraction was 62.6 ± 4.8%. Over half of the participants (66.4%) exhibited decreased septal e’ velocity < 7 cm/s. Three hundred twenty-three participants (11.9%) had a septal E/e’ ≥15, and 197 participants (7.3%) presented with mitral annular calcification. The proportion of participants with septal E/e’ ≥15 was significantly higher among those with mitral annular calcification than those without (39.1% vs. 9.8%, p < 0.001) (Supplementary Table S2). The mean LA volume index was 31.5 ± 11.2 ml/m2. The mean LVGLS, LASr, LAScd, and LASct were 17.4 ± 2.5, 28.9 ± 8.9, 15.7 ± 7.7, and 13.4 ± 5.5%, respectively.
The average HFA-PEFF score in the total population was 2.4 ± 1.6 points, and 238 (8.8%) had a high HFA-PEFF score. LASr, previously identified as a useful LAS parameter, was moderately correlated with the HFA-PEFF score (r = −0.50, p < 0.001). In the receiver operating characteristic curve analysis, LASr had good diagnostic performance to estimate the HFA-PEFF score [sensitivity 71.8%, specificity 68.9%, area under the curve 0.75, 95% confidence interval (CI) 0.72–0.78]. The area under the curve of LASr was similar to or higher than that of traditional diastolic function parameters [0.77 (0.74–0.79) in septal E/e’, 0.75 (0.72–0.78) in septal e’, 0.70 (0.66–0.74) in maximal velocity of tricuspid regurgitation (TR-Vmax)] (Figure 2).
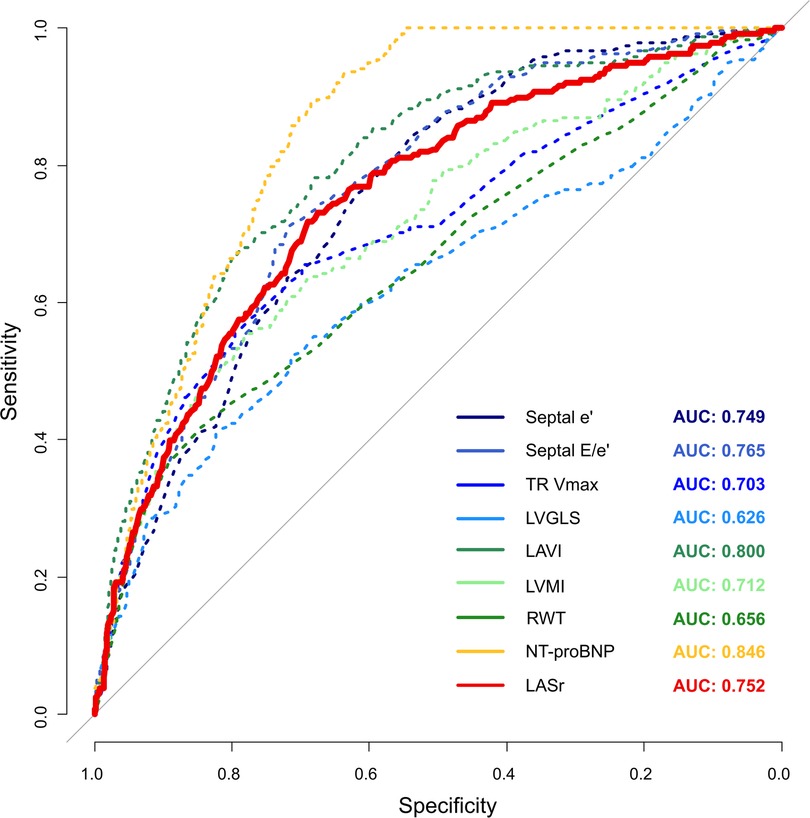
Figure 2. Diagnostic performance of LASr to estimate a high HFA-PEFF score with other components consisting of the HFA-PEFF diagnostic algorithm.
Among diagnostic function parameters, there were moderate correlations between LASr and three of four major diastolic function parameters [r = 0.55 in septal e’, r = −0.43 in septal E/e’, r = −0.41 in LA volume index (LAVI); all p’s < 0.001] and LVGLS (r = 0.37, p < 0.001). LASr was found to be mildly correlated with the peak velocity of tricuspid regurgitation (r = −0.25, p < 0.001) and relative wall thickness (RWT; r = −0.26, p < 0.001) and had a very weak correlation with NT-proBNP (r = −0.12. p < 0.001). In addition, LASr was found to be was moderately correlated with the number of abnormal diastolic function parameters from the ASE/EACVI 2016 diastolic function guideline (r = −0.52, p < 0.001).
We performed a multivariable logistic regression analysis of two different models to identify the relative importance of LASr with components of the ASE/EACVI 2016 diastolic function guideline and the HFA-PEFF diagnostic algorithm (Table 3). In model 1 (2016 ASE/EACVI diastolic function guideline and LASr), LASr was an independent parameter to estimate a high HFA-PEFF score with other diastolic function parameters [odds ratio (OR) 2.39, 95% CI 1.72–3.30, p < 0.001]. The OR of LARs < 25.2% was higher than that of septal E/e’ (OR 1.41, 95% CI 1.00–1.99, p = 0.049), which is a useful parameter to estimate LV filling pressure in real clinical practice. In model 2 (HFA-PEFF diagnostic algorithm and LASr), impaired LASr < 25.2% also had a significant role in predicting a high HFA-PEFF score with covariates (OR 1.74, 95% CI 1.23–2.47; p = 0.002) (Table 3). LASr < 25.5% had a higher OR than septal E/e’ (OR 1.41), LVGLS < 16% (OR 1.59), and RWT (OR 1.20).
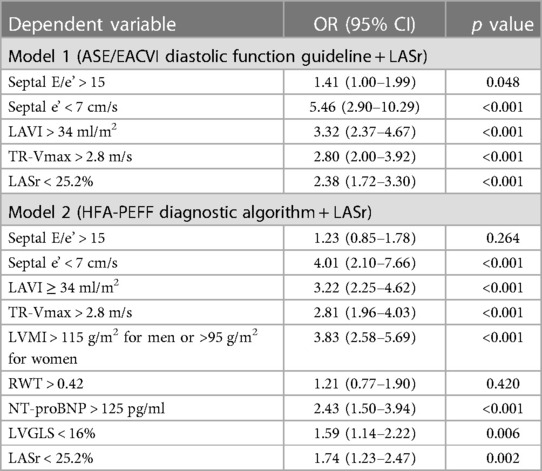
Table 3. Multivariable logistic regression models showing the association between a high HFA-PEFF score of 5 or 6 and the LA reservoir strain with parameters consisting of the 2016 ASE/EACVI diastolic function guideline and the HFA-PEFF score.
We conducted random forest analysis to analyze the relative importance of LAS (LASr, LAScd, and LASct) in predicting a high HFA-PEFF score in relation to 33 other demographic, clinical, laboratory, and echocardiographic covariates. The best-predicting model generated from the training cohort was built on a model of 1,500 tree with an area under the curve of 0.91 (95% CI 0.89–0.92), as evaluated on the validation cohort. Along with LAVI, NT-proBNP, age, LVGLS, hemoglobin, and glomerular filtration rate, LASr had a higher importance in predicting a high HFA-PEFF score than traditional diastolic parameters such as E/e’, TR-Vmax, LV mass index (LVMI), RWT, and septal e’ (Figure 3 and Supplementary Table S3). Presuming the significance of the highest variable (LAVI) to be 100, the relative importance of LASr in a high HFA-PEFF score was 40.3, which was similar to or higher than that of traditional diastolic function parameters (41.9 in septal E/e’, 32.0 in TR-Vmax, 31.3 in LVMI, 27.8 in RWT, and 24.7 in septal e’).
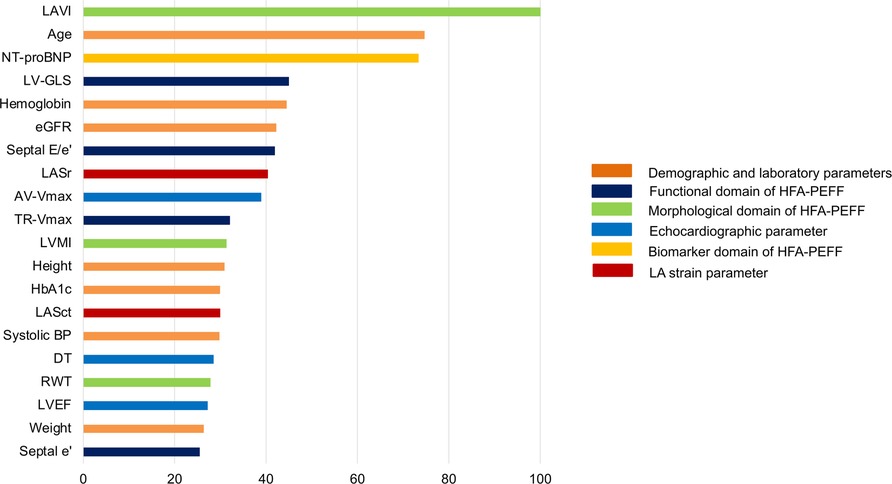
Figure 3. Relative importance of LAS in predicting a high HFA-PEFF score of 5 or 6 as analyzed in random forest analysis. Among 36 variables, only the top 20 parameters are shown. The highest important variable (LAVI) was set as 100, and other variables were compared to identify the relative importance.
Incorporating LASr into existing models for predicting diastolic dysfunction and HFpEF removed variables with lower ORs or statistical insignificance and demonstrated the enhanced diagnostic performance of these revised models to estimate high HFA-PEFF scores (Figure 4 and Table 4). In the newly constructed model (model 3) where LASr replaced septal E/e’, which had a lower OR value in the previous 2016 diastolic function guideline model (model 1’), the performance for predicting a high HFA-PEFF score improved—an increase in the C-statistics value from 0.824 to 0.875, and the NRI and IDI of the new model exhibited enhanced diagnostic performance with values of 0.937 (95% CI 0.814–1.061) and 0.081 (95% CI 0.063–0.099), respectively (Table 4 and Figure 4A). Another newly formulated model (model 4), which incorporated LASr while excluding septal E/e’ and RWT—variables that had lower OR values than LASr in the original HFA-PEFF model (model 2’)—demonstrated statistically significant superior performance compared to the traditional HFA-PEFF algorithm [C-statistics 0.834–0.875; p < 0.001, NRI 0.910 (95% CI 0.784–1.035), IDI (0.074, 95% CI 0.058–0.091)] (Table 4 and Figure 4B).
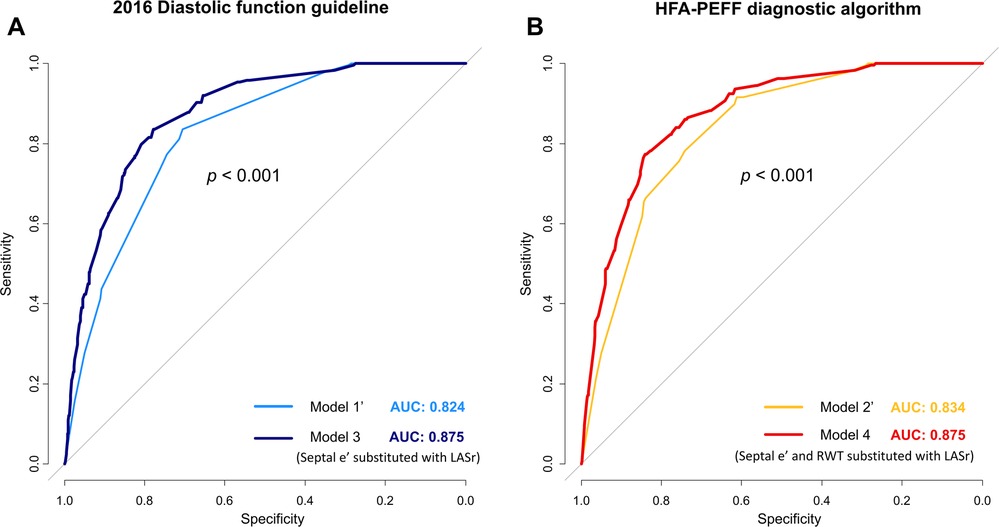
Figure 4. Changes in diagnostic performance for predicting a high HFA-PEFF score upon substituting LASr for variables in the 2016 diagnostic function guideline (A) and HFA-PEFF diagnostic algorithm (B).
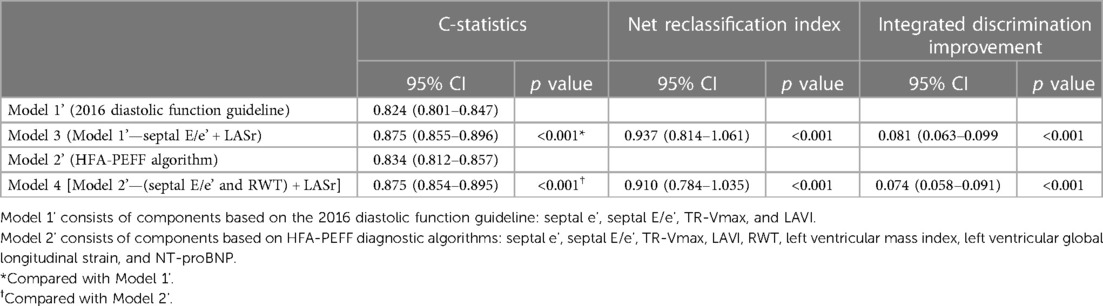
Table 4. Comparison of diagnostic performance of models predicting an HFA-PEFF score of 5 or 6 points.
The present study showed that LAS, especially LASr, was independently associated with a high HFA-PEFF score and good performance to diagnose HFpEF and was similar or a more important parameter than traditional diastolic function parameters such as septal E/e’. In contrast to previous studies that focused on LASr as an independent or incremental predictor for prognosis in patients with heart failure, this study aimed to examine the relative importance of LAS compared to existing parameters for assessing diastolic function. Although LAS is not yet used in the diagnosis algorithm or guidelines for HFpEF, demonstrating the usefulness of LAS could serve as evidence for its potential utilization in the diagnosis of HFpEF.
LAS is a useful indicator to predict a poor cardiovascular prognosis, including readmission, in patients with heart failure (HF) (12, 20). A previous study suggested that LASr is the most important predictor of poor cardiovascular outcomes among cardiac mechanics indicators in patients with HFpEF (21). LASr has also proven helpful in predicting incident HF in individuals with asymptomatic risk factors (22). Moreover, replacing LAVI with LASr more effectively reclassified indeterminate decisions to normal in all cases of diastolic function analysis (22). Conversely, impaired LAS has been reported to predict worse NYHA functional class and elevated estimated right ventricular systolic pressure, even in patients with normal LAVI (23). As LASr progressively changes according to the severity of diastolic dysfunction, it effectively categorizes diastolic dysfunction grading in the existing ASE/EACVI 2016 algorithm (24). In our study, LASr demonstrated similar importance to existing parameters in predicting the possibility of HFpEF; therefore, it may be used in HFpEF diagnosis in the future. In addition to LASr, LAScd and LASct are highly associated with tissue Doppler parameters and represent indicators of LA afterload (LV end-diastolic pressure) and LA pumping function (25, 26). Our results in random forest analysis also demonstrated that LASct had comparable importance to previous important parameters, thus helping to explain the clinical utility of LAS.
The HFA-PEFF diagnostic algorithm was developed to diagnose HFpEF more accurately, which was previously difficult and challenging (4). The algorithm confirms the likelihood of HFpEF through a pre-test assessment of the ambulatory setting, followed by score calculation using TTE and NT-proBNP. The algorithm was validated in two independent prospective cohorts and was demonstrated to be helpful in diagnosing HFpEF (6). Unlike the H2FPEF, another algorithm to diagnose HFpEF, the HFA-PEFF includes a wider range of diastolic function-associated echocardiographic parameters, which increases accuracy while retaining the ASE/EACVI 2016 algorithm (3, 4). However, when tested on a large cohort of patients presenting with dyspnea, the HFA-PEFF diagnostic algorithm was unable to exclude a significant number of healthy participants and diagnosed a group of patients that did not overlap with those diagnosed based on the ASE/EACVI 2016 and H2FPEF algorithms (27). Our study demonstrated the importance of LAS that was comparable to the parameters required for the HFA-PEFF in HFpEF diagnoses. Therefore, LAS can be used as an additional auxiliary or substitute tool in HFpEF diagnosis.
The E/e’ is a long-standing predictor of LV filling pressure and is an important indicator for evaluating diastolic function. However, conditions such as annular calcification, mitral regurgitation, and pericardial disease may not accurately reflect LV filling pressure (3). Among the participants of our study, those with mitral annular calcification also tended to have higher septal E/e’; thus, the predictive power of E/e’ to estimate a high HFA-PEFF score may have been lower than that of LASr. The LASr cutoff value in our study predicted a high HFA-PEFF score of 25.2, which was higher than the previously proposed score of 18 (9); however, this was similar to the average value of 24.6 in the PARAMOUNT trial and the average of the apical four- and two-chamber strain value of 26.0 in the TOPCAT trial (12, 28). There are two reasons to explain this discordance. First, differences in the software used to measure LAS may account for the variation. However, a recent study showed no significant difference in strain values depending on the software used for analysis (29). Second, we tried to trace only the thin LA wall to avoid the pericardial tissue and set the ROI as light as possible by setting the ROI to the default value of 3 mm or less while ensuring that tracing was possible (Supplementary Figure S1) (17). Among speckle-tracking echocardiographic strain analysis software, TOMTEC tends to show higher strain values than EchoPAC (30). However, LAS values would be similar to those measured by TOMTEC if the ROIs were set as thin as possible to trace. Moreover, the main focus of our study was not the LASr cutoff value but rather the potential of LAS to provide additional assistance in diagnosing diastolic dysfunction and HFpEF.
AF patients, who were excluded from our study, show a significant decrease in LASr compared to that in patients in sinus rhythm, a phenomenon that can be explained mechanistically by the absence of booster pump action and the atrioventricular dyssynchrony. In previous studies, LASr in patients with AF was lower than those in the healthy population, with LASr values averaging approximately 10%–15% (31, 32). Another study observed a decrease in LASr even in AF patients with normal invasively measured LV filling pressure (9). This potential decrease in LASr in patients with AF in our study cohort may confound the main purpose of our study, which was to help in the differential diagnosis of HFpEF patients with sinus rhythm. Previous studies also consistently excluded patients with AF, other supraventricular arrhythmias, and pacemaker rhythms from analysis of LA strain (11, 33).
Our study had some limitations. First, the study was designed retrospectively; thus, the power of the evidence may be weaker than that of a prospective or randomized control study. However, we tried to overcome any shortcomings of a retrospective study by measuring LAS across a large cohort. Furthermore, various models and statistical methods were used to demonstrate the concordant significance of LAS. Second, the primary endpoint of our study was not an indicator measured through invasive measurement or a hard endpoint, such as cardiovascular mortality. However, the HFA-PEFF score is an excellent and validated diagnostic algorithm. In addition, for scores of five or higher, the sensitivity and positive predictive value for diagnosing HFpEF were very high, at 93% and 98%, respectively (6). Third, interactions between LAS and other diastolic functions and echocardiographic parameters are possible. Recent studies have suggested that LAS is closely associated with not only LV function but also various indices such as e’ and a’ (9, 25, 26). However, LAS can be used in clinical practice as a reproducible, single index that can integrate various echocardiographic indices and produce an easily understood numerical value. Based on our results, well-designed future studies are needed to accumulate evidence to include LAS in the guidelines for diagnosing HFpEF.
LASr is a relatively important factor with components of the HFA-PEFF score, a useful tool to assess patients with HFpEF. Ultimately, additional efforts are needed to incorporate LAS into the diagnostic process for HFpEF.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Yonsei University College of Medicine, Yongin Severance Hospital, Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MK, SB, JP, and IJ designed the research. JP performed the left atrial strain analysis. MK, SB, and IJ recruited clinical data. MK performed the statistical analysis. MK wrote the manuscript. IJ supervised all aspects of the study. MK, SB, JP, and IJ reviewed, revised the manuscript, and approved the submitted version. All authors contributed to the article and approved the submitted version.
The authors extend their gratitude to Seok-Jae Heo, Ph.D. (Division of Biostatistics, Department of Biomedical Systems Informatics, Yonsei University College of Medicine) for his invaluable assistance with statistical consultation during the revision process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1213557/full#supplementary-material
1. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14(10):591–602. doi: 10.1038/nrcardio.2017.65
2. Obokata M, Reddy YNV, Borlaug BA. Diastolic dysfunction and heart failure with preserved ejection fraction: understanding mechanisms by using noninvasive methods. JACC Cardiovasc Imaging. (2020) 13(1 Pt 2):245–57. doi: 10.1016/j.jcmg.2018.12.034
3. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2016) 17(12):1321–60. doi: 10.1093/ehjci/jew082
4. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European society of cardiology (ESC). Eur Heart J. (2019) 40(40):3297–317. doi: 10.1093/eurheartj/ehz641
5. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. (2018) 138(9):861–70. doi: 10.1161/CIRCULATIONAHA.118.034646
6. Barandiaran Aizpurua A, Sanders-van Wijk S, Brunner-La Rocca HP, Henkens M, Heymans S, Beussink-Nelson L, et al. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur J Heart Fail. (2020) 22(3):413–21. doi: 10.1002/ejhf.1614
7. Vieira MJ, Teixeira R, Goncalves L, Gersh BJ. Left atrial mechanics: echocardiographic assessment and clinical implications. J Am Soc Echocardiogr. (2014) 27(5):463–78. doi: 10.1016/j.echo.2014.01.021
8. Choi YJ, Kim D, Rhee TM, Lee HJ, Park JB, Lee SP, et al. Left atrial reservoir strain as a novel predictor of new-onset atrial fibrillation in light-chain-type cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. (2023) 24(6):751–8. doi: 10.1093/ehjci/jeac269
9. Inoue K, Khan FH, Remme EW, Ohte N, Garcia-Izquierdo E, Chetrit M, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. (2021) 23(1):61–70. doi: 10.1093/ehjci/jeaa415
10. Maffeis C, Morris DA, Belyavskiy E, Kropf M, Radhakrishnan AK, Zach V, et al. Left atrial function and maximal exercise capacity in heart failure with preserved and mid-range ejection fraction. ESC Heart Fail. (2021) 8(1):116–28. doi: 10.1002/ehf2.13143
11. Frydas A, Morris DA, Belyavskiy E, Radhakrishnan AK, Kropf M, Tadic M, et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail. (2020) 7(4):1956–65. doi: 10.1002/ehf2.12820
12. Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. (2016) 9(4):e002763. doi: 10.1161/CIRCHEARTFAILURE.115.002763
13. Hwang IC, Cho GY, Choi HM, Yoon YE, Park JJ, Park JB, et al. H2FPEF score reflects the left atrial strain and predicts prognosis in patients with heart failure with preserved ejection fraction. J Card Fail. (2021) 27(2):198–207. doi: 10.1016/j.cardfail.2020.09.474
14. Ye Z, Miranda WR, Yeung DF, Kane GC, Oh JK. Left atrial strain in evaluation of heart failure with preserved ejection fraction. J Am Soc Echocardiogr. (2020) 33(12):1490–9. doi: 10.1016/j.echo.2020.07.020
15. Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new chronic kidney disease epidemiology collaboration equation in comparison with the modification of diet in renal disease study equation. Am Heart J. (2011) 162(3):548–54. doi: 10.1016/j.ahj.2011.06.006
16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
17. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19(6):591–600. doi: 10.1093/ehjci/jey042
18. Stekhoven DJ, Buhlmann P. MissForest—non-parametric missing value imputation for mixed-type data. Bioinformatics. (2012) 28(1):112–8. doi: 10.1093/bioinformatics/btr597
19. Yin J, Tian L. Joint confidence region estimation for area under ROC curve and Youden index. Stat Med. (2014) 33(6):985–1000. doi: 10.1002/sim.5992
20. Park JH, Hwang IC, Park JJ, Park JB, Cho GY. Prognostic power of left atrial strain in patients with acute heart failure. Eur Heart J Cardiovasc Imaging. (2021) 22(2):210–9. doi: 10.1093/ehjci/jeaa013
21. Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, et al. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circ Cardiovasc Imaging. (2016) 9(3):e003754. doi: 10.1161/CIRCIMAGING.115.003754
22. Potter EL, Ramkumar S, Kawakami H, Yang H, Wright L, Negishi T, et al. Association of asymptomatic diastolic dysfunction assessed by left atrial strain with incident heart failure. JACC Cardiovasc Imaging. (2020) 13(11):2316–26. doi: 10.1016/j.jcmg.2020.04.028
23. Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, et al. Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging. (2018) 11(10):1405–15. doi: 10.1016/j.jcmg.2017.07.029
24. Singh A, Addetia K, Maffessanti F, Mor-Avi V, Lang RM. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging. (2017) 10(7):735–43. doi: 10.1016/j.jcmg.2016.08.014
25. Malaescu GG, Mirea O, Capota R, Petrescu AM, Duchenne J, Voigt JU. Left atrial strain determinants during the cardiac phases. JACC Cardiovasc Imaging. (2022) 15(3):381–91. doi: 10.1016/j.jcmg.2021.09.009
26. Tayal B, Malahfji M, Buergler JM, Shah DJ, Nagueh SF. Hemodynamic determinants of left atrial strain in patients with hypertrophic cardiomyopathy: a combined echocardiography and CMR study. PLoS One. (2021) 16(2):e0245934. doi: 10.1371/journal.pone.0245934
27. Nikorowitsch J, Bei der Kellen R, Kirchhof P, Magnussen C, Jagodzinski A, Schnabel RB, et al. Applying the ESC 2016, H(2) FPEF, and HFA-PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the general population. ESC Heart Fail. (2021) 8(5):3603–12. doi: 10.1002/ehf2.13532
28. Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. (2014) 16(10):1096–103. doi: 10.1002/ejhf.147
29. Rhee T-M, Kim H-K, Choi Y-J, Lee H-J, Hwang I-C, Yoon YE, et al. Agreement of two vendor-independent strain analysis software platforms in assessing left ventricular global longitudinal strain. Int J Cardiovasc Imaging. (2022) 38(9):1939–50. doi: 10.1007/s10554-022-02589-w
30. Park JH. Two-dimensional echocardiographic assessment of myocardial strain: important echocardiographic parameter readily useful in clinical field. Korean Circ J. (2019) 49(10):908–31. doi: 10.4070/kcj.2019.0200
31. Liao JN, Chao TF, Hung CL, Chen SA. The decrease in peak atrial longitudinal strain in patients with atrial fibrillation as a practical parameter for stroke risk stratification. Heart Rhythm. (2021) 18(4):538–44. doi: 10.1016/j.hrthm.2020.12.026
32. Hopman L, Mulder MJ, van der Laan AM, Demirkiran A, Bhagirath P, van Rossum AC, et al. Impaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J Cardiovasc Magn Reson. (2021) 23(1):131. doi: 10.1186/s12968-021-00820-6
Keywords: left atrial function, heart failure with preserved ejection, reservoir function, left atrial longitudinal strain, HFA-PEFF score
Citation: Kim M, Bae S, Park JH and Jung IH (2023) Relative importance of left atrial reservoir strain compared with components of the HFA-PEFF score: a cross-sectional study. Front. Cardiovasc. Med. 10:1213557. doi: 10.3389/fcvm.2023.1213557
Received: 28 April 2023; Accepted: 27 September 2023;
Published: 12 October 2023.
Edited by:
Riccardo M. Inciardi, University of Brescia, ItalyReviewed by:
Yasufumi Nagata, Massachusetts General Hospital and Harvard Medical School, United States© 2023 Kim, Bae, Park and Jung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: In Hyun Jung c2F2ZWhlYXJ0QHl1aHMuYWM=; ZHJqaWhAbmF2ZXIuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.