
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 September 2023
Sec. Pediatric Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1212882
A correction has been applied to this article in:
Corrigendum: Use of the index of pulmonary vascular disease for predicting longterm outcome of pulmonary arterial hypertension associated with congenital heart disease
 Ayako Chida-Nagai1,2,3,†
Ayako Chida-Nagai1,2,3,† Naoki Masaki2,†
Naoki Masaki2,† Kay Maeda2
Kay Maeda2 Konosuke Sasaki2
Konosuke Sasaki2 Hiroki Sato4,5
Hiroki Sato4,5 Jun Muneuchi6
Jun Muneuchi6 Yoshie Ochiai7
Yoshie Ochiai7 Hiroomi Murayama8
Hiroomi Murayama8 Masahiro Tahara9
Masahiro Tahara9 Atsuko Shiono10
Atsuko Shiono10 Atsushi Shinozuka11
Atsushi Shinozuka11 Fumihiko Kono12
Fumihiko Kono12 Daisuke Machida13
Daisuke Machida13 Shinichi Toyooka14
Shinichi Toyooka14 Seiichiro Sugimoto14
Seiichiro Sugimoto14 Kazufumi Nakamura15
Kazufumi Nakamura15 Satoshi Akagi15
Satoshi Akagi15 Maiko Kondo16
Maiko Kondo16 Shingo Kasahara17
Shingo Kasahara17 Yasuhiro Kotani17
Yasuhiro Kotani17 Junichi Koizumi18
Junichi Koizumi18 Katsuhiko Oda19
Katsuhiko Oda19 Masako Harada20
Masako Harada20 Daisuke Nakajima21
Daisuke Nakajima21 Akira Murata22
Akira Murata22 Hazumu Nagata23
Hazumu Nagata23 Koichi Yatsunami24
Koichi Yatsunami24 Tomio Kobayashi25
Tomio Kobayashi25 Yoshikiyo Matsunaga26
Yoshikiyo Matsunaga26 Takahiro Inoue27
Takahiro Inoue27 Hiroyuki Yamagishi28
Hiroyuki Yamagishi28 Naomi Nakagawa29
Naomi Nakagawa29 Katsuki Ohtani30
Katsuki Ohtani30 Masaki Yamamoto31
Masaki Yamamoto31 Yushi Ito32
Yushi Ito32 Tatsunori Hokosaki33
Tatsunori Hokosaki33 Yuta Kuwahara34
Yuta Kuwahara34 Satoshi Masutani35,36
Satoshi Masutani35,36 Koji Nomura37
Koji Nomura37 Tsutomu Wada38
Tsutomu Wada38 Hirofumi Sawada39
Hirofumi Sawada39 Masayuki Abiko40
Masayuki Abiko40 Tatsunori Takahashi40
Tatsunori Takahashi40 Yuichi Ishikawa41
Yuichi Ishikawa41 Seigo Okada42
Seigo Okada42 Atsushi Naitoh43
Atsushi Naitoh43 Takako Toda44
Takako Toda44 Tatsuya Ando45
Tatsuya Ando45 Akihiro Masuzawa46
Akihiro Masuzawa46 Shinsuke Hoshino47
Shinsuke Hoshino47 Masaaki Kawada48
Masaaki Kawada48 Yuichi Nomura49
Yuichi Nomura49 Kentaro Ueno50
Kentaro Ueno50 Naoki Ohashi51
Naoki Ohashi51 Tsuyoshi Tachibana52
Tsuyoshi Tachibana52 Yuchen Cao52
Yuchen Cao52 Hideaki Ueda53
Hideaki Ueda53 Sadamitsu Yanagi53
Sadamitsu Yanagi53 Masaaki Koide54
Masaaki Koide54 Norie Mitsushita55
Norie Mitsushita55 Kouji Higashi56
Kouji Higashi56 Yoshihiro Minosaki57
Yoshihiro Minosaki57 Tomohiro Hayashi58
Tomohiro Hayashi58 Takashi Okamoto59
Takashi Okamoto59 Kenji Kuraishi60
Kenji Kuraishi60 Eiji Ehara61
Eiji Ehara61 Hidekazu Ishida62
Hidekazu Ishida62 Hitoshi Horigome63
Hitoshi Horigome63 Takashi Murakami63
Takashi Murakami63 Kohta Takei64
Kohta Takei64 Taku Ishii65,66
Taku Ishii65,66 Gen Harada3
Gen Harada3 Yasutaka Hirata67
Yasutaka Hirata67 Jun Maeda68
Jun Maeda68 Shunsuke Tatebe69
Shunsuke Tatebe69 Chiharu Ota70
Chiharu Ota70 Yasunobu Hayabuchi71
Yasunobu Hayabuchi71 Hisanori Sakazaki72
Hisanori Sakazaki72 Takashi Sasaki73
Takashi Sasaki73 Keiichi Hirono74
Keiichi Hirono74 Sayo Suzuki75
Sayo Suzuki75 Masahiro Yasuda76
Masahiro Yasuda76 Atsuhito Takeda1
Atsuhito Takeda1 Madoka Sawada77
Madoka Sawada77 Kagami Miyaji78
Kagami Miyaji78 Atsushi Kitagawa79
Atsushi Kitagawa79 Yosuke Nakai80
Yosuke Nakai80 Nobuyuki Kakimoto81
Nobuyuki Kakimoto81 Kouta Agematsu82
Kouta Agematsu82 Atsushi Manabe1,‡
Atsushi Manabe1,‡ Yoshikatsu Saiki2,‡*
Yoshikatsu Saiki2,‡*
Aims: Limited data exist on risk factors for the long-term outcome of pulmonary arterial hypertension (PAH) associated with congenital heart disease (CHD-PAH). We focused on the index of pulmonary vascular disease (IPVD), an assessment system for pulmonary artery pathology specimens. The IPVD classifies pulmonary vascular lesions into four categories based on severity: (1) no intimal thickening, (2) cellular thickening of the intima, (3) fibrous thickening of the intima, and (4) destruction of the tunica media, with the overall grade expressed as an additive mean of these scores. This study aimed to investigate the relationship between IPVD and the long-term outcome of CHD-PAH.
Methods: This retrospective study examined lung pathology images of 764 patients with CHD-PAH aged <20 years whose lung specimens were submitted to the Japanese Research Institute of Pulmonary Vasculature for pulmonary pathological review between 2001 and 2020. Clinical information was collected retrospectively by each attending physician. The primary endpoint was cardiovascular death.
Results: The 5-year, 10-year, 15-year, and 20-year cardiovascular death-free survival rates for all patients were 92.0%, 90.4%, 87.3%, and 86.1%, respectively. The group with an IPVD of ≥2.0 had significantly poorer survival than the group with an IPVD <2.0 (P = .037). The Cox proportional hazards model adjusted for the presence of congenital anomaly syndromes associated with pulmonary hypertension, and age at lung biopsy showed similar results (hazard ratio 4.46; 95% confidence interval: 1.45–13.73; P = .009).
Conclusions: The IPVD scoring system is useful for predicting the long-term outcome of CHD-PAH. For patients with an IPVD of ≥2.0, treatment strategies, including choosing palliative procedures such as pulmonary artery banding to restrict pulmonary blood flow and postponement of intracardiac repair, should be more carefully considered.
Pulmonary hypertension (PH) is a progressive and serious disease, defined as a mean pulmonary arterial pressure >20 mmHg at rest (1). Pulmonary arterial hypertension associated with congenital heart disease (CHD-PAH) accounts for the majority of PH in children, unlike in adults (2). Several reports have examined the long-term outcome of patients with CHD-PAH (2–4), but the risk factors remain unclear.
As a prognostic factor for patients with CHD-PAH, we focused on pulmonary artery pathology. The first method developed to assess pulmonary arterial pathology in PH was the Heath–Edwards (HE) classification (5). However, Yamaki et al. considered that the HE classification was based on using only the most severe lesion in the lung specimens, so the classification was a qualitative taxonomy and inappropriate for assessing the entirety of pulmonary arterial lesions (6). Therefore, he developed the index of pulmonary vascular disease (IPVD) (6).
We showed that the IPVD is useful in assessing severity in some cases of patients with CHD-PAH (7–9), however, the relationship between the IPVD and long-term outcome is unclear. Therefore, this study is aimed to examine the association between the IPVD and long-term outcome in patients with CHD-PAH.
This retrospective cohort study was based on clinical data that accompanied each patient's pulmonary pathology specimen submitted to the Japanese Research Institute of Pulmonary Vasculature within the Department of Cardiovascular Surgery at Tohoku University. Each lung specimen was requested for pathological review from 79 medical centres in Japan. Additional clinical data on the patients was obtained from each patient's attending physician up to 30 June 2022. The study period started on 1 March 2021 and ended on 30 June 2022. For each therapeutic drug or home oxygen therapy administration, the total number of cases of current or past use was included.
Study patients were children aged <20 years with CHD-PAH who underwent pulmonary pathology studies between 2001 and 2020 at the Japanese Research Institute of Pulmonary Vasculature in the Department of Cardiovascular Surgery at Tohoku University (Figure 1). The study population was narrowed down to 764 patients with the latest PH classification Group 1.4.4. pulmonary arterial hypertension associated with congenital heart disease (1). The diagnosis of pulmonary arterial hypertension is made by the respective attending physician before the lung biopsy. The breakdown of congenital heart diseases is listed in Supplementary Table S1.
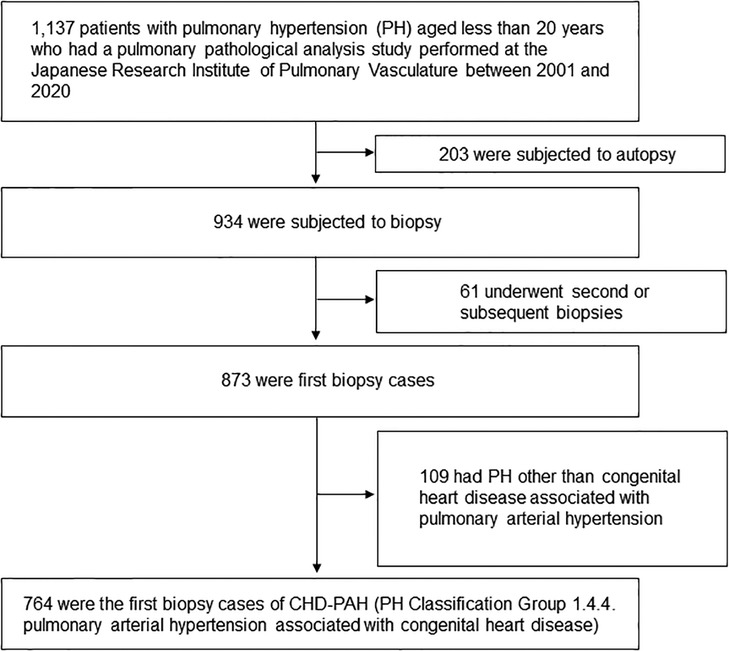
Figure 1. Patient flow chart. The latest pulmonary hypertension classification was quoted by reference (1).
The IPVD classifies pulmonary vascular lesions into four categories based on severity: (1) no intimal thickening, (2) cellular thickening of the intima, (3) fibrous thickening of the intima, and (4) destruction of the tunica media, with all small pulmonary arteries on the histopathology specimen given their grade and expressed as an additive mean of these scores (Figure 2) (6). HE classification and IPVD scoring were performed by a skilled pulmonary pathology expert immediately upon receipt of each pulmonary pathology specimen. Autopsy cases were excluded. In addition, cases of congenital heart disease, such as obstructed total anomalous pulmonary venous return but judged to be “Group 2.4 Congenital/acquired cardiovascular conditions leading to post-capillary PH” in the latest PH clinical classification (1), were excluded. For patients who had submitted pulmonary pathology specimens more than once, only the initial assessment was included in the analysis. Based on previous reports, 13 trisomy, 18 trisomy, 21 trisomy, 22q11.2 deletion, Noonan syndrome, CHARGE syndrome, and VACTERL association (10–12), which are closely associated with the development of PH, are summarised as “congenital anomaly syndromes related to PH”.
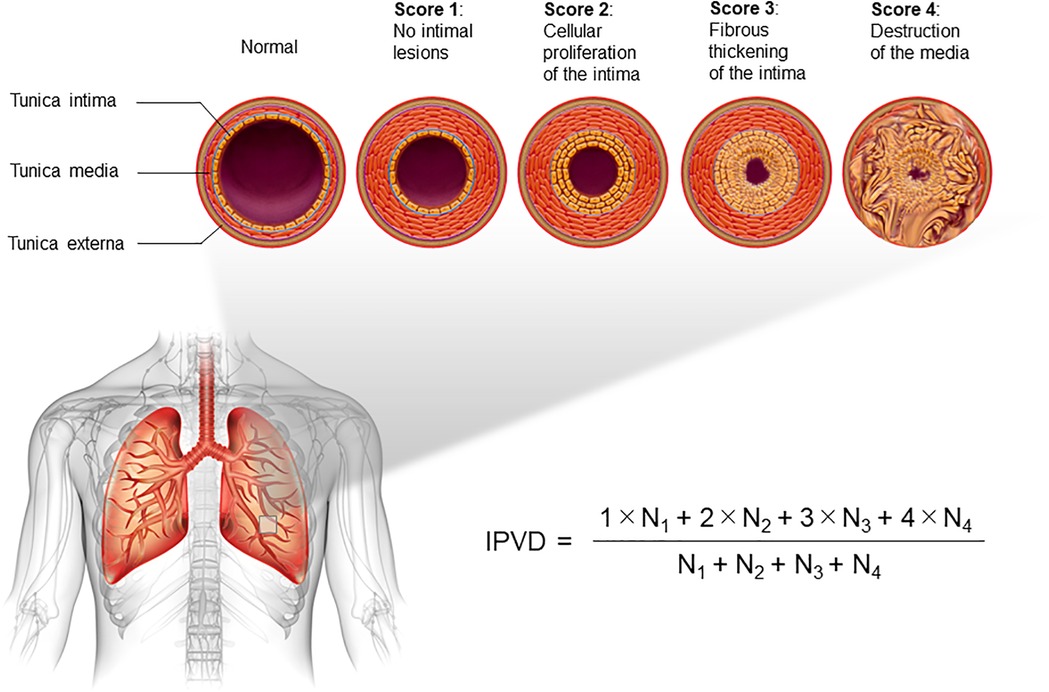
Figure 2. Description of the index of pulmonary vascular disease (IPVD). All pulmonary arteries on pathology specimens are scored according to the severity, from 1 to 4. The additive mean is defined as the IPVD.
The primary outcome of the study was cardiovascular death. In HE classification, two groups were divided into Grade 0 (Normal) to Grade 2 and Grade 3–6. Grade 1–2 are considered to be reversible lesions (5, 13). Taking into account past report of 5 cases of death in transposition of the great arteries with an IPVD of 2.3 or higher (7), a report indicating that an IPVD rating of 2.2 in Down's syndrome and 2.1 without the syndrome were considered as the upper permissible limits for surgical intervention based on lung autopsy or biopsy results in 49 cases with ventricular septal defect and/or paten ductus arteriosus (8), and a report of 67 patients with atrioventricular septal defect where surgical survivors had an IPVD below 2.0 (9), we divided patients into one of two categories according to a cut-off value of the IPVD of 2.0. To determine whether it was statistically valid to perform a two-group survival analysis using an IPVD of 2.0, a sensitivity analysis was performed in two groups with IPVDs ranging from 1.7 to 2.2.
Clinical features are presented as median with interquartile range or count and proportion, as appropriate. For continuous variables, the Wilcoxon signed-rank test was used to compare differences between the two groups. For categorical variables, Fisher's exact test was used. Kaplan–Meier plots were constructed to demonstrate the association between the HE classification and outcome, and the data were compared using the log-rank test. The relationship between the IPVD and outcome was also examined, as was that between the HE classification and outcome. Furthermore, a multivariable Cox proportional hazards regression model adjusted for presence of congenital anomaly syndromes related to PH, year of performing a lung biopsy, and age at lung biopsy was used to estimate the association between the IPVD and outcome. Time zero was defined as corresponding to the time at which each patient submitted a pulmonary pathology specimen. As a sensitivity analysis, Cox regression analysis was performed with cut-off values for the IPVD of 1.7, 1.8, 1.9, 2.1, and 2.2. Statistical analyses were performed using JMP Pro for Windows, version 16 and SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
This study was performed in line with the principles of the Declaration of Helsinki. The Institutional Review Board of Tohoku University Graduate School of Medicine for clinical research approved this study (approval no. 2020-1-357, 2022-1-968). Information regarding the present study was disclosed on the Tohoku University website with an opt-out option because some patients had already died or were lost to follow-up. The requirement for informed consent was waived because of the retrospective nature of the study.
In total, 764 patients with CHD-PAH were included in this study. Cardiovascular deaths occurred in 54 out of 568 who could be observed (9.5%). None of the patients received lung transplantation. Five-, 10-, 15-, and 20-year cardiovascular death-free survival rates of all patients were 92.0%, 90.4%, 87.3%, and 86.1%, respectively (Figure 3A). Age at which lung biopsies were performed was 150 days (interquartile range, 60–395 days), and the duration of observation was 8.3 years (interquartile range, 3.3–13.1 years; Table 1).
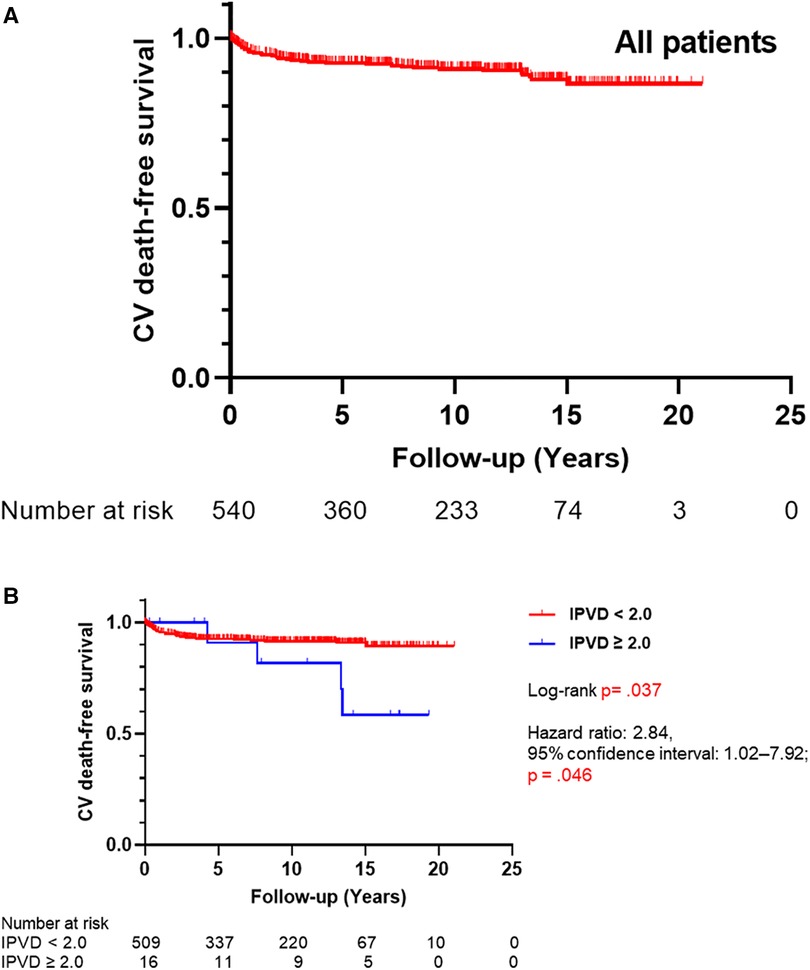
Figure 3. (A) Kaplan–Meier curves for cardiovascular death-free survival of all patients (N = 543). (B) Kaplan–Meier curves for cardiovascular death-free survival by the index of pulmonary vascular disease (IPVD) (N = 528). CV, cardiovascular.
First, the relationship between the HE classification and long-term outcome was examined. The proportion of freedom from cardiovascular death according to Kaplan–Meier curve analysis is shown in Supplementary Figure S1. No significant differences in long-term outcome were found between the two groups (log-rank P = .826, hazard ratio 1.08; 95% confidence interval 0.55–2.12; P = .826).
Next, we investigated the impact of IPVD on long-term outcome (Table 2). Presence of congenital anomaly syndromes related to PH, year of performing a lung biopsy, age at lung biopsy, mean pulmonary arterial pressure, pulmonary vascular resistance index, experience of using endothelin receptor antagonist, experience of using phosphodiesterase 5 inhibitor, experience of using selexipag, experience of using home oxygen therapy, and cardiovascular death were significantly different between the groups with IPVD <2.0 and ≥2.0.
A univariate Cox proportional-hazards model for time to death indicated that there was no significant difference between HE classification ≥3 group and ≤2 group. Similar results were obtained for the presence of congenital anomaly syndromes related to PH, year of performing a lung biopsy, and age at lung biopsy. In contrast, the IPVD ≥2.0 group had worse outcome than the IPVD <2.0 group significantly (hazard ratio 2.84; 95% confidence interval 1.02–7.92; P = .046) (Supplementary Table 2).
As shown in Figure 3B, cardiovascular death-free survival was worse in the group with an IPVD ≥2.0 than in the group with an IPVD <2.0 (P = .037). Five-, 10-, and 15-year cardiovascular death-free survival rates were 91.9%, 91.0%, and 90.3% in the group with an IPVD <2.0 and 90.9%, 81.8%, 58.4% in the group with an IPVD ≥2.0, respectively. As shown in Table 3, multivariable Cox regression analysis indicated that an IPVD ≥2.0 was independently associated with poor outcome (hazard ratio 4.46; 95% confidence interval: 1.45–13.73; P = 0.009).
A sensitivity analysis was performed in two groups with IPVDs ranging from 1.7 to 2.2, and similar results were shown (Supplementary Table 3).
This study showed that patients with CHD-PAH with an IPVD ≥2.0 had a significantly poorer outcome than those with an IPVD <2.0. The results were also independent of age at the time of lung biopsy, year of performing a lung biopsy, or the presence or absence of congenital anomaly syndromes related to PH (Table 3). The previously used HE classification did not show any relationship with long-term outcome. Therefore, we were able to demonstrate the superiority of using the IPVD in clinical practice compared to the HE classification. We suppose that IPVD is superior to the HE classification in predicting long-term prognosis because, unlike the HE classification, IPVD evaluates the pulmonary arterioles in the entire sample. The IPVD of 2.0 as the cut-off value, which our group has found in the past in a few patients with CHD-PAH, proved to be useful for predicting the long-term outcome of CHD-PAH as a whole.
The risks and benefits of lung biopsy in paediatric PH need to be carefully considered, based on a 1990 report of a 20% mortality rate (14). According to a report from 2013, the mortality rate associated with lung biopsies was 0%, but complications such as bleeding and infections were observed in approximately 30% of cases (15). Haworth stated that lung biopsy in children with PH is essentially not justified, with possible exceptions in children with suspected pulmonary veno-occlusive disease/pulmonary capillary haemangiomatosis, children with persistent PH of the newborn syndrome, and children with PH complicated by congenital heart disease for whom surgery is indicated (16). Moreover, Davies et al. conducted a retrospective review of 64 paediatric lung biopsies, including 18 cases of PH. The results suggest that lung biopsy is recommended for children who have been on extracorporeal membrane oxygenation for >2 weeks and for children with PH and pulmonary parenchymal disease (17).
Our research group and collaborators have performed lung biopsies in children with moderate or severe CHD-PAH, either simultaneously when performing a palliative operation (e.g., pulmonary artery banding) or independently. The pulmonary pathology has been reviewed, and based on the results, the final decision on whether and when to perform intracardiac repair has been made. For the 764 cases reported here, no fatal accidents due to lung biopsy have been observed, so lung biopsy can be performed safely. We believe that advancements in perioperative management, including the use of inhaled nitric oxide, and improved surgical procedures have led to more appropriate care and contributed to a reduction in mortality by preventing pulmonary hypertensive crisis. These developments have the potential to significantly improve mortality rates.
In our study, despite the presence of severe patients with CHD-PAH warranting lung biopsy, we observed a remarkably favourable long-term outcome compared with that of reports from other countries (2–4). The 5-year survival rate, free from cardiovascular-related mortality, was 92.0%, while the 10-year survival rate reached 90.4%. The absence of patients who underwent lung transplantation in the study population can be attributed to the low number of lung transplantations in Japan, especially in pediatric cases, where there are a few cases per year (18). This could be a potential factor contributing to the results of this study.
We recognize there are several limitations in this study. First, the variation in the biopsies, including the location of the sample collection, which may differ between patients. Next, no deaths related to lung biopsies were observed among the subjects in this study, but follow-up data on complications such as bleeding and infections were unavailable. Also, this study's success mainly depends on the skills of the pulmonary pathology expert. In addition, this study only examined cardiovascular death as the primary outcome and the exact number of individuals who underwent cardiac repair surgery after lung biopsy is unknown. Future works may be needed to assess the co-morbidities or clinical course of the disease using the IPVD. Futhermore, in this study, we included year of biopsy in the analysis to account for any potential impact of advancements in pulmonary vasodilator therapy on long-term outcomes. As shown in Table 2, year of biopsy did not have a significant effect on outcome. However, it is possible that in the future, with the widespread use of pulmonary vasodilators following intracardiac repair for CHD-PAH, there may be an impact on long-term outcomes.
In summary, the IPVD scoring system developed by our group is a determinant of the long-term outcome of patients with CHD-PAH. In cases of severe CHD-PAH, confirming IPVD can be beneficial in cases where there is uncertainty regarding the treatment approach. We suggest that the thorough evaluation of pulmonary vascular pathology in patients with CHD-PAH before formulating a treatment plan could potentially contribute to the favorable long-term outcome. However, it is essential to acknowledge the limitations of our study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Institutional Review Board of Tohoku University Graduate School of Medicine for clinical research. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Information regarding the present study was disclosed on the Tohoku University website with an opt-out option because some patients had already died or were lost to follow-up. The requirement for informed consent was waived because of the retrospective nature of the study.
ACN wrote the manuscript. ACN and NM designed the study and analyzed the data. NM and KM performed the pulmonary artery pathological diagnosis. KS and HS analyzed the data. AM and YS designed the study and critically reviewed the manuscript. Other co-authors recruited the patients and collected the clinical information. All authors contributed to the article and approved the submitted version.
This study was supported by JSPS KAKENHI (grant number: JP22K08225), the Takeda Science Foundation, and Miyata Cardiac Research Promotion Foundation.
We would like to thank the late Shigeo Yamaki, a great pioneer in the field of pulmonary arterial histopathology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1212882/full#supplementary-material
1. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53(1):1801913. doi: 10.1183/13993003.01913-2018
2. van Loon RL, Roofthooft MT, Hillege HL, ten Harkel AD, van Osch-Gevers M, Delhaas T, et al. Pediatric pulmonary hypertension in the Netherlands: epidemiology and characterization during the period 1991 to 2005. Circulation. (2011) 124:1755–64. doi: 10.1161/CIRCULATIONAHA.110.969584
3. Haworth SG, Hislop AA. Treatment and survival in children with pulmonary arterial hypertension: the UK pulmonary hypertension service for children 2001–2006. Heart (British Cardiac Soc). (2009) 95:312–7. doi: 10.1136/hrt.2008.150086
4. Barst RJ, McGoon MD, Elliott CG, Foreman AJ, Miller DP, Ivy DD. Survival in childhood pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Circulation. (2012) 125:113–22. doi: 10.1161/CIRCULATIONAHA.111.026591
5. Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. (1958) 18:533–47. doi: 10.1161/01.CIR.18.4.533
6. Yamaki S, Tezuka F. Quantitative evaluation of hypertensive pulmonary arterial change. Acta Pathol Jpn. (1979) 29:61–6.433591
7. Yamaki S, Horiuchi T, Ishizawa E, Mohri H, Fukuda M, Tezuka F. Indication for total correction of complete transposition of the great arteries with pulmonary hypertension. J Thorac Cardiovasc Surg. (1980) 79:890–5. doi: 10.1016/S0022-5223(19)37862-6
8. Yamaki S, Mohri H, Haneda K, Endo M, Akimoto H. Indications for surgery based on lung biopsy in cases of ventricular septal defect and/or patent ductus arteriosus with severe pulmonary hypertension. Chest. (1989) 96:31–9. doi: 10.1378/chest.96.1.31
9. Yamaki S, Yasui H, Kado H, Yonenaga K, Nakamura Y, Kikuchi T, et al. Pulmonary vascular disease and operative indications in complete atrioventricular canal defect in early infancy. J Thorac Cardiovasc Surg. (1993) 106:398–405. doi: 10.1016/S0022-5223(19)34071-1
10. Ma L, Chung WK. The genetic basis of pulmonary arterial hypertension. Hum Genet. (2014) 133:471–9. doi: 10.1007/s00439-014-1419-3
11. Tahara M, Sanada K, Morita R, Hawaka H, Urayama K, Sugino M, et al. Insufficient development of vessels and alveoli in lungs of infants with trisomy 18-features of pulmonary histopathological findings from lung biopsy. Am J Med Genet A. (2021) 185:1059–66. doi: 10.1002/ajmg.a.62060
12. Saji T. Clinical characteristics of pulmonary arterial hypertension associated with down syndrome. Pediatr Int. (2014) 56:297–303. doi: 10.1111/ped.12349
13. Burrows FA, Rabinovitch M. The pulmonary circulation in children with congenital heart disease: morphologic and morphometric considerations. Can Anaesth Soc J. (1985) 32:364–73. doi: 10.1007/BF03011341
14. Wilson NJ, Seear MD, Taylor GP, LeBlanc JG, Sandor GG. The clinical value and risks of lung biopsy in children with congenital heart disease. J Thorac Cardiovasc Surg. (1990) 99:460–8. doi: 10.1016/S0022-5223(19)36976-4
15. Beghetti M, Berger RM, Schulze-Neick I, Day RW, Pulido T, Feinstein J, et al. Diagnostic evaluation of paediatric pulmonary hypertension in current clinical practice. Eur Respir J. (2013) 42:689–700. doi: 10.1183/09031936.00140112
16. Haworth SG. The management of pulmonary hypertension in children. Arch Dis Child. (2008) 93:620–5. doi: 10.1136/adc.2007.120493
17. Davies L, Dolgin S, Kattan M. Morbidity and mortality of open lung biopsy in children. Pediatrics. (1997) 99:660–4. doi: 10.1542/peds.99.5.660
Keywords: index of pulmonary vascular disease, pulmonary arterial hypertension, congenital heart disease, pediatrics, outcome
Citation: Chida-Nagai A, Masaki N, Maeda K, Sasaki K, Sato H, Muneuchi J, Ochiai Y, Murayama H, Tahara M, Shiono A, Shinozuka A, Kono F, Machida D, Toyooka S, Sugimoto S, Nakamura K, Akagi S, Kondo M, Kasahara S, Kotani Y, Koizumi J, Oda K, Harada M, Nakajima D, Murata A, Nagata H, Yatsunami K, Kobayashi T, Matsunaga Y, Inoue T, Yamagishi H, Nakagawa N, Ohtani K, Yamamoto M, Ito Y, Hokosaki T, Kuwahara Y, Masutani S, Nomura K, Wada T, Sawada H, Abiko M, Takahashi T, Ishikawa Y, Okada S, Naitoh A, Toda T, Ando T, Masuzawa A, Hoshino S, Kawada M, Nomura Y, Ueno K, Ohashi N, Tachibana T, Cao Y, Ueda H, Yanagi S, Koide M, Mitsushita N, Higashi K, Minosaki Y, Hayashi T, Okamoto T, Kuraishi K, Ehara E, Ishida H, Horigome H, Murakami T, Takei K, Ishii T, Harada G, Hirata Y, Maeda J, Tatebe S, Ota C, Hayabuchi Y, Sakazaki H, Sasaki T, Hirono K, Suzuki S, Yasuda M, Takeda A, Sawada M, Miyaji K, Kitagawa A, Nakai Y, Kakimoto N, Agematsu K, Manabe A and Saiki Y (2023) Use of the index of pulmonary vascular disease for predicting long-term outcome of pulmonary arterial hypertension associated with congenital heart disease. Front. Cardiovasc. Med. 10:1212882. doi: 10.3389/fcvm.2023.1212882
Received: 27 April 2023; Accepted: 21 August 2023;
Published: 4 September 2023.
Edited by:
Ruth Heying, University Hospital Leuven, BelgiumReviewed by:
Zhi-Cheng Jing, Peking Union Medical College Hospital (CAMS), China© 2023 Chida-Nagai, Masaki, Maeda, Sasaki, Sato, Muneuchi, Ochiai, Murayama, Tahara, Shiono, Shinozuka, Kono, Machida, Toyooka, Sugimoto, Nakamura, Akagi, Kondo, Kasahara, Kotani, Koizumi, Oda, Harada, Nakajima, Murata, Nagata, Yatsunami, Kobayashi, Matsunaga, Inoue, Yamagishi, Nakagawa, Ohtani, Yamamoto, Ito, Hokosaki, Kuwahara, Masutani, Nomura, Wada, Sawada, Abiko, Takahashi, Ishikawa, Okada, Naitoh, Toda, Ando, Masuzawa, Hoshino, Kawada, Nomura, Ueno, Ohashi, Tachibana, Cao, Ueda, Yanagi, Koide, Mitsushita, Higashi, Minosaki, Hayashi, Okamoto, Kuraishi, Ehara, Ishida, Horigome, Murakami, Takei, Ishii, Harada, Hirata, Maeda, Tatebe, Ota, Hayabuchi, Sakazaki, Sasaki, Hirono, Suzuki, Yasuda, Takeda, Sawada, Miyaji, Kitagawa, Nakai, Kakimoto, Agematsu, Manabe and Saiki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshikatsu Saiki eW9zaGlzYWlraUBtZWQudG9ob2t1LmFjLmpw
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.