- 1Department of Cardiovascular Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 2Department of Cardiovascular Surgery, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China
- 3Department of Anesthesiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 4Department of Anesthesiology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Anesthesia and Respiratory Critical Medicine, Jinan, China
- 5Imaging Department, Pingyin Chinese Medicine Hospital, Jinan, China
- 6Department of Pathology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Here, we report an unusual case of left atrial myxoma presented with morphology of cavernous hemangioma supplied by the right coronary artery. Surgical resection of the left atrium myxoma was performed, and the patient experienced an uneventful recovery during hospitalization.
Introduction
The incidence of primary cardiac tumors has been reported to be as low as approximately 0.02% (1–5). Myxomas account for nearly 77% of all primary cardiac tumors (6–8). Echocardiography, cardiac MRI, coronary computed tomography angiography (CTA), and coronary angiography are available diagnostic modalities for cardiac myxoma (8–10). Here, we reported an uncommon case of an adult female patient in whom a left atrial myxoma presenting with partial morphology of cavernous hemangioma was supplied by the right coronary artery. Surgical treatment was performed to completely excise the myxoma. The patient had a smooth recovery following surgery and did not experience any significant complications during hospitalization.
Case description
A 68-year-old female was admitted to the hospital with a chief complaint of paroxysmal chest tightness, shortness of breath, and palpitation for over a month. The patient had no history of hypertension or diabetes mellitus and other comorbidities. Preoperative echocardiography showed an intracardiac mass with medium-level echogenicity attached to the upper part of the interatrial septum in the left atrium, with a wide base, regular shape, and fixed position (Figure 1A). The coronary CTA showed a spherical lesion of equal density in the left atrium, closely related to the upper part of the interatrial septum, with right coronary artery blood supply (Figure 1B). Cardiac magnetic resonance imaging (MRI) depicted a spherical lesion with mixed isointense T1 and hyperintense T2 signal in the left atrium, with a clear edge and a size of approximately 2.5 cm × 2.6 cm × 2.7 cm. Late gadolinium-enhanced (LGE) imaging demonstrated patchy and uneven delayed enhancement in the left atrial mass (Figure 1C). Coronary angiography prior to surgery revealed the blood perfusion from the distal right coronary artery to the left atrial mass (Figure 2).
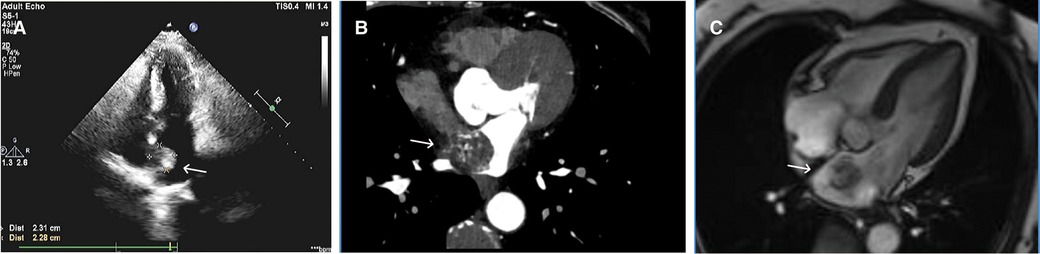
Figure 1. Preoperative echocardiography, coronary CTA, and cardiac MRI. (A) Echocardiography showed a medium echogenic mass attached to the upper part of the interatrial septum in the left atrium, with a wide base, regular shape, and fixed position. (B) Coronary CTA demonstrated a spherical lesion in the left atrium with right coronary artery blood supply. (C) LGE imaging depicted a spherical mass having a size of approximately 2.5 cm × 2.6 cm × 2.7 cm in the left atrium, with an abnormal signal showing a clear edge and patchy and uneven delayed enhancement.
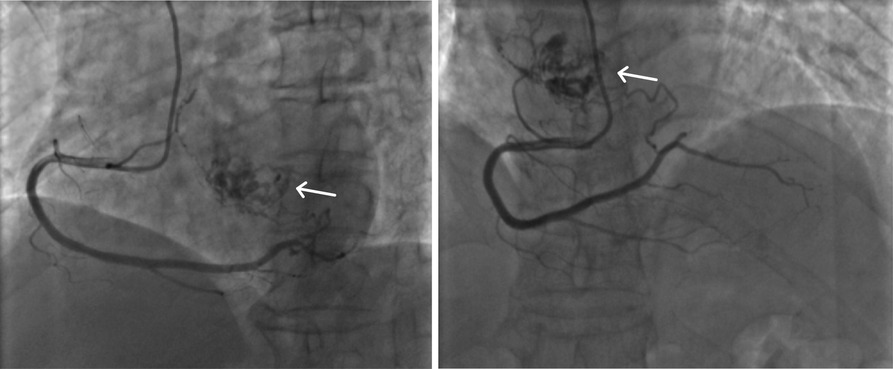
Figure 2. Characterization of the left atrial mass and its blood supply on coronary angiography. Preoperative coronary angiography detected a tumor-like mass in the left atrium and blood supply from the distal right coronary artery to the mass.
The surgery was performed under general anesthesia with extracorporeal circulation. During the operation, the right atrium and interatrial septum were opened, revealing an irregular, nodular mass with a size of approximately 2.5 cm × 3.0 cm × 3.0 cm in the left atrium. The mass had a broad base and was located at the posterior and inferior portion of the interatrial septum, close to the orifice of the right inferior pulmonary vein. The mass also involved the posterior wall of the left atrium and was covered with a cyst-like, fluid-filled capsule. The lesion was completely excised along with a portion of the interatrial septum and posterior wall of the left atrium (Figure 3A). A bovine pericardium patch was used to repair the posterior wall of the left atrium and the atrial septal incision was continuously sutured. The surgery was successful, with aortic clamping and extracorporeal circulation lasting 40 and 61 min respectively.
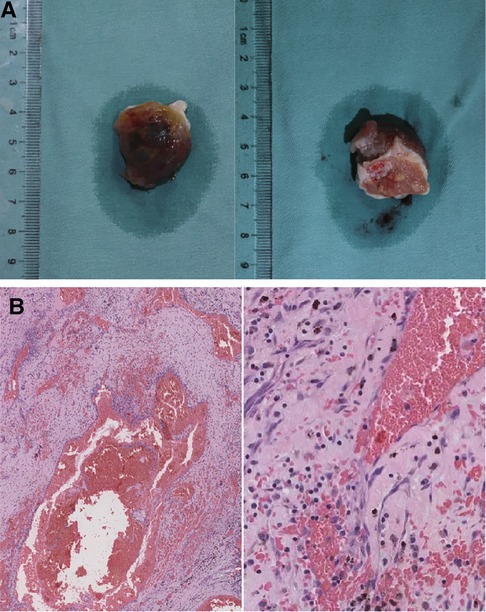
Figure 3. Gross pathological specimen and histopathology of the left atrial mass. (A) The mass resected from the left atrium. (B) Histopathology showed cavernous hemangioma with vascular dilation and congestion based on background of cardiac myxoma. The magnifications in (B) are ×40 (left) and ×200 (right).
Postoperative histopathology demonstrated that the lesion tissue was a left atrial myxoma, presenting with partial cavernous hemangioma showing vascular dilation and congestion (Figure 3B). Postoperative echocardiography did not detect any residual tumor tissue, and cardiac chamber structure and function were normal (Figure 4).
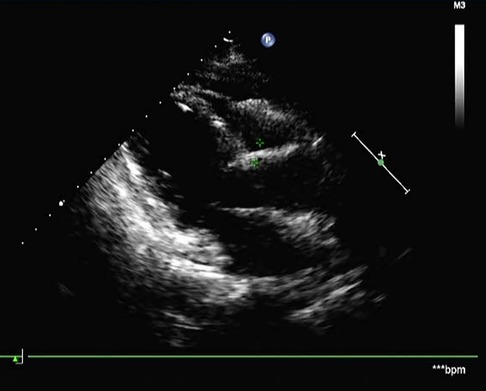
Figure 4. Postoperative echocardiography showed no residual tumor tissue and a normal cardiac chamber structure.
The recovery following surgery was smooth, and the patient did not experience any significant complications during hospitalization. Upon discharge 6 days after surgery, the symptoms such as chest tightness and shortness of breath were completely relieved. During a 3-month follow-up, her chest tightness, shortness of breath, and palpitation had resolved completely. Echocardiography showed that the cardiac myxoma did not recur.
Discussion
Cardiac myxoma is the most common type of adult primary heart tumor. In recent years, the possibility of misdiagnosis of cardiac myxoma has decreased significantly due to the utilization of diagnostic modality such as echocardiography, coronary CTA, and cardiac MRI (8–10). Coronary CTA and angiography could reveal the blood supply of cardiac myxoma (11, 12). Although cardiac myxoma is benign, it may grow toward the heart cavity and result in blood flow obstruction and heart valve dysfunction. The detachment of myxoma may even cause severe systemic embolism. Surgical incision of cardiac myxoma is required once the diagnosis is established, and cardiac myxoma may still recur after surgery; thus, regular postoperative follow-up is necessary.
Cardiac myxoma is also the most common benign tumor of the heart (13). Although cardiac cavernous hemangioma is a rare benign tumor, there have also been reports (14). However, the combination of cardiac myxoma and cavernous hemangioma has not been reported yet. In this case, the left atrial tumor included two portions: both myxoma and cavernous hemangioma. The myxoma could be observed in the area consisting of sparse irregular spindle-shaped and star-shaped fibroblasts, with small and deeply stained nuclei, scattered tumor cells, loose mucinous stroma, and varying numbers of small blood vessels. In the background of myxoma, thin-walled large blood vessels with different diameters could be detected. The wall of the vessels was composed of flat endothelial cells without atypia, with smooth muscles also visible. Thrombosis could be noticed in the vascular lumen, and fibrous septa seen in the large lumen. This case needs to be differentiated from simple atrial myxoma, which also shows blood vessels, but mostly thick-walled small vessels and rarely thin-walled large vessels (15, 16). Cavernous hemangioma also needs to be distinguished from well-differentiated angiosarcoma. Angiosarcoma often manifests infiltrative growth, with vascular endothelial cells arranged in an atypical or multiple layers and visible nuclear mitotic, and has a poor prognosis (17).
Both myxomas and hemangiomas belong to mesenchymal tumors and thus may have the same origin. The ultrastructural examination of cardiac myxoma shows that it is derived from multipotential mesenchymal cells. On the one hand, immunohistochemistry indicates that myxoma expresses vascular endothelial markers, such as CD31 and CD34, which are considered markers of mesenchymal cells differentiating into endothelial cells (18). On the other hand, studies have found that myxoma expresses epithelial markers such as carcinoembryonic antigen (CEA) and epithelial membrane antibody (EMA), and cardiomyocyte-specific transcription factors, which indicate myocardial differentiation (19). Overall, myxoid of cardiac myxoma may originate from mesenchymal cells with multi-directional differentiation potential. These mesenchymal cells can not only differentiate into myxoid cells but also secrete angiogenic factors such as vascular endothelial growth factor (VEGF), promoting angiogenesis. Finally, the myxoma has a rich angioma-like thin-walled vessel filled with mucus such as chondroitin sulfate at the same time, forming a morphological myxoma structure (20). Hemangiomas arise from multipotent stem cells and these hemangioma stem cells that give rise to the endothelial cells are also the essential source of adipocytes during hemangioma involution (21). Based on the two types of morphologies, myxomas and hemangiomas are still considered independent diseases and we state that our case has its special pathological features.
Coronary angiography is a common and effective method for diagnosing coronary artery disease and is a safe and reliable invasive diagnostic technique, which is now widely used in clinical practice. Preoperative coronary angiography for cardiac myxoma patients not only excludes coronary artery disease but also detects the artery feeding the cardiac myxoma. In a review of coronary angiography in 19 patients with cardiac myxoma, seven had angiographically visible tumor vascularity. One patient had significant coronary artery disease of the circumflex coronary artery and another patient had a thrombus-like lesion in the proximal third of the left anterior descending coronary artery (22). In another retrospective study of 42 cardiac myxoma patients performed by Lee et al., 21 (50%) of the patients were discovered to have artery feeding to the myxoma. The feeding arteries in this study included right coronary artery (RCA) (57.1%), left circumflex coronary artery (LCx) (28.6%), and both (14.3%) (23). In addition, coronary angiography can reveal the distinctive morphology of the myxoma feeding artery, which can be helpful in surgical planning (24). Indeed cardiac myxomas having blood supply from the right coronary artery are not uncommon, since the blood perfusion of atrial septum depends upon the dominant right coronary artery pattern. However, we state that in this case, the myxoma has a unique feature of rich and extensive blood supply from the right coronary artery. First, coronary angiography showed a more dense distribution of vessels in the myxoma. LGE imaging also demonstrated obvious, patchy, and uneven delayed enhancement in the left atrial mass. In addition, sufficient blood supply of the myxoma leads to the coronary steal which may explain the paroxysmal chest tightness and palpitation symptoms of the patient.
Stiver et al. reported a case of a large intracardiac left atrial mass supplied by two anomalous coronary arteries causing coronary steal (25). Coronary steal, a rare phenomenon of unbalanced blood flow, is primarily seen in patients with prior coronary artery bypass grafting specifically in the left internal mammary artery (LIMA) to the left anterior descending artery (LAD) graft and simultaneously with significant subclavian artery stenosis. Very few cases reported that cardiac myxomas supplied by coronary arteries can cause coronary steal, even though no coronary lesions were observed in coronary angiography (25–27). First, patients may present with fatigue, weakness, exertional chest heaviness, or angina, and coronary steal explains the symptoms of cardiac ischemia. Second, it was reported that the mechanisms of coronary steal included highly vascularized myxoma (neovascularization), hemorrhage, and associated fistula formation in myxoma. Coronary angiography reveals the details of the blood supply. It is remarkable and verifiable that not only the total incision of the myxoma but also the ligation of the supplying branches helps solving the symptoms of patients (25–27).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81800255 and 82000306), the Nature Science Foundation of Shandong Province (ZR2020MH044, ZR2021MH112, and ZR2021QH016), and the Jinan Science and Technology Plan Project (202019165 and 202225050).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int. (2014) 111(12):205–11. doi: 10.3238/arztebl.2014.0205
2. Poterucha TJ, Kochav J, O’connor DS, Rosner GF. Cardiac tumors: clinical presentation, diagnosis, and management. Curr Treat Options Oncol. (2019) 20(8):66. doi: 10.1007/s11864-019-0662-1
3. Bussani R, Castrichini M, Restivo L, Fabris E, Porcari A, Ferro F, et al. Cardiac tumors: diagnosis, prognosis, and treatment. Curr Cardiol Rep. (2020) 22(12):169. doi: 10.1007/s11886-020-01420-z
4. Mylonas KS, Ziogas IA, Avgerinos DV. Microenvironment in cardiac tumor development: what lies beyond the event horizon? Adv Exp Med Biol. (2020) 1226:51–6. doi: 10.1007/978-3-030-36214-0_4
5. Uetani T, Inaba S, Nishiyama H, Yamaguchi O. Metastatic cardiac tumor-induced acute coronary syndrome. JACC Cardiovasc Interv. (2020) 13(20):e179–80. doi: 10.1016/j.jcin.2020.08.016
6. Alizade E, Çakır H, Açar G, Pirmammadova C. Giant left atrial myxoma with left and right coronary system blood supply accompanying mitral stenosis; real-time three- dimensional echocardiography imaging. Anadolu Kardiyol Derg. (2013) 13(5):E27–9. doi: 10.5152/akd.2013.163
7. Lee PT, Hong R, Pang PY, Chua YL, Ding ZP. Clinical presentation of cardiac myxoma in a Singapore national cardiac centre. Singapore Med J. (2021) 62(4):195–8. doi: 10.11622/smedj.2020041
8. Mahavar RK, Arora D, Singh A, Mishra M. Recurrent cardiac myxoma: a case report. Ann Card Anaesth. (2021) 24(4):490–2. doi: 10.4103/aca.ACA_7_20
9. Haji K, Nasis A. Radiological characteristics of atrial myxoma in cardiac computed tomography. J Cardiovasc Comput Tomogr. (2017) 11(3):234–6. doi: 10.1016/j.jcct.2017.02.002
10. Yokoyama S, Nagao K, Higashida A, Aoki M, Yamashita S, Fukuda N, et al. Diagnosis of carney complex following multiple recurrent cardiac myxomas. Gen Thorac Cardiovasc Surg. (2022) 70(1):87–91. doi: 10.1007/s11748-021-01719-w
11. Nasser SB, Doeblin P, Doltra A, Schnackenburg B, Wassilew K, Berger A, et al. Cardiac myxomas show elevated native T1, T2 relaxation time and ECV on parametric CMR. Front Cardiovasc Med. (2020) 7:602137. doi: 10.3389/fcvm.2020.602137
12. Gi WT, Sedaghat-Hamedani F, Shirvani Samani O, Kayvanpour E, Herpel E, Arif R, et al. Cardiac myxoma in a patient with hypertrophic cardiomyopathy. JACC Case Rep. (2020) 2(3):378–83. doi: 10.1016/j.jaccas.2019.11.072
13. Samanidis G, Khoury M, Balanika M, Perrea DN. Current challenges in the diagnosis and treatment of cardiac myxoma. Kardiol Pol. (2020) 78(4):269–77. doi: 10.33963/KP.15254
14. Wen Y, Ren S, Yan Q, Ma G. Surgical resection of primary cardiac cavernous hemangioma: a case report. Heart Surg Forum. (2022) 25(5):E753–5. doi: 10.1532/hsf.4969
15. Mcallister BJ. Multi modality imaging features of cardiac myxoma. J Cardiovasc Imaging. (2020) 28(4):235–43. doi: 10.4250/jcvi.2020.0027
16. Zhang M, Ding L, Liu Y, Xue L. Cardiac myxoma with glandular elements: a clinicopathological and immunohistochemical study of five new cases with an emphasis on differential diagnosis. Pathol Res Pract. (2014) 210(1):55–8. doi: 10.1016/j.prp.2013.09.018
17. Leduc C, Jenkins SM, Sukov WR, Rustin JG, Maleszewski JJ. Cardiac angiosarcoma: histopathologic, immunohistochemical, and cytogenetic analysis of 10 cases. Hum Pathol. (2017) 60:199–207. doi: 10.1016/j.humpath.2016.10.014
18. Donato G, Conforti F, Camastra C, Ammendola M, Donato A, Renzulli A. The role of mast cell tryptases in cardiac myxoma: histogenesis and development of a challenging tumor. Oncol Lett. (2014) 8(1):379–83. doi: 10.3892/ol.2014.2104
19. Kodama H, Hirotani T, Suzuki Y, Ogawa S, Yamazaki K. Cardiomyogenic differentiation in cardiac myxoma expressing lineage-specific transcription factors. Am J Pathol. (2002) 161(2):381–9. doi: 10.1016/S0002-9440(10)64193-4
20. Di Vito A, Mignogna C, Donato G. The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology. Histopathology. (2015) 66(3):321–32. doi: 10.1111/his.12531
21. Kleiman A, Keats EC, Chan NG, Khan ZA. Evolution of hemangioma endothelium. Exp Mol Pathol. (2012) 93(2):264–72. doi: 10.1016/j.yexmp.2012.04.020
22. Van Cleemput J, Daenen W, De Geest H. Coronary angiography in cardiac myxomas: findings in 19 consecutive cases and review of the literature. Cathet Cardiovasc Diagn. (1993) 29(3):217–20. doi: 10.1002/ccd.1810290308
23. Lee SY, Lee SH, Jung SM, Choi JH, Chon MK, Hwang KW, et al. Value of coronary angiography in the cardiac myxoma. Clin Anat. (2020) 33(6):833–8. doi: 10.1002/ca.23527
24. Omar HR. The value of coronary angiography in the work-up of atrial myxomas. Herz. (2015) 40(3):442–6. doi: 10.1007/s00059-013-3930-z
25. Stiver K, Bittenbender P, Whitson BA, Bush CA. Left atrial myxoma causing coronary steal: an atypical cause of angina. Tex Heart Inst J. (2015) 42(3):270–2. doi: 10.14503/THIJ-14-4220
26. Su Y, Song H. Hemorrhage and associated fistula formation in a left atrial myxoma causing coronary artery steal syndrome. Echocardiography. (2019) 36(6):1191–3. doi: 10.1111/echo.14351
Keywords: cardiac tumors, cardiac myxoma, cardiac surgery, coronary steal, cardiac pathology, coronary angiography
Citation: Zhang S, Wang J, Song F, Yang F, Li F, Liu S, Ma J, Zhang H and Ma X (2023) Case report: Left atrial myxoma with morphology of cavernous hemangioma supplied by the right coronary artery. Front. Cardiovasc. Med. 10:1207339. doi: 10.3389/fcvm.2023.1207339
Received: 21 April 2023; Accepted: 17 July 2023;
Published: 1 August 2023.
Edited by:
Enyi Shi, China Medical University, ChinaReviewed by:
Gaetano Thiene, University of Padua, ItalyXiaobing Liu, Shanghai Jiao Tong University, China
© 2023 Zhang, Wang, Song, Yang, Li, Liu, Ma, Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiwei Ma bWFqaXdlaTkwMDJAMTYzLmNvbQ== Haizhou Zhang emh6X2RvY3RvckAxNjMuY29t Xiaochun Ma bXhjbXhjMjAwOEAxNjMuY29t
†These authors have contributed equally to this work
 Shouji Zhang
Shouji Zhang Junlin Wang3,†
Junlin Wang3,† Fahang Song
Fahang Song Jiwei Ma
Jiwei Ma Xiaochun Ma
Xiaochun Ma