- 1Department of Cardiovascular Surgery, Fujian Medical University Union Hospital, Fuzhou, China
- 2Key Laboratory of Cardio-Thoracic Surgery (Fujian Medical University), Fujian Province University, Fuzhou, China
- 3Fujian Provincial Center for Cardiovascular Medicine, Fuzhou, China
Background: The aim of this study was to identify the predictive factors for adverse clinical events after surgery in patients with acute type A aortic dissection (AAAD), and to explore the predictive value of preoperative prognostic nutritional index (PNI) combined with D-dimer for these events.
Methods: This study was a retrospective analysis of clinical data of 153 patients with AAAD who underwent emergency surgery at our center from January 2019 to January 2022. Patients were divided into adverse event group and non-adverse event group based on whether they experienced adverse clinical events after surgery. Univariate and multivariable logistic regression analyses were performed to identify the risk factors for adverse events, and the predictive efficacy was evaluated by the area under the receiver operating characteristic curve (ROC-AUC).
Results: A total of 153 AAAD patients were included in the study, and were divided into the adverse event group (n = 46) and the non-adverse events group (n = 107) based on whether or not they experienced clinical adverse events after surgery. The optimal cutoff value was determined using ROC curves, and multivariate logistic regression analysis was performed. Ultimately, it was found that preoperative PNI < 42.45 and D-dimer > 15.05 were independent predictors of postoperative clinical adverse events in AAAD patients. The odd ratios (OR) value for preoperative PNI < 42.45 is 3.596 [95% Confidence Interval (CI): 1.508–8.923, p = 0.004], while the OR value for D-dimer > 15.05 is 7.572 [95% CI: 3.094–20.220, p < 0.001]. The combination of these two indicators has a high predictive value (AUC = 0.843, 95% CI: 0.774–0.912, p < 0.001) and is superior to using either variable alone.
Conclusion: Preoperative PNI < 42.45 and D-dimer > 15.05 are independent predictive factors for postoperative adverse events during hospitalization in patients with AAAD. The combination of these two indicators can improve the predictive accuracy, which is superior to using either variable alone.
Introduction
Acute aortic dissection is a highly lethal and high-risk cardiovascular emergency. Among them, acute type A aortic dissection (AAAD) is the most dangerous situation (1–3). The previous research results showed that the overall incidence rate of AAAD is approximately 4.7 per 100,000 population, but there are significant regional variations in the incidence rates (4). A study by Meszaros et al. indicated that the average age of onset of AAAD is around 50 years old (5). Its clinical features include an acute onset, rapid progression, and high mortality rate. Some studies have reported that the in-hospital mortality rate associated with AAAD is approximately 22% (6), and without surgical treatment, it is as high as 82% at 1 year (7). The only effective treatment for AAAD is emergency surgical repair. Although surgical techniques and perioperative management have made great progress compared to the past, the prognosis of AAAD after surgery is significantly worse compared to conventional cardiovascular surgery due to the characteristics of the disease and the complexity of the surgery (7–9). Therefore, it is necessary to identify sensitive preoperative prognostic indicators to predict the prognosis of AAAD patients, which can effectively improve perioperative treatment measures and help clinical doctors better evaluate the early outcomes after AAAD surgery.
Inflammatory responses and changes in coagulation function are integral to the development of aortic dissection and are closely related to its pathogenesis and prognosis (10, 11). Previous studies have shown that some inflammation-related biomarkers, such as C-reactive protein (CRP) and D-dimer, are considered to be associated with an adverse prognosis after AAAD surgery (12–14). The prognostic nutritional index (PNI) is a new systemic inflammation marker calculated based on serum albumin levels and peripheral lymphocyte count. It was originally widely used to evaluate the long-term outcomes and prognosis of gastrointestinal cancer patients (15, 16). In recent years, PNI has been shown to be associated with the prognosis of heart failure and coronary heart disease patients (17–19). Studies have found that a low PNI during the perioperative period is a risk factor for in-hospital mortality in AAAD patients (20, 21). However, as a single indicator, the accuracy of using PNI to predict postoperative outcomes after AAAD surgery is not sufficient. Therefore, it is necessary to combine other indicators for a systematic evaluation. Previous studies have shown that the coagulation function is closely associated with the prognosis of AAAD. D-dimer, as a major indicator reflecting the coagulation status of the body, can serve as a key biomarker for predicting postoperative outcomes (22). Through univariate and multivariate regression analysis, we found that PNI and D-dimer are independent risk factors for clinical adverse events after AAAD surgery. Therefore, we speculate that the composite index formed by PNI combined with D-dimer can provide effective clinical predictive information for clinical adverse events after AAAD surgery. The aim of this study is to explore the application of two indicators combined in predicting the risk of adverse clinical events after AAAD surgery.
Materials and methods
Study design and setting
A single center retrospective study was used to investigate the clinical data of AAAD patients admitted to our center. These patients were admitted to hospital for emergency surgery from January 2019 to January 2022. Since this is a retrospective study and there is no need to obtain informed consent, this study was approved by the Ethics Committee of the affiliated Union Hospital of Fujian Medical University, which is in line with the Helsinki Declaration.
The inclusion criteria of this study were: AAAD diagnosed by computed tomography thoracic aortography or magnetic resonance imaging; over 18 years of age; emergency surgical treatment after admission. Patients with the following conditions are excluded: patients whose time from onset to hospitalization is more than 48 h; patients with long-term use of drugs that affect blood cell count; patients with malignant tumors, autoimmune diseases, severe infectious diseases and chronic organ dysfunction. The serological samples of all patients were drawn from venous blood without medication before emergency operation.
By measuring the level of D-dimer, serum albumin and lymphocyte count, the formula (10 × serum albumin (g/dL) + 0.005 × lymphocyte count (per mm3)) was used to calculate PNI (16), and the relationship between PNI, D-dimer and clinical adverse events after operation was analyzed.
Definition of clinical adverse events
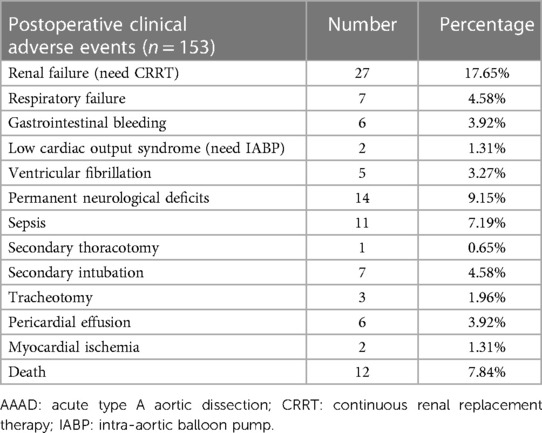
Clavien–Dindo grading is a general surgical complication grading system, which can also be used to grade the severity of complications after cardiovascular surgery (23). The postoperative clinical adverse events in this study were defined as complications of Clavien–Dindo grade III or above, including single or multiple organ dysfunction and postoperative death (24). Single organ dysfunction includes renal insufficiency requiring dialysis treatment, cardiac dysfunction requiring left ventricular assist device or intra-aortic balloon pump (IABP) therapy, neurological deficits requiring reintubation, tracheostomy or radiological and neurosurgical interventions, irreversible spinal cord injury, and intestinal ischemia requiring surgical intervention. Multiple organ dysfunction is defined as simultaneous or sequential dysfunction of two or more organs or systems caused by various clinical factors (25).
Data collection
We collected clinical data of each patient from the hospital's medical record system and observed and summarized various indicators before and during surgery. Preoperative indicators include: (1) demographic data: gender, age, body mass index (BMI); (2) past medical history: smoking history, drinking history; (3) comorbidities: hypertension, diabetes, coronary artery disease, history of cerebrovascular disease, chronic obstructive pulmonary disease, Marfan syndrome, hepatic dysfunction and renal insufficiency; (4) preoperative general condition: aortic valve regurgitation (moderate or above), pericardial effusion (moderate or above), left ventricular ejection fraction (LVEF). Preoperative laboratory tests: red blood cell count, white blood cell count, leukomonocyte, hemoglobin, platelet, albumin, alanine aminotransferase, aspartate aminotransferase, serum creatinine, D-dimer, prothrombin time (PT), B-type natriuretic peptide, C-reactive protein, troponin-I. Intraoperative indicators include: processing method of the aortic root, total operation time, cardiopulmonary bypass time, aortic cross-clamp time, cerebral perfusion time time, deep hypothermic circulatory arrest time and intraoperative blood product input (red blood cells, plasma, platelets). The postoperative complications were classified into two groups based on whether or not Clavien–Dindo grade III or higher surgical complications occurred: the no-adverse events group and the adverse events group.
Surgical technique
The surgery was performed under general anesthesia and cardiopulmonary bypass support. The specific surgical procedure was described in detail in Chen et al.'s previous study, including reconstruction of the aortic root, replacement of the ascending aorta, and implantation of a modified triple-branch stent graft (26, 27).
Statistical analysis
All statistical analyses were performed using SPSS 23.0 and R software (4.2.2). Continuous variables were expressed as mean ± standard deviation or interquartile range, while categorical variables were expressed as frequency, ratio, and percentage. The Kolmogorov–Smirnov test was used to check the normality of the distribution of continuous variables. Student t-tests were used for intergroup comparison of continuous variables that followed a normal distribution, while Mann–Whitney U tests were used for those that did not follow a normal distribution. The chi-square test or Fisher's exact test was used for categorical variables. Predictive variables with p < 0.05 in the univariate analysis were included in a multivariate logistic regression analysis to identify independent risk factors for postoperative adverse events. Receiver operating characteristic (ROC) curves were constructed to determine the optimal cutoff values for PNI, D-dimer, and combination variables in predicting postoperative adverse events, and the area under the curve (AUC) was calculated. The predictive performance of the combined indicators will be evaluated using AUC, net reclassification improvement (NRI), and integrated discrimination improvement index (IDI). A difference was considered statistically significant when p < 0.05.
Results
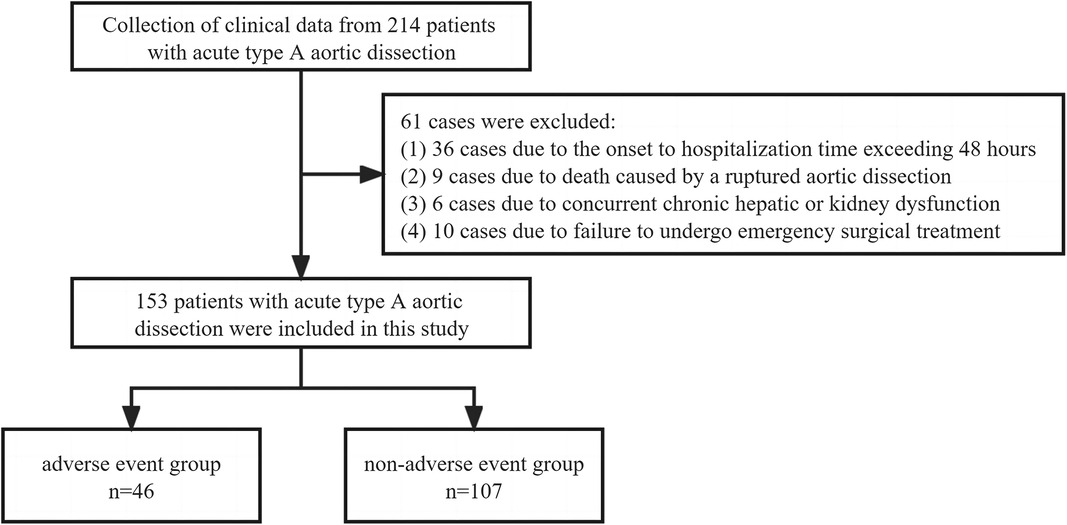
This study diagnosed a total of 214 patients with AAAD between January 2019 and January 2022. Among them, 36 cases were excluded due to the onset-to-hospitalization time exceeding 48 h, 9 cases were excluded due to death caused by a ruptured aortic dissection, 6 cases were excluded due to concurrent chronic hepatic or kidney dysfunction, and 10 cases were excluded due to failure to undergo emergency surgical treatment. In the end, a total of 153 patients were included in this study (Figure 1).
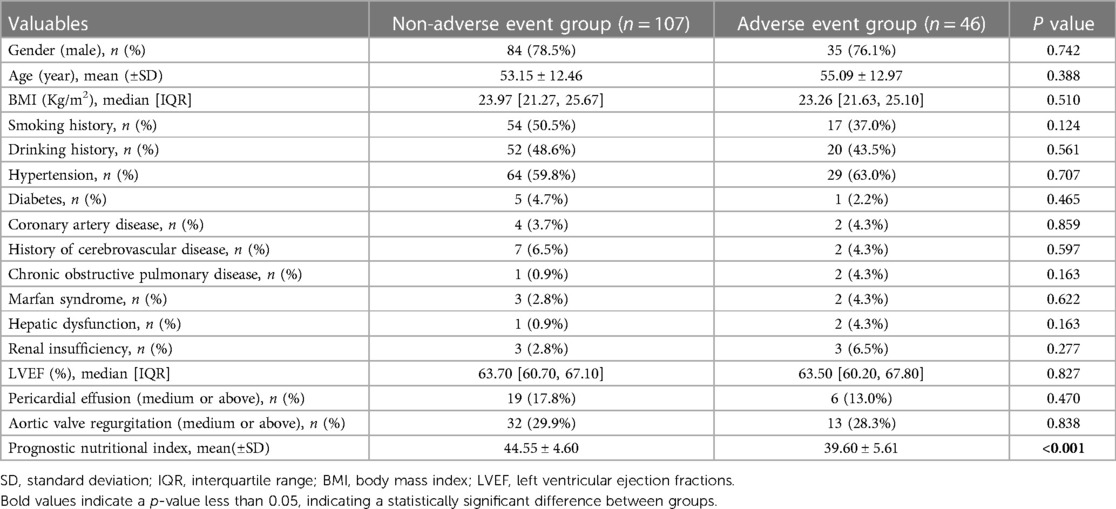
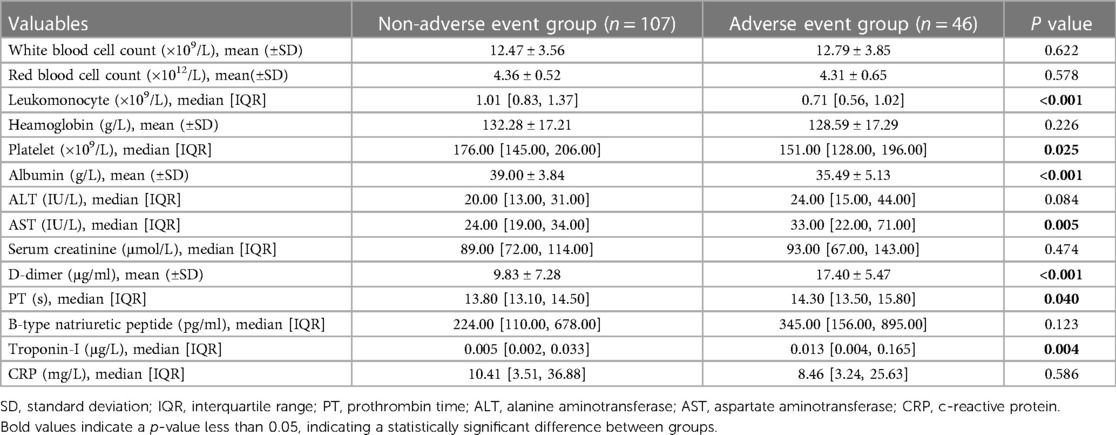
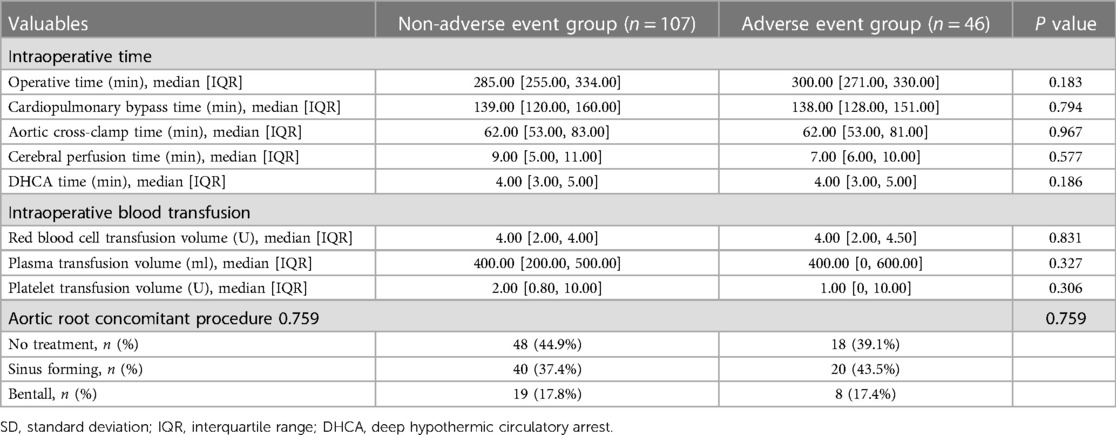
The baseline data comparison of the patients showed that the two groups of patients had similar baseline data such as gender, age, and body mass index (BMI), but the preoperative PNI of the adverse event group was significantly lower than that of the non-adverse event group (39.60 ± 5.68 vs. 44.55 ± 4.63, p < 0.001). Laboratory examination results showed that the leukomonocyte [0.71 (0.56, 1.02) vs. 1.01 (0.83, 1.37)], albumin (35.49 ± 5.13 vs. 39.00 ± 3.84), and platelet [151.00 (128.00, 196.00) vs. 176.00 (145.00, 206.00)] of the adverse event group were significantly lower than those of the non-adverse event group, while D-dimer (17.40 ± 5.47 vs. 9.83 ± 7.28), AST [33.00 (22.00, 71.00) vs. 24.00 (19.00, 34.00)], PT [14.30 (13.50, 15.80) vs. 13.80 (13.10, 14.50)], and troponin-I [0.013 (0.004, 0.165) vs. 0.005 (0.002, 0.033)] were significantly higher than those of the non-adverse event group. The differences in the clinical indicators between the two groups were statistically significant (p < 0.05) (Tables 1, 2). The intraoperative comparison results showed that there were no significant differences in the total operation time, cardiopulmonary bypass (CPB) time, aortic cross-clamp (ACC) time, cerebral perfusion time, and deep hypothermic circulatory arrest (DHCA) time between the two groups of patients. The aortic root handling methods and intraoperative blood product transfusion were also similar between the two groups (Table 3). The occurrence of postoperative complications in the adverse event group is shown in Table 4.
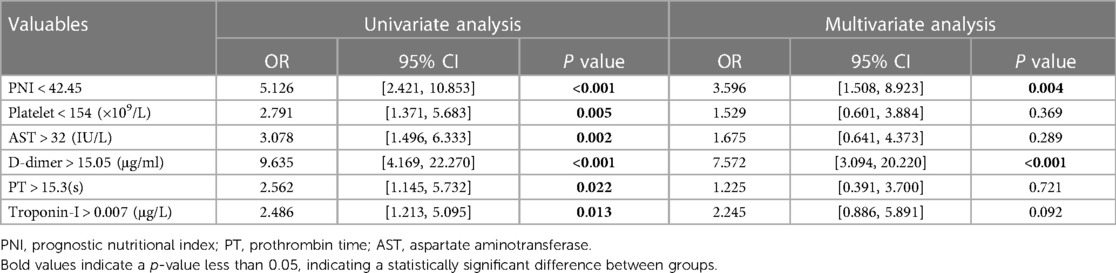
The optimal cut-off values of the clinical characteristic variables for which there were statistically significant differences between groups were calculated by drawing a ROC curve. Subsequently, univariate and multivariate logistic regression analyses were performed, and the results are presented in Table 5. A forest plot depicting the odds ratio (OR) and their 95% confidence interval (CI) was generated based on the results, as shown in Figure 2.
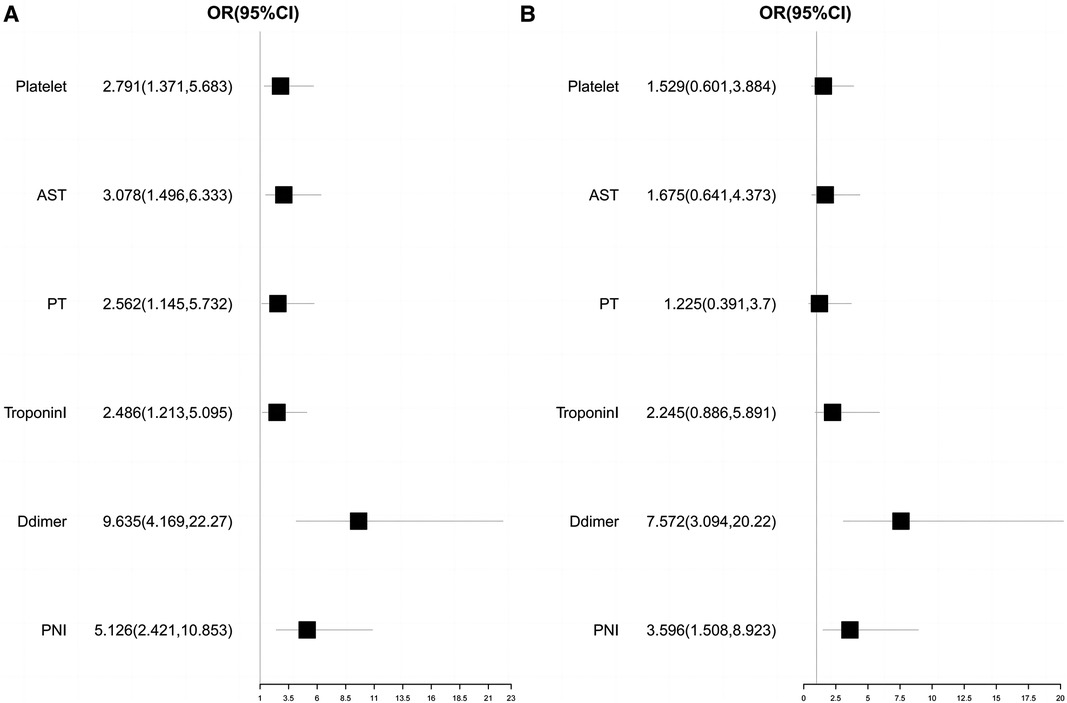
Figure 2. Univariate and multivariate logistics regression analysis forest plot. (A) Univariate logistics regression analysis forest plot; (B) Multivariate logistics regression analysis forest plot.
We found that when the optimal cutoff value was 42.45, the sensitivity of preoperative PNI was 0.692, specificity was 0.696, and the AUC value was 0.752; the optimal cutoff value for D-dimer was 15.05, with a sensitivity of 0.826, specificity of 0.701, and an AUC value of 0.770. The results of the multivariate logistic regression analysis showed that a PNI < 42.45 and a D-dimer > 15.05 μg/ml were independent risk factors for postoperative clinical adverse events in AAAD patients.
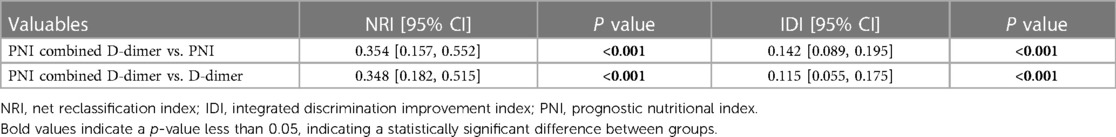
In order to better predict postoperative clinical adverse events, we combined the above clinical indicators (preoperative PNI combined D-dimer) and calculated the AUC of the combined index to be 0.843 (95% CI was 0.774–0.912, p < 0.001), with a sensitivity of 0.826 and specificity of 0.738. The AUC value of the combined index was more predictive than using a single indicator alone (Table 6). We also assessed the effectiveness and accuracy of the combined indicators in clinical prediction using the NRI and IDI. The results, shown in Table 7, indicate that the combined indicators have higher predictive accuracy compared to traditional individual predictors such as PNI or D-dimer. In order to compare the predictive performance of the combined index with traditional single biochemical indicators, we drew ROC curves to compare the combined index (preoperative PNI combined D-dimer) with commonly used clinical indicators of inflammation severity (WBC, CRP) and nutritional status (ALB). The results are shown in Figure 3, and the combined index still outperforms the single biochemical indicators in predicting outcomes.
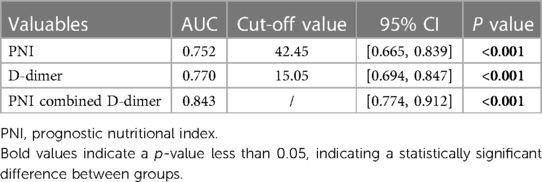
Table 6. Predictive value of preoperative PNI combined D-dimer for postoperative clinical adverse events.
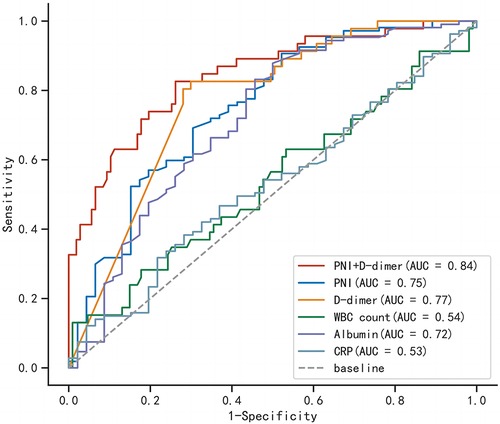
Figure 3. Comparison of receiver operating characteristic curves for combined indicators and single indicators.
In summary, preoperative PNI and D-dimer are effective predictive indicators for adverse events after AAAD surgery, and their combined use is more accurate.
Discussion
AAAD is an extremely dangerous cardiovascular emergency. If damaged aorta is not repaired by surgery in a timely manner, the mortality rate within 48 h is about 50% (28). Despite emergency surgery being performed, the mortality rate remains high due to numerous postoperative complications. Therefore, early risk prediction of clinical adverse events and taking timely and effective measures for treatment can greatly help reduce in-hospital mortality. It is crucial to identify predictive factors that can forecast the risk of clinical adverse events after AAAD surgery. Currently, many studies have shown that certain blood biochemical indicators may have important significance in predicting the postoperative prognosis of AAAD, such as inflammatory factors and coagulation function indicators (29, 30). However, the clinical accuracy of predicting with a single indicator still needs to be improved. Therefore, this study aims to explore the clinical application value of combining multiple indicators to predict clinical adverse events after AAAD surgery.
Previous studies have shown that local and systemic inflammatory responses play a crucial role in the development of AAAD (31–33). Inflammatory factors such as interleukin-6, procalcitonin, CRP are significantly elevated in the serum of most AAAD patients, and the degree of inflammatory response is often closely related to the prognosis (34, 35). As a composite index of systemic inflammation, PNI takes into account the degree of current inflammatory response and corresponding nutritional status, and the immune and nutritional status is closely related to the progression and prognosis of cardiovascular disease (36, 37). In recent years, in addition to being an independent predictor of postoperative mortality and prognosis in gastrointestinal tumors, the potential application value of PNI in cardiovascular diseases has also been increasingly recognized (17, 19). Although some studies have shown that PNI can be used to predict short-term prognosis for certain heart surgeries, due to the urgency and complexity of AAAD disease, the predictive value of PNI in such cardiovascular emergencies is still unknown. Our study is also the first retrospective study on the predictive value of PNI for postoperative clinical adverse events in AAAD (38, 39).
Currently, the pathogenesis of aortic dissection (AD) is not clear. Many studies believe that the degradation of extracellular matrix in the middle layer of the aorta is closely related to the occurrence of AD (40). Activated lymphocytes can induce matrix metalloproteinase expression in smooth muscle cells of the aortic media, which is an important factor in promoting extracellular matrix degradation and plays an important role in the inflammatory response of AD, closely related to its prognosis (41, 42). Therefore, we believe that lymphocyte counts have potential value in predicting the prognosis of AD outcomes. At the same time, albumin, as an important indicator in clinical biochemistry testing, is usually used to evaluate the current nutritional status of the body, and can also indirectly reflect the degree of consumption of the body caused by disease, as most patients with inflammation-related diseases have acute or chronic consumption in their bodies (43). In this study, the PNI calculated by combining lymphocyte count and serum albumin was used to predict postoperative clinical adverse events. We believe that PNI has more accurate predictive value than its individual components. Currently, we have found that preoperative PNI < 42.45 is an independent risk factor for clinical adverse events after AAAD surgery. This finding can help clinicians make more appropriate clinical decisions in emergency situations of AAAD.
D-dimer is a non-specific fibrin degradation product that can reflect hyperfibrinolysis and hypercoagulable state in the human body. It is widely used for the diagnosis, efficacy evaluation, and prognosis prediction of thrombotic diseases (44, 45). During the occurrence of AD, due to the tearing and damage of the inner layer of the aorta, the coagulation system is rapidly activated, forming a false lumen thrombus, triggering a cascade reaction of coagulation, and activating the fibrinolysis system, leading to a rapid increase in the level of D-dimer in the serum. This has a good reference value in the diagnosis and differential diagnosis of AAAD (46). However, due to the fact that D-dimer is a highly sensitive but low-specificity detection indicator, its levels will significantly increase in cancer, infections, or any condition that may affect the body's coagulation function. Therefore, the reliability of using it as a prognostic indicator for AAAD still needs further verification. Research has also reported a correlation between the risk of in-hospital mortality in AAAD and the level of D-dimer (47). This may be due to the association between elevated D-dimer levels and serious complications such as postoperative acute renal failure, severe infection, and gastrointestinal bleeding. The level of elevated D-dimer can reflect the degree of disorder in the body's coagulation function to some extent. Although using D-dimer alone to predict the clinical outcome of AAAD has low specificity, our research results indicate that D-dimer > 15.05 μg/ml is an independent risk factor for postoperative clinical adverse events in AAAD, and has potential value in predicting the prognosis of AAAD. Therefore, we hope to increase its predictive reliability by combining it with other clinical indicators.
The results of this study indicate that preoperative PNI combined D-dimer are effective indicators for predicting postoperative clinical adverse events in patients with AAAD. Preoperative PNI < 42.45 and D-dimer > 15.05 μg/ml are independent risk factors for patients to experience postoperative clinical adverse events, which may provide more valuable predictive evaluation for the prognosis of patients with AAAD. This study is the first to apply PNI to the prediction of prognosis outcomes in cardiovascular emergencies such as AAAD. This composite inflammation-related indicator comprehensively considers the current nutritional status of the body, which can more reliably evaluate the prognosis outcomes. At the same time, we also found that the combination of PNI and the coagulation function indicator D-dimer had a significantly better predictive effect than using any single indicator alone (AUC = 0.843). The combination of the two indicators mentioned above for predicting the postoperative outcomes of AAAD patients has clear advantages, mainly because these clinical indicators do not increase the patient's medical costs or cause additional trauma, and are easy to obtain results in practical operations. This advantage also increases the clinical application value of these indicators.
The limitations of this study are as follows: (1) This is a single-center retrospective study, and the results may be limited by factors such as sample size, only representing the experience of this center in predicting adverse events of AAAD. In the future, more multi-center randomized controlled trials with larger sample sizes are needed to further verify the conclusions of this study; (2) This study only explored the relationship between preoperative PNI and D-dimer levels and postoperative adverse events, without further studying whether they have practical value in predicting the mid-to-long-term prognosis of AAAD, which is our next research direction.
Conclusion
Preoperative PNI < 42.45 and D-dimer > 15.05 are independent predictive factors for adverse clinical events in patients with AAAD after surgery, and have potential application value for predicting postoperative prognosis. The combined use of these two indicators can further improve their predictive value.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the affiliated Union Hospital of Fujian Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study and there is no need to obtain informed consent. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because this is a retrospective study and there is no need to obtain informed consent.
Author contributions
LX, JH, and DJ designed the study, and drafted the manuscript. XL, XZ, and ZZ collected the clinical data and performed the statistical analysis. LX and DJ provide technical support. All authors contributed to the article and approved the submitted version.
Funding
This research was sponsored by the Fujian Provincial Special Reserve Talents Fund (2021–25) and the Startup Fund for Scientific Research, Fujian Medical University (2022QH2019).
Acknowledgments
We highly acknowledge the contribution by the participating doctors: Yue Shen and Fan Xu.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. (2015) 385:800–11. doi: 10.1016/S0140-6736(14)61005-9
2. Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the international registry of acute aortic dissection score. Ann Thorac Surg. (2007) 83:55–61. doi: 10.1016/j.athoracsur.2006.08.007
3. Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. (2018) 137:1846–60. doi: 10.1161/CIRCULATIONAHA.117.031264
4. Pacini D, Di Marco L, Fortuna D, Belotti LM, Gabbieri D, Zussa C, et al. Acute aortic dissection: epidemiology and outcomes. Int J Cardiol. (2013) 167:2806–12. doi: 10.1016/j.ijcard.2012.07.008
5. Mészáros I, Mórocz J, Szlávi J, Schmidt J, Tornóci L, Nagy L, et al. Epidemiology and clinicopathology of aortic dissection. Chest. (2000) 117:1271–8. doi: 10.1378/chest.117.5.1271
6. Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: the international registry of acute aortic dissection experience. J Thorac Cardiovasc Surg. (2005) 129:112–22. doi: 10.1016/j.jtcvs.2004.09.005
7. Pagni S, Ganzel BL, Trivedi JR, Singh R, Mascio CE, Austin EH, et al. Early and midterm outcomes following surgery for acute type A aortic dissection. J Card Surg. (2013) 28:543–9. doi: 10.1111/jocs.12170
8. Shang W, Ma M, Ge YP, Liu N, Zhu JM, Sun LZ. Analysis of risk factors of type a aortic dissection (TAAD) operation of frozen elephant trunk and total arch replacement. Eur Rev Med Pharmacol Sci. (2016) 20:4586–92.27874962
9. Halstead JC, Spielvogel D, Meier DM, Rinke S, Bodian C, Malekan R, et al. Composite aortic root replacement in acute type A dissection: time to rethink the indications? Eur J Cardiothoracic Surg. (2005) 27:626–32. discussion 632–3. doi: 10.1016/j.ejcts.2004.12.059
10. Nagareddy P, Smyth SS. Inflammation and thrombosis in cardiovascular disease. Curr Opin Hematol. (2013) 20:457–63. doi: 10.1097/MOH.0b013e328364219d
11. Levi M, van der Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Semin Thromb Hemost. (2008) 34:459–68. doi: 10.1055/s-0028-1092876
12. Liu Y, Han L, Li J, Gong M, Zhang H, Guan X. Consumption coagulopathy in acute aortic dissection: principles of management. J Cardiothorac Surg. (2017) 12:50. doi: 10.1186/s13019-017-0613-5
13. Guan XL, Wang XL, Liu YY, Lan F, Gong M, Li HY, et al. Changes in the hemostatic system of patients with acute aortic dissection undergoing aortic arch surgery. Ann Thorac Surg. (2016) 101:945–51. doi: 10.1016/j.athoracsur.2015.08.047
14. Okina N, Ohuchida M, Takeuchi T, Fujiyama T, Satoh A, Sakamoto T, et al. Utility of measuring C-reactive protein for prediction of in-hospital events in patients with acute aortic dissection. Heart Vessels. (2013) 28:330–5. doi: 10.1007/s00380-012-0257-2
15. Nogueiro J, Santos-Sousa H, Pereira A, Devezas V, Fernandes C, Sousa F, et al. The impact of the prognostic nutritional index (PNI) in gastric cancer. Langenbecks Arch Surg. (2022) 407:2703–14. doi: 10.1007/s00423-022-02627-0
16. Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.6438478
17. Li H, Cen K, Sun W, Feng B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. (2021) 33:1477–86. doi: 10.1007/s40520-020-01656-3
18. Raposeiras Roubín S, Abu Assi E, Cespón Fernandez M, Barreiro Pardal C, Lizancos Castro A, Parada JA, et al. Prevalence and prognostic significance of malnutrition in patients with acute coronary syndrome. J Am Coll Cardiol. (2020) 76:828–40. doi: 10.1016/j.jacc.2020.06.058
19. Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, et al. Prognostic nutritional Index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. (2017) 6(6):e004876. doi: 10.1161/JAHA.116.004876
20. Lin Y, Chen Q, Peng Y, Chen Y, Huang X, Lin L, et al. Prognostic nutritional index predicts in-hospital mortality in patients with acute type A aortic dissection. Heart Lung. (2021) 50:159–64. doi: 10.1016/j.hrtlng.2020.06.004
21. Keskin HA, Kurtul A, Esenboğa K, Çiçek MC, Katırcıoğlu SF. Prognostic nutritional index predicts in-hospital mortality in patients with acute Stanford type A aortic dissection. Perfusion. (2021) 36:710–6. doi: 10.1177/0267659120961937
22. Nazerian P, Mueller C, Soeiro AM, Leidel BA, Salvadeo SAT, Giachino F, et al. Diagnostic accuracy of the aortic dissection detection risk score plus D-dimer for acute aortic syndromes: the ADvISED prospective multicenter study. Circulation 137 (2018) 250–8. doi: 10.1161/CIRCULATIONAHA.117.029457
23. Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
24. Wu Q, Li J, Chen L, Yan LL, Qiu Z, Shen Y, et al. Efficacy of interleukin-6 in combination with D-dimer in predicting early poor postoperative prognosis after acute Stanford type A aortic dissection. J Cardiothorac Surg. (2020) 15:172. doi: 10.1186/s13019-020-01206-y
25. Gourd NM, Nikitas N. Multiple organ dysfunction syndrome. J Intensive Care Med. (2020) 35:1564–75. doi: 10.1177/0885066619871452
26. Chen LW, Dai XF, Wu XJ, Liao DS, Hu YN, Zhang H, et al. Ascending aorta and hemiarch replacement combined with modified triple-branched stent graft implantation for repair of acute DeBakey type I aortic dissection. Ann Thorac Surg. (2017) 103:595–601. doi: 10.1016/j.athoracsur.2016.06.017
27. Chen LW, Wu XJ, Dai XF, Liao DS, Li C, Wang QM, et al. A self-adaptive triple-branched stent graft for arch repair during open type A dissection surgery. J Thorac Cardiovasc Surg. (2015) 149:1278–83.e1. doi: 10.1016/j.jtcvs.2014.11.079
28. Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. (2012) 33:26–35b. doi: 10.1093/eurheartj/ehr186
29. Lu J, Li P, Ma K, Li Y, Yuan H, Zhu J, et al. OPG/TRAIL ratio as a predictive biomarker of mortality in patients with type A acute aortic dissection. Nat Commun. (2021) 12:3401. doi: 10.1038/s41467-021-23787-5
30. Tang Z, Liu H, Shao Y. Efficacy of CRP in combination with D-dimer in predicting adverse postoperative outcomes of patients with acute Stanford type A aortic dissection. J Cardiothorac Surg. (2022) 17:71. doi: 10.1186/s13019-022-01818-6
31. Luo F, Zhou XL, Li JJ, Hui RT. Inflammatory response is associated with aortic dissection. Ageing Res Rev. (2009) 8:31–5. doi: 10.1016/j.arr.2008.08.001
32. Kalkan ME, Kalkan AK, Gündeş A, Yanartaş M, Oztürk S, Gurbuz AS, et al. Neutrophil to lymphocyte ratio: a novel marker for predicting hospital mortality of patients with acute type A aortic dissection. Perfusion. (2017) 32:321–7. doi: 10.1177/0267659115590625
33. Zhou Q, Chai XP, Fang ZF, Hu XQ, Tang L. Association of plasma pentraxin-3 levels on admission with in-hospital mortality in patients with acute type A aortic dissection. Chin Med J. (2016) 129:2589–95. doi: 10.4103/0366-6999.192785
34. Zeng T, Shi L, Ji Q, Shi Y, Huang Y, Liu Y, et al. Cytokines in aortic dissection. Clin Chim Acta. (2018) 486:177–82. doi: 10.1016/j.cca.2018.08.005
35. Hsieh WC, Henry BM, Hsieh CC, Maruna P, Omara M, Lindner J. Prognostic role of admission C-reactive protein level as a predictor of in-hospital mortality in type-A acute aortic dissection: a meta-analysis. Vasc Endovascular Surg. (2019) 53:547–57. doi: 10.1177/1538574419858161
36. Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. (2017) 14:133–44. doi: 10.1038/nrcardio.2016.185
37. Casas R, Castro-Barquero S, Estruch R, Sacanella E. Nutrition and cardiovascular health. Int J Mol Sci. (2018) 19(12):3988. doi: 10.3390/ijms19123988
38. Wada H, Dohi T, Miyauchi K, Jun S, Endo H, Doi S, et al. Relationship between the prognostic nutritional index and long-term clinical outcomes in patients with stable coronary artery disease. J Cardiol. (2018) 72:155–61. doi: 10.1016/j.jjcc.2018.01.012
39. Lee SI, Ko KP, Choi CH, Park CH, Park KY, Son KH. Does the prognostic nutritional index have a predictive role in the outcomes of adult cardiac surgery? J Thorac Cardiovasc Surg. (2020) 160:145–153.e3. doi: 10.1016/j.jtcvs.2019.08.069
40. Wu X, Ye J, Cai W, Yang X, Zou Q, Lin J, et al. LDHA mediated degradation of extracellular matrix is a potential target for the treatment of aortic dissection. Pharmacol Res. (2022) 176:106051. doi: 10.1016/j.phrs.2021.106051
41. Oviedo-Orta E, Bermudez-Fajardo A, Karanam S, Benbow U, Newby AC. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology. (2008) 124:42–50. doi: 10.1111/j.1365-2567.2007.02728.x
42. Schönbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res. (1997) 81:448–54. doi: 10.1161/01.RES.81.3.448
43. Ohsuzu F. The roles of cytokines, inflammation and immunity in vascular diseases. J Atheroscler Thromb. (2004) 11:313–21. doi: 10.5551/jat.11.313
44. Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. (2017) 70:2411–20. doi: 10.1016/j.jacc.2017.09.024
45. Favresse J, Lippi G, Roy PM, Chatelain B, Jacqmin H, Ten Cate H, et al. D-dimer: preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. (2018) 55:548–77. doi: 10.1080/10408363.2018.1529734
46. Sodeck G, Domanovits H, Schillinger M, Ehrlich MP, Endler G, Herkner H, et al. D-dimer in ruling out acute aortic dissection: a systematic review and prospective cohort study. Eur Heart J. (2007) 28:3067–75. doi: 10.1093/eurheartj/ehm484
Keywords: acute type A aortic dissection, prognostic nutrition index, D-dimer, clinical adverse events, predictive efficacy
Citation: Xie L, He J, Lin X, Zhang Z, Zhuang X and Jiang D (2023) Efficacy of prognostic nutrition index in combination with D-dimer in predicting postoperative clinical adverse events after acute type A aortic dissection: a single center retrospective study. Front. Cardiovasc. Med. 10:1210725. doi: 10.3389/fcvm.2023.1210725
Received: 23 April 2023; Accepted: 25 September 2023;
Published: 9 October 2023.
Edited by:
Wolf-Hans Eilenberg, Medical University of Vienna, AustriaReviewed by:
Barbara Ruszkowska-Ciastek, Nicolaus Copernicus University in Toruń, PolandGian Marco Rosa, San Martino Hospital (IRCCS), Italy
© 2023 Xie, He, Zhuang, Lin, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Debin Jiang MjU4NTMxMjU4QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Linfeng Xie1,2,3,†
Linfeng Xie1,2,3,† Jian He
Jian He Xinghui Zhuang
Xinghui Zhuang Debin Jiang
Debin Jiang