- Department of Cardiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
The aim of this study was to develop a predictive model for severe thrombocytopenia after transfemoral transcatheter aortic valve replacement (TAVR). A total of 155 patients treated with TAVR at our center were retrospectively enrolled in this study. The incidence of severe thrombocytopenia after TAVR was 25.16%, and most patients suffered from severe thrombocytopenia on 4 days after procedure. Multivariate regression analysis showed that weight <60 kg, New York Heart Association Functional Classification (NYHAFC IV), major vascular complications, and lower first post-procedural platelet count were independent risk factors for severe thrombocytopenia after TAVR. The c-statistic for the area under the curve was 0.758, the sensitivity was 0.744, the specificity was 0.784, and the negative predictive value of the model was 91.38%. The overall predictive value was 76.77%. The predictive model developed from this cohort data could effectively identify patients at high risk of severe thrombocytopenia after TAVR, and might be applicable to patients with aortic regurgitation (AR) and severe thrombocytopenia with different definitions.
1. Introduction
A large body of evidence demonstrated that thrombocytopenia is inevitable after transcatheter aortic valve replacement (TAVR) (1–4), and that severe thrombocytopenia are significantly associated with worse clinical outcomes (1–3, 5–8). With the development of TAVR technology, TAVR is an established treatment for selected severe symptomatic aortic stenosis (AS), and its safety and efficacy in patients with aortic regurgitation (AR) has also been explored (9–11). Although platelet activation and systemic inflammatory response are possible explanations for the pathophysiology of thrombocytopenia after TAVR (2, 12, 13), however, the clinical characteristics of patients, the definition of severe thrombocytopenia and reported risk factors varied widely in previous studies (1–8, 14–17). At present, there is no clinical tool that can be used to assess the risk of severe thrombocytopenia after TAVR. Therefore, we attempted to further explore the risk factors of severe thrombocytopenia after transfemoral TAVR in patients with AS or AR, and develop a prediction model to predict the probability of severe thrombocytopenia after TAVR, so as to provide reference for clinical decision-making and prevention.
2. Materials and methods
2.1. Study design and data collection
Between January 2019 and 2023 January, 169 patients with severe aortic valve disease, including severe AS and AR, were treated with transfemoral TAVR at our center. Patients with periprocedural death and baseline platelet count <50 × 109/L were excluded, and finally a total of 155 patients participated in the development of the predictive model. All patients received general anesthesia and unfractionated heparin to maintain a minimum active clotting time of more than 250 s. The type and size of the implanted valve is determined by the cardiologist. Femoral artery access and closure was performed with the PROGLIDE suture-mediated closure system (Abbott Vascular Inc.,Santa Clara, Ca, USA). The procedural time was recorded accoring to “Skin to skin” (time 0 was the opening of the operative pathway and the end time was the closing of the operative pathway). Preprocedural and postprocedural antiplatelet regimens are at the discretion of the cardiologist, except for those requiring chronic oral anticoagulation.
2.2. Event and thrombocytopenia criteria
Periprocedural endpoints such as major vascular complications and severe bleeding (Type II/III) were classified according to the definition of Valve Academic Research Consortium 3 (VARC-3)[ (18)]. The lowest recorded platelet count during hospitalization was the nadir platelet count. Formula for decreased platelet count (DPC): [%DPC = 100 × (baseline platelet count—nadir platelet count)/baseline platelet count]. We divided the study population into severe thrombocytopenia (STP) and non-severe thrombocytopenia (NSTP). Severe thrombocytopenia (STP) was defined as DPC > 50% and platelet count <100 × 109/L based on previous studies (7, 19, 20).
2.3. Statistical analysis
Continuous variables were tested for normality using the Shapiro-Wilk test, and categorical variables were compared using chi-square statistics or Fisher's exact test. Data that did not satisfy the normal distribution were tested using the Mann-Whitney U test. The parameters with significant univariate analysis (p < 0.05) and the variables of interest were subjected to multivariate analysis using two-way-stepwise regression method before variable adjustment. After variable adjustment, the regression coefficient, OR value and confidence interval were calculated by Binary logistic regression. According to the predictive value of multivariate analysis, with STP as the endpoint, the receiver-operator characteristic curve (ROC) was generated, and the ROC was further evaluated by c-statistics. Sensitivity, specificity, negative predictive value, and positive predictive value were calculated by specific cut-off values, using Youden's index generated from the ROC based on STP prediction probabilities. All analyses were considered significant with a 2-tail p < 0.05. The SPSSAU project(2021). SPSSAU(Version 21.0)[Online Application Software]. Retrieved from https://www.spssau.com. were used to perform all statistical evaluations.
3. Results
3.1. The clinical characteristics
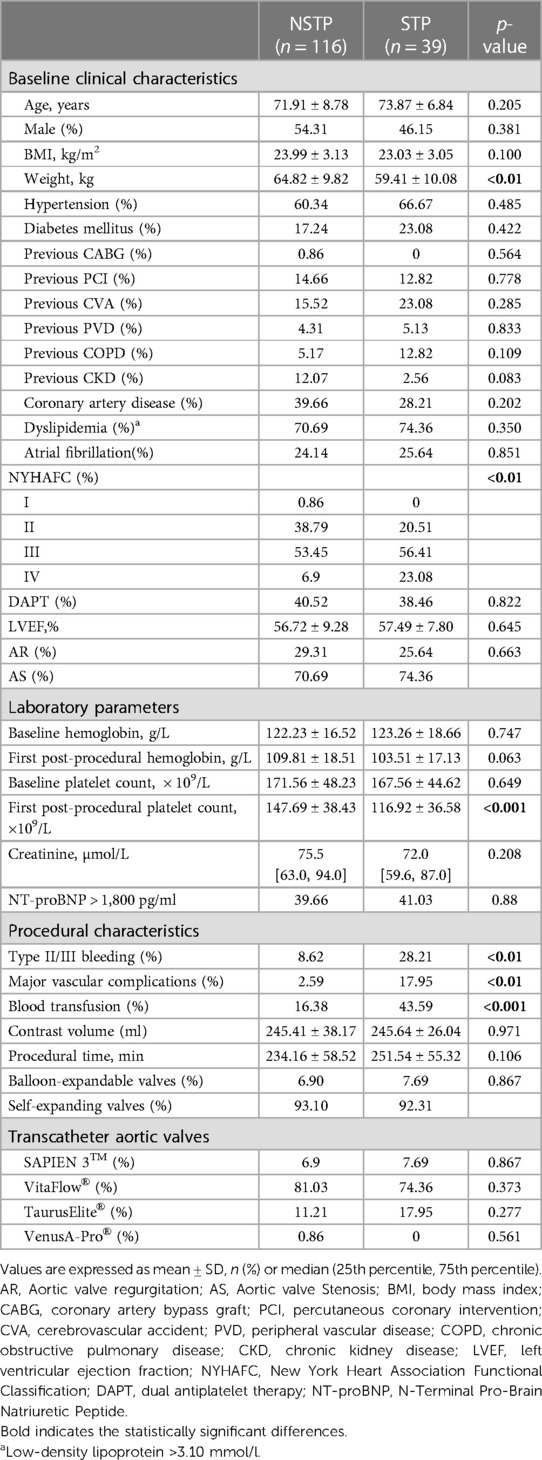
The clinical and procedural characteristics of the 155 participants are presented in Table 1. 96.8% of patients experienced varying decreased degrees of platelet count after TAVR. The total platelet count decreased by 40.2% ± 18.8% and the mean nadir platelet count was 100.26 ± 37.01 × 109/L. The platelet count decreased by 33% ± 15% and the nadir platelet count was 112.67 ± 32.28 × 109/L in NSTP group, while the platelet count decreased by 62% ± 9% and the nadir platelet count was 63.33 ± 22.98 × 109/L in STP group (p < 0.01). STP occurred in 39 patients (25.16%), mainly on the fourth postprocedural day. The body weight of patients in STP group was significantly lower than that in NSTP group (59.41 ± 10.08 vs. 64.82 ± 9.82, p < 0.01). There was significant difference in New York Heart Association Functional Classification (NYHAFC) between the two groups (p < 0.01). The first postprocedural platelet count in STP group was significantly lower than that in NSTP group (116.92 ± 36.58 vs. 147.69 ± 38.43 × 109/L, p < 0.001). The incidence of severe bleeding, major vascular complications and blood transfusion in STP group was higher than that in NSTP group (28.21% vs. 8.62%, p < 0.01; 17.95% vs. 2.59%, p < 0.001; 43.59% vs. 16.38%, p < 0.001). Clinical and procedural characteristics were almost similar between patients with AS and those with AR (Supplementary Table S1). There was no significant difference in the nadir platelet count (102.98 ± 35.15 vs. 99.18 ± 37.82 × 109/L, p = 0.566) and the incidence of STP between two groups (22.73% vs. 26.12%, p = 0.660). However, patients with AS were more prone to severe bleeding (8.62% vs. 28.21%, p = 0.039).
3.2. Variable analysis
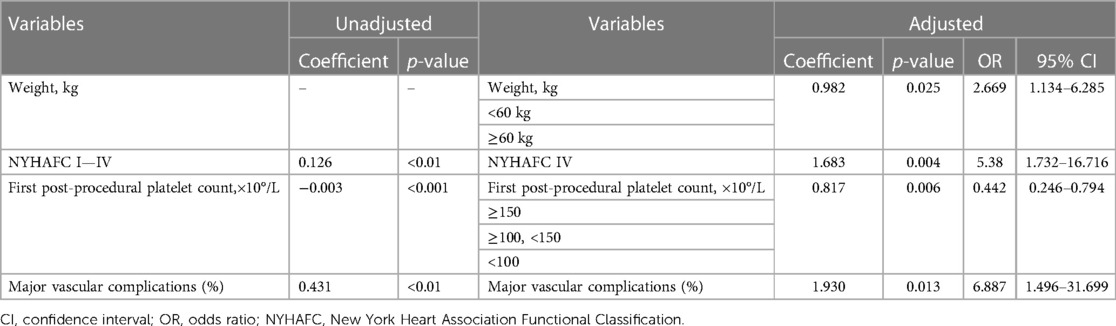
The results of multivariate analysis before and after variable adjustment are shown in Table 2. NYHAFC (p < 0.01), first post-procedural platelet count (p < 0.001), and major vascular complications (p < 0.01) were significantly associated with STP before adjustment, with the highest correlation being for major vascular complications (coefficient: 0.431, p < 0.01). Multivariate analysis after variable transformation of body weight, NYHAFC, and first post-procedural platelet count showed that body weight <60 kg (p = 0.02), NYHAFC IV (p < 0.01), low first post-procedural platelet count (p < 0.01), and major vascular complications (p < 0.01) were independent risk factors for STP after TAVR, accounting for 28.4% of the change in STP. Binary logistic regression showed weight <60 kg was 2.669 times higher of STP after TAVR compared with weight ≥60 kg (OR:2.669; 95% CI: 1.134–6.285; p = 0.025). Patients with NYHAFC IV was associated with 5.380 times higher risk of STP after TAVR than NYHAFC I-III (OR:5.380; 95% CI: 1.732–16.716; p = 0.004). Moreover, patients with major vascular complications (OR: 6.887; 95%CI: 1.496–31.699; p = 0.013) had a nearly 7 times increased risk of STP during hospitalization.
3.3. Model development and performance
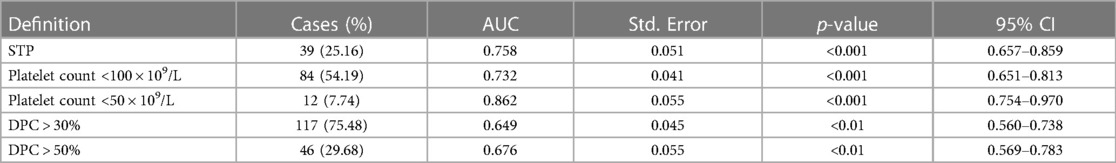
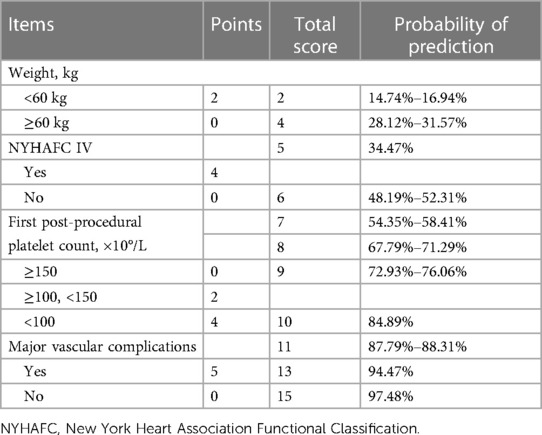
Due to a previously reported incidence of STP of 26.9%–56.5% (3, 14, 17), we use 30% probability and 10 events per parameter (EPP) rule to develop the predictive model with four parameters included. The criteria and results of the test are shown in Table 3, including STP, platelet count <100 × 109/L, platelet count <50 × 109/L, DPC > 30%, and DPC > 50%. The model had better discrimination for STP during hospitalization (AUC = 0.758; 95% CI: 0.657–0.859, p < 0.001), with a sensitivity of 0.744, a specificity of 0.784, a negative predictive value of 91.38%, a positive predictive value of 33.33%, and the overall probability of 76.77%. There was better discrimination for platelet count <100 × 109/L (AUC = 0.732; 95% CI: 0.651–0.813, p < 0.001) and platelet count <50 × 109/L (AUC = 0.862; 95% CI: 0.754–0.970, p < 0.001). However, the discrimination ability of DPC > 30% (AUC = 0.649; 95% CI: 0.560–0.738, p < 0.01) and DPC > 50% (AUC = 0.676; 95% CI: 0.569–0.783, p < 0.01) was not significant. Finally, based on the regression coefficients of multivariate analysis, we created a scoring chart to provide reference for clinical decision-making and prevention, as seen in Table 4. The estimation of scores and prediction probabilities is based on the Framingham Study Risk Score (21).
4. Discussion
The findings of this study were as follows: (1) The incidence of STP after TAVR was 25.16%, and the overall platelet count decreased by 40.2% ± 18.8%; (2) There was no significant difference in the incidence of STP after TAVR between AR and AS patients; (3) Independent risk factors for STP after TAVR were weight <60 kg, NYHAFC IV, first post-procedural platelet count and major vascular complications; (4) The combination of these risk factors can effectively predict STP after TAVR, and has certain predictive value for thrombocytopenia under different criteria.
It still remains controversial that patients with lower weight are associated with thrombocytopenia after TAVR. Eight studies have evaluated the effect of BMI on thrombocytopenia after TAVR (1, 3, 4, 6–8, 16, 19), and only two have shown a significant association between BMI and thrombocytopenia. Although almost all studies used BMI as a measurement of body weight and our study showed no association between BMI and STP, there was a significant correlation when the body weight was less than 60 kg. This leads us to wonder whether STP after TAVR is really independent of body weight. The main explanations for STP in low-weight patients after TAVR are platelet loss and hemodilution. As our study could not fully record the blood loss during the procedural, and STP is unlikely to be related to platelet loss based on the results of hemoglobin reduction before and after procedure, which is consistent with the report of Yamada et al. (17). Thus, the most likely explanation is hemodilution, which explains why our findings are not related to BMI, but are significantly related to the body weight. Body weight is a better indicator of a person's volume and blood volume than BMI. Patients with lower weight have smaller volumes, and large amounts of perioperative fluids have a more significant effect on hemodilution. Although we cannot exclude the differences in weight and infusion management after TAVR between Asian and European populations, in any case, patients with lower weight are more likely to have adverse outcomes after TAVR (22).
The present study found an association between vascular complications and STP after TAVR, which is consistent with previous studies, and relevant studies described its possible mechanism as platelet activation and hemodilution (6, 14). In addition, the occurrence of STP after TAVR may be the result of rapid platelet depletion in adverse events, including vascular complications and bleeding, and can be considered as a marker of systemic inflammatory response after TAVR (14, 23).
To our knowledge, this is the first study to report an association between first post-procedural platelet count and in-hospital STP. The first post-procedural platelet count after TAVR may be the result of physiological stress reaction under the combined action of different events such as procedural events, such as vascular complications, bleeding, blood transfusion, etc. This is likely to be a very sensitive marker reflecting the immediate clinical status of patients after TAVR. Therefore, it is logical that patients with lower first post-procedural platelet count after TAVR are more likely to develop severe thrombocytopenia.
Previous studies have reported LVEF as an independent risk factor for STP (5). Although we tried to use LVEF and NT-proBNP to more objectively reflect the cardiac function and evaluate the effect of deterioration of cardiac function on STP, the results showed that NYHAFC IV had a more positive effect. We have not found relevant studies describing the mechanism of STP after TAVR in patients with heart failure, and recent studies have shown a strong correlation between left ventricular end-diastolic pressure increase and platelet count decrease in patients with heart failure, suggesting that pressure overload of the lung may interfere with the function of the lung as a site of platelet biosynthesis (24).
Of particular note, this is the first report to compare STP after TAVR in patients with AR and AS, and this is a good response to the controversy about the role of endothelial injury and shear stress in STP after TAVR (1, 5, 16, 17). In theory, endothelial injury and shear stress after valve release should be more pronounced in patients with AS, but the final results showed no correlation between AS and AR. Due to the small sample size and limited statistical ability of our study, the controversy surrounding the explanation of endothelial damage and shear stress still requires future research to verify.
The model did not perform as expected in DPC > 30% and DPC > 50%. Considering that the average platelet decrease in our study was 40.2% ± 18.8%, so we focused on DPC > 50%, which is a more clinically meaningful index. Although DPC > 50% may reflect a decrease in platelet count due to some factors in this group of patients, it cannot be ruled out that platelet count may be normal in a significant proportion of these patients. Therefore, DPC > 50% as an outcome index cannot explain the confusion in clinical diagnosis and treatment. Of course, the best definition of severe thrombocytopenia after TAVR and its associated prognosis remain to be determined. Although our goal is to maximize the clinical value of our findings and enable patients to recover safely and quickly, the statistical power of the study may be insufficient due to sample size and population limitations, and more data are needed to validate and optimize the model to help more patients.
5. Conclusions
Severe thrombocytopenia occurring at high rates after TAVR, is consistently associated with poor clinical outcomes. This study demonstrated that weight <60 kg, NYHAFC IV, major vacular complications, and lower first post-procedural platelet count were independent risk factors for severe thrombocytopenia after TAVR. Further clinical trials are warranted to validate these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (or Ethics Committee) of Nanjing First Hospital, Nanjing Medical University. The Ethics Committee waived the requirement of written informed consent for participation.
Author contributions
Study concept and design: LSM, SLP and ZXM; acquisition of data: LSM, WYF, and WJJ; analysis and interpretation of data: LSM, WYF and SLP; drafting and critical revision of the manuscript: LSM, SLP, and ZXM. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1213248/full#supplementary-material
References
1. Hernandez-Enriquez M, Regueiro A, Romaguera R, Andrea R, Gomez-Hospital JA, Pujol-Lopez M, et al. Thrombocytopenia after transcatheter aortic valve implantation. A comparison between balloon-expandable and self-expanding valves. Catheter Cardiovasc Interv. (2019) 93:1344–51. doi: 10.1002/ccd.27907
2. Gallet R, Seemann A, Yamamoto M, Hayat D, Mouillet G, Monin JL, et al. Effect of transcatheter (via femoral artery) aortic valve implantation on the platelet count and its consequences. Am J Cardiol. (2013) 111:1619–24. doi: 10.1016/j.amjcard.2013.01.332
3. Takahashi S, Yokoyama N, Watanabe Y, Katayama T, Hioki H, Yamamoto H, et al. Predictor and mid-term outcome of clinically significant thrombocytopenia after transcatheter aortic valve selection. Circ J. (2020) 84:1020–7. doi: 10.1253/circj.CJ-19-0875
4. Abu Khadija H, Gandelman G, Ayyad O, Poles L, Jonas M, Paz O, et al. Trends in transfemoral aortic valve implantation related thrombocytopenia. J Clin Med. (2022) 11:726. doi: 10.3390/jcm11030726
5. Hernandez-Enriquez M, Chollet T, Bataille V, Campelo-Parada F, Boudou N, Bouisset F, et al. Comparison of the frequency of thrombocytopenia after transfemoral transcatheter aortic valve implantation between balloon-expandable and self-expanding valves. Am J Cardiol. (2019) 123:1120–6. doi: 10.1016/j.amjcard.2018.12.036
6. Tirado-Conte G, Salazar CH, McInerney A, Cruz-Utrilla A, Jimenez-Quevedo P, Cobiella J, et al. Incidence, clinical impact and predictors of thrombocytopenia after transcatheter aortic valve replacement. Int J Cardiol. (2022) 352:21–6. doi: 10.1016/j.ijcard.2022.01.072
7. Zhu Q, Liu X, He W, He Y, Tang M, Sun Y, et al. Predictors of thrombocytopenia after self-expandable transcatheter aortic valve replacement: a single-center experience from China. Cardiology. (2018) 139:151–8. doi: 10.1159/000484627
8. Kyranis SJ, Markiham R, Savage M, Crowhurst J, Murdoch D, Poon K, et al. Thrombocytopenia post transcatheter aortic valve insertion: clinical and prognostic significance. Structural Heart. (2019) 3:150–4. doi: 10.1080/24748706.2019.1569794
9. Lee CH, Inohara T, Hayashida K, Park DW. Transcatheter aortic valve replacement in Asia: present Status and future perspectives. JACC Asia. (2021) 1:279–93. doi: 10.1016/j.jacasi.2021.10.006
10. Markham R, Ghodsian M, Sharma R. TAVR In patients with pure aortic regurgitation: ready to use? Curr Cardiol Rep. (2020) 22:98. doi: 10.1007/s11886-020-01338-6
11. Soong EL, Ong YJ, Ho JSY, Chew NWS, Kong WKF, Yeo TC, et al. Transcatheter aortic valve replacement for aortic regurgitation in Asians: TAVR for aortic regurgitation in Asians. AsiaIntervention. (2021) 7:103–11. doi: 10.4244/aij-d-21-00007
12. Abu Khadija H, Gandelman G, Ayyad O, Poles L, Jonas M, Paz O, et al. Comparative analysis of the kinetic behavior of systemic inflammatory markers in patients with depressed versus preserved left ventricular function undergoing transcatheter aortic valve implantation. J Clin Med. (2021) 10:4148. doi: 10.3390/jcm10184148
13. Sexton TR, Wallace EL, Chen A, Charnigo RJ, Reda HK, Ziada KM, et al. Thromboinflammatory response and predictors of outcomes in patients undergoing transcatheter aortic valve replacement. J Thromb Thrombolysis. (2016) 41:384–93. doi: 10.1007/s11239-015-1326-z
14. Dvir D, Genereux P, Barbash IM, Kodali S, Ben-Dor I, Williams M, et al. Acquired thrombocytopenia after transcatheter aortic valve replacement: clinical correlates and association with outcomes. Eur Heart J. (2014) 35:2663–71. doi: 10.1093/eurheartj/ehu082
15. Kisacik H, Tok D, Balci KG, Demirkan B, Karakurt M, Açar B, et al. Evaluation of acquired thrombocytopenia according to the balloon-expandable versus self-expandable valves in patients undergoing transcatheter aortic valve replacement. Angiology. (2020) 72:290–4. doi: 10.1177/0003319720953048
16. Sugiura A, Treiling L, Al-Kassou B, Shamekhi J, Wilde N, Sinning JM, et al. Spleen size and thrombocytopenia after transcatheter aortic valve implantation. Am J Cardiol. (2021) 157:85–92. doi: 10.1016/j.amjcard.2021.07.021
17. Yamada Y, Miura D, Takamori A, Nogami E, Yunoki J, Sakaguchi Y. Predictors of short-term thrombocytopenia after transcatheter aortic valve implantation: a retrospective study at a single Japanese center. BMC Res Notes. (2020) 13:536. doi: 10.1186/s13104-020-05386-7
18. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. Eur Heart J. (2021) 42:1825–57. doi: 10.1093/eurheartj/ehaa799
19. Flaherty MP, Mohsen A, Moore JBT, Bartoli CR, Schneibel E, Rawasia W, et al. Predictors and clinical impact of pre-existing and acquired thrombocytopenia following transcatheter aortic valve replacement. Catheter Cardiovasc Interv. (2015) 85:118–29. doi: 10.1002/ccd.25668
20. McCabe JM, Huang PH, Riedl LA, Devireddy SR, Grondell J, Connors AC, et al. Incidence and implications of idiopathic thrombocytopenia following transcatheter aortic valve replacement with the Edwards Sapien(©) valves: a single center experience. Catheter Cardiovasc Interv. (2014) 83:633–41. doi: 10.1002/ccd.25206
21. Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: the framingham study risk score functions. Stat Med. (2004) 23:1631–60. doi: 10.1002/sim.1742
22. Tezuka T, Higuchi R, Hagiya K, Saji M, Takamisawa I, Nanasato M, et al. Midterm outcomes of underweight patients undergoing transcatheter aortic valve implantation: insight from the LAPLACE-TAVR registry. JACC Asia. (2023) 3:78–89. doi: 10.1016/j.jacasi.2022.08.014
23. Sinning JM, Scheer AC, Adenauer V, Ghanem A, Hammerstingl C, Schueler R, et al. Systemic inflammatory response syndrome predicts increased mortality in patients after transcatheter aortic valve implantation. Eur Heart J. (2012) 33:1459–68. doi: 10.1093/eurheartj/ehs002
Keywords: transcatheter aortic valve replacement, platelet count, thrombocytopenia, predictive model, aortic regurgitation
Citation: Li SM, Wu YF, Wang JJ, She LP and Zheng XM (2023) Predictive model for severe thrombocytopenia after transfemoral transcatheter aortic valve replacement. Front. Cardiovasc. Med. 10:1213248. doi: 10.3389/fcvm.2023.1213248
Received: 29 April 2023; Accepted: 19 July 2023;
Published: 9 August 2023.
Edited by:
Alexander E. Berezin, Zaporizhia State Medical University, UkraineReviewed by:
Anna Olasinska-Wisniewska, Poznan University of Medical Sciences, PolandPablo Codner, Rabin Medical Center, Israel
© 2023 Li, Wu, Wang, She and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping She c2hlbGlwaW5nODBAMTYzLmNvbQ== Xuemei Zheng eG16aGVuZ0AxNjMuY29t
†These authors have contributed equally to this work
 Shaoman Li
Shaoman Li Yafeng Wu†
Yafeng Wu† Jinju Wang
Jinju Wang