
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 15 June 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1196348
This article is part of the Research Topic Epigenetic and Genetic Mechanisms Underlying Cardiovascular Diseases and Neurodevelopmental Disorders View all 11 articles
Background: Serum miR-183-5p levels are associated with carotid atherosclerosis, while less is known about the relationship between circulating miR-183-5p levels and stable coronary artery disease (CAD).
Methods: In this cross-sectional study, consecutive patients with chest pain who underwent coronary angiograms from January 2022 to March 2022 at our center were enrolled. Those presenting acute coronary syndrome or had a prior CAD were excluded. Clinical presentations, laboratory parameters, and angiographic findings were collected. Serum miR-183-5p levels were measured using quantitative real-time polymerase chain reaction. CAD severity was displayed as the number of diseased vessels and further evaluated by the Gensini score system.
Results: Overall, 135 patients (median age, 62.0 years; male, 52.6%) were included in the present study. Stable CAD was identified in 85.2% of the study population, with 45.9% having 1-vessel disease, 21.5% having 2-vessel disease, and 17.8% having 3-vessel or left main disease. Serum miR-183-5p levels were significantly increased in CAD patients with different severities than non-CAD patients (all adjusted p < 0.05). Serum miR-183-5p levels increased as tertiles of the Gensini score progressed (all adjusted p < 0.05). Importantly, serum miR-183-5p levels could predict the presence of CAD and 3-vessel or left main disease in the receiver operating characteristic curve analysis (both p < 0.01), and also in multivariate analysis adjusting for age, sex, body mass index, diabetes, hypersensitive-C-reactive protein (both p < 0.05).
Conclusion: Serum miR-183-5p levels are independently and positively correlated with CAD presence and severity.
Coronary artery disease (CAD) remains a major cause of mortalities and morbidities worldwide (1). Approximately 11% of adults ≥ 45 years and 17% of adults ≥ 65 years are probably to have CAD, and around 800,000 suffer a myocardial infarction (MI) every year in the U.S. (2). In 2018, CAD mortality was 365,744 and MI mortality was 108,610 in the U.S. (2). These lead to a significant healthcare burden, expected to increase to >$177 billion by 2040 in the U.S. (3). Importantly, in-hospital mortality did not improve in patients with ST-segment elevation MI (STEMI) undergoing percutaneous coronary intervention (PCI) (4). Similarly, overall mortality of acute MI continued to increase since 2002 in both the urban and rural area of China. Given the increasing prevalence of CAD and its risk factors (e.g., advanced age, obesity), there is an unmet need to discover an optimal biomarker predicting the presence and severity of CAD.
MicroRNA (miRNA or miR) has been known to regulate gene expression at the post-transcriptional level (5). More than half of human protein-coding genes are estimated to be modulated by miRNA, given one miRNA can regulate the expression of several transcripts (6). Moreover, miRNA can influence cell proliferation, differentiation, and death in the circulatory system (7). Meanwhile, several circulating miRNAs have shown promising for early detection, severity evaluation, and outcome prediction of CAD (8).
miR-183-5p is already known as an oncomir, highly expressed in tumor tissues (9–11). Recently publications revealed that patients with carotid atherosclerosis had higher serum miR-183-5p levels compared with health individuals (12, 13). Meanwhile, elevated expression of circulating miR-183-5p was also detected in both patients with acute coronary syndrome (ACS) and non-ST-segment elevation MI (NSTEMI) (14, 15). Thus, circulating miR-183-5p could potentially be a promising biomarker for atherosclerotic and/or thrombotic disease. However, serum miR-183-5p levels have never been investigated in patients with stable CAD, the most common category of CAD. To fill this gap, we performed this cross-sectional study to examine the relationship between serum miR-183-5p levels and the presence of CAD, as well as the severity of CAD.
We prospectively enrolled consecutive patients with chest pain who underwent invasive coronary angiograms to determine the presence of stable CAD from January 2022 to March 2022 at Beijing Renhe Hospital, Beijing, China. Patients were excluded from the present study if they had: (1) age < 18 or ≥ 80 years old; (2) history of established arteriosclerotic cardiovascular disease (ASCVD) or vascular revascularization; (3) ACS at this admission; (4) major organ failure (e.g., heart, liver, kidney); (5) active infectious disease, autoimmune diseases, and malignancy.
Fasting venous blood was collected for measuring plasma total triglyceride, total cholesterol, low-density lipoprotein cholesterol (LDL-C), glycosylated hemoglobin, creatinine, and hypersensitive-C-reactive protein (hs-CRP). The blood serum was isolated and stored at −80°C. We also collected the patient's clinical characteristics and risk factors for CAD. Body mass index (BMI) was calculated as weight divided by the square height. Echocardiography was used to evaluate cardiac function and structure using a GE ViVid E7 ultrasonography (GE Healthcare, USA). This study was approved by the Ethics Committee of Beijing Renhe Hospital (RH20220103), and performed following the Declaration of Helsinki. Written informed consent was obtained from all participants.
Total RNA was extracted from the preserved serum samples using Trizol reagent (Invitrogen, USA). RNA (0.5 μg) was reverse transcribed with PrimeScript RT Reagent Kit (Takara, Japan). Then, quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the CFX Real-Time PCR Detection System (Bio-Rad, USA). The thermocycling amplification protocol was as follows: denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and 30 s at 60°C. Expression of miR-183-5p was calculated based on the 2−ΔΔCt method. The primer sequences were as follows: miR-183-5p, forward 5′-CGCGGTATGGCACTGGTAGA-3′, reverse 5′-AGTGCAGGGTCCGAGGTATTC-3′; U6 (internal control), forward 5′-CTCGCTTCGGCAGCACAT-3′, reverse 5′-TTTGCGTGTCATCCTTGCG-3′.
Invasive coronary procedures and periprocedural management were performed by international guidelines (16). Briefly, diagnostic coronary angiography was performed by experienced interventionists, who were blind to the patient's serum miR-183-5p levels, using the transradial or transfemoral approach. The location and severity of each coronary artery stenosis were assessed by two independent interventionists, and discrepancies were solved with discussion.
CAD was defined as any major epicardial coronary stenosis ≥ 50%. Thus, patients were divided according to the number of diseased vessels: 0-vessel disease (i.e., non-CAD), 1-vessel disease, 2-vessel disease, 3-vessel or left main (LM) disease. In addition, we quantified the severity of CAD using the Gensini score system (17). Thus, in the current study, CAD severity was presented using either the different numbers of diseased vessels or the tertiles of Gensini score. Finally, the decision of coronary intervention was made at the discretion of the interventionist.
Continuous variables were shown as median (interquartile range), and compared using the Kruskal-Wallis H test. The Bonferroni correction was used to adjust the p value in multiple-comparison analysis. Categorical variables were expressed as numbers (percentages), and compared using the Chi-square test or Fisher's exact test. To investigate the relationship between serum miR-183-5p levels and CAD severity, we firstly compared serum miR-183-5p levels among a varied number of diseased vessels and tertiles of Gensini score (low tertile, <15.3; middle tertile, 15.3-30.0; high tertile, >30.0). Then, this relationship was examined using Spearman's correlation analysis, shown as r and its 95% confidence interval (CI). Meanwhile, we also determined different CAD severity and extent across the tertile of serum miR-183-5p levels. The receiver operating characteristic (ROC) curve was performed to explore the diagnostic value of the serum miR-183-5p level for the presence and severity of CAD. The area under the ROC curve (AUC) with its 95% CI was used to measure the predictive ability of miR-183-5p. The Youden index was used to determine the optimal diagnostic threshold of miR-183-5p, and its corresponding sensitivity and specificity. Finally, the predictive value of miR-183-5p on the presence and severity of CAD was investigated in a multivariate model using logistical regression analysis, in which co-variables included age, sex, BMI, and those with a p -value < 0.1 in the univariate analysis. A two-sided p-value < 0.05 was considered statistically significant. All statistical analyzes were conducted using SPSS 20.0 software (IBM, Armonk, New York).
Overall, 135 patients (median age, 62.0 years; male, 52.6%) were included in the present study, who underwent invasive coronary artery angiogram to determine whether CAD was the origin of chest pain (Table 1). Hypertension (71.9%), hyperlipidemia (54.1%) and, current smoking (45.2%) were common risk factors for CAD. Notably, baseline total cholesterol (median, 5.1 mmol/L) and LDL-C (median, 3.2 mmol/L) levels were relatively high (Table 1), resulting in 92.6% of patients prescribed statins (Table 2). In addition, left ventricular systolic function (median left ventricular ejection fraction, 66.0%) and structure (median left ventricular end-diastolic diameter, 46.0 mm) were preserved (Table 1). Importantly, baseline characteristics were comparable across different CAD severities (p > 0.05), except for hs-CRP levels (p = 0.005). In particular, higher hs-CRP levels were observed in 3-vessel or LM disease (adjusted p < 0.05) and 2-vessel disease subgroups (adjusted p < 0.05), compared to non-CAD patients (Table 1).
After coronary angiography, CAD was identified in 115 patients (85.2%), with 45.9% of patients having 1-vessel disease, 21.5% of 2-vessel disease, and 17.8% of 3-vessel or LM disease (Table 1). These findings were consistent with an increased overall Gensini score (median, 20.0) (Table 2). Coronary artery stenosis was most likely to present in the left anterior descending artery (78.3%), followed by the right coronary artery (44.3%) and left circumflex artery (43.5%). Three patients (2.6%) had LM disease, which represented a severe type of CAD. Therefore, PCI was performed in 85.2% of CAD patients, with 75.7% having stent implantation (Table 2).
Firstly, we found that serum miR-183-5p levels in any CAD subgroup were significantly increased than non-CAD patients (all adjusted p < 0.05) (Figure 1A). Among CAD subgroups, significantly increased miR-183-5p levels were observed in 3-vessel or LM than 1-vessel disease [3.75 [interquartile range (IQR): 2.45–4.90] vs. 2.25 (IQR: 1.45–3.20), adjusted p = 0.002] (Figure 1A). Moreover, a significant step-wise increased pattern between the Gensini score tertiles and miR-183-5p was showed [middle vs. low tertile: 2.10 (IQR: 1.60–2.80) vs. 1.20 (IQR: 0.80–2.55), adjusted p = 0.044], [high vs. middle tertile: 3.50 (IQR: 2.60–5.50) vs. 2.10 (IQR: 1.60–2.80), adjusted p < 0.01], [high vs. low tertile: 3.50 (IQR: 2.60–5.50) vs. 1.20 (IQR: 0.80–2.55), adjusted p < 0.01] (Figure 1B).
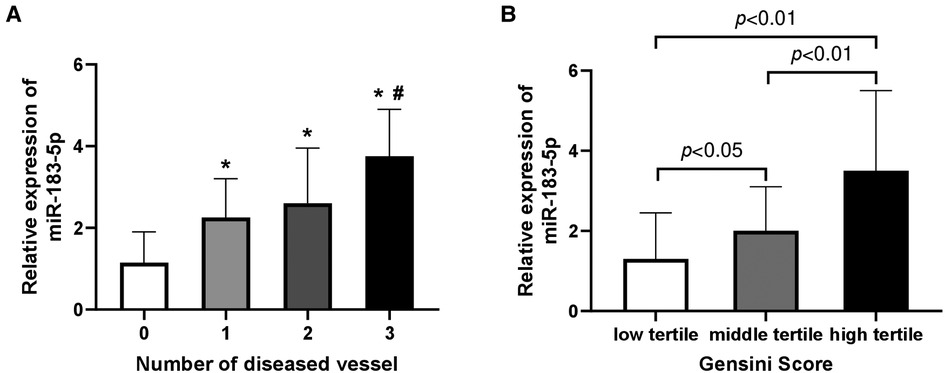
Figure 1. Relative expression of serum miR-183-5p levels according to number of diseased vessel (A) and gensini score tertiles (B). (A) *indicated Bonferroni adjusted p < 0.05 when compared to 0-vessel disease; #indicated Bonferroni adjusted p < 0.01 when compared to 1-vessel disease. (B) all p-values adjusted by Bonferroni correction.
Firstly, we found that, across tertiles of serum miR-183-5p levels, the proportion of multiple vessel disease increased, while single or none vessel disease decreased (all p < 0.05) (Figure 2A). Similarly, a significant step-wise increased pattern between miR-183-5p tertiles and the Gensini score was observed [middle vs. low tertile: 20.0 (IQR: 15.0–34.5) vs. 12.0 (IQR: 7.0–20.0), adjusted p = 0.003] [high vs. middle tertile: 36.0 (IQR: 25.0–52.0) vs. 20.0 (IQR: 15.0–34.5), adjusted p = 0.007] [high vs. middle tertile: 36.0 (IQR: 25.0–52.0) vs. 12.0 (IQR: 7.0–20.0), adjusted p < 0.01] (Figure 2B). Moreover, serum miR-183-5p levels were positively correlated with the Gensini score (r = 0.65, 95% CI: 0.54–0.74, p < 0.01) (Figure 3A) and hs-CRP (r = 0.63, 95% CI: 0.51–0.73, p < 0.01) (Figure 3B).
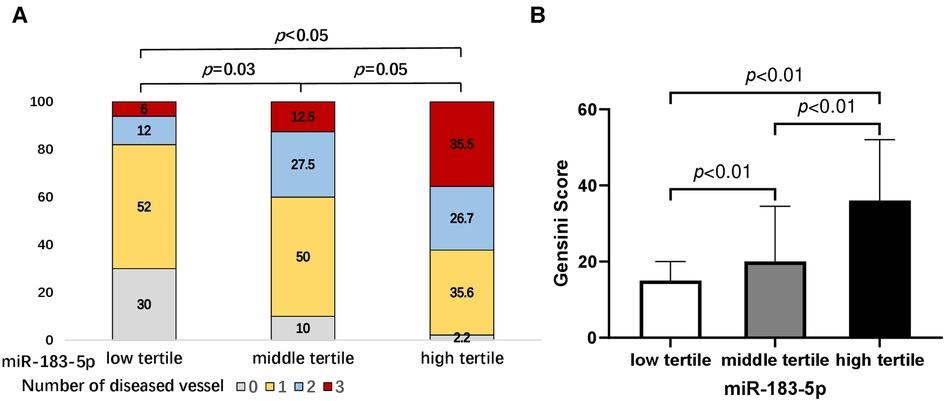
Figure 2. Proportions of number of diseased vessel (A) and gensini score (B) according to tertiles of serum miR-183-5p levels. All p-values adjusted by Bonferroni correction.
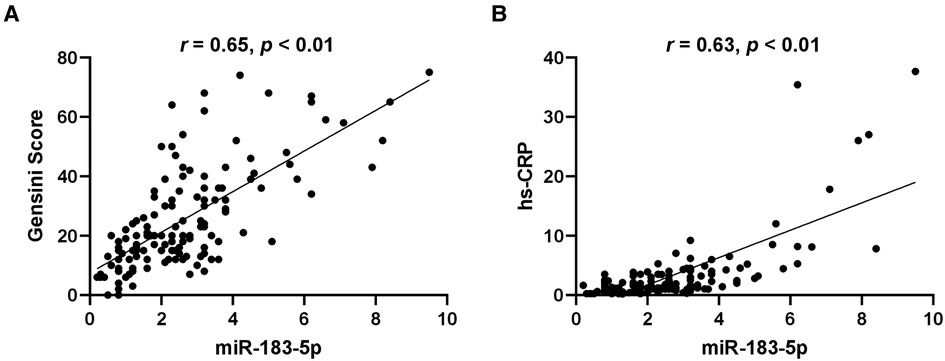
Figure 3. Correlation of serum miR-183-5p levels with gensini score (A) and hypersensitive-C-reactive protein (hs-CRP) (B).
The predictive values of serum miR-183-5p levels on the presence of CAD (AUC 0.82, 95% CI 0.71–0.92, p < 0.01) and 3-vessel or LM disease (AUC 0.76, 95% CI 0.65–0.86, p < 0.01) were confirmed in the ROC curve analysis, respectively (Figure 4A). The optimal cut-off values of miR-183-5p predicting CAD presence and severity were 1.40 (sensitivity 82.6%, specificity 70.0%) and 3.65 (sensitivity 54.2%, specificity 88.3%), respectively (Figure 4B).
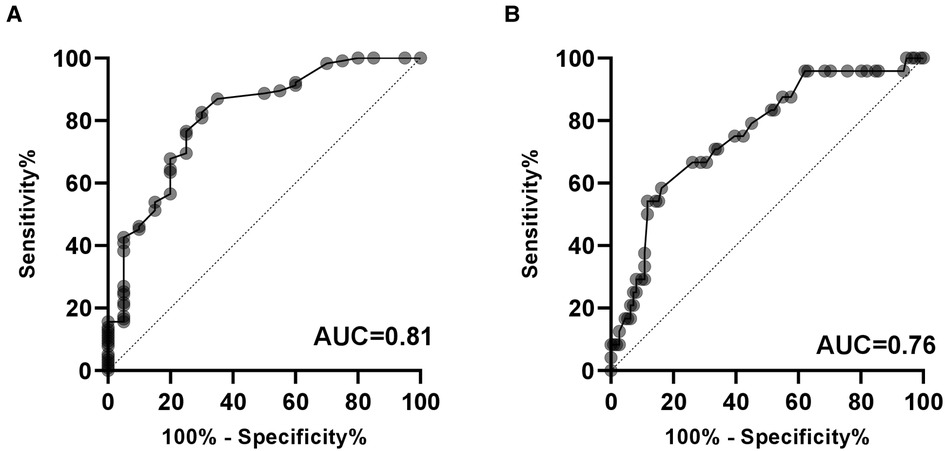
Figure 4. Diagnostic value of serum miR-183-5p levels on the presence of CAD (A) and severe CAD (i.e., 3-vessel or LM disease). (B) AUC, area under the receiver operating characteristic curve; CAD, coronary artery disease.
Furthermore, in multivariate analysis adjusting for age, sex, BMI, diabetes, hs-CRP, tertile of serum miR-183-5p levels showed increased predictive value on the presence of CAD (middle vs. low tertile, HR 3.81, 95% CI 1.07–13.56, p = 0.039; high vs. low tertile, HR 14.57, 95% CI 1.38–153.65, p = 0.026; p for trend = 0.024) and 3-vessel or LM disease (middle vs. low tertile, HR 2.11, 95% CI 0.46–9.70, p = 0.34; high vs. low tertile, HR 6.59, 95% CI 1.59–27.25, p = 0.009; p for trend = 0.020) (Table 3).
To the best of our knowledge, this is the first study investigating the relationship between serum miR-183-5p and stable CAD. The main findings of the present study included: (1) a significant step-wise increased pattern existed between the Gensini score tertiles and serum miR-183-5p levels; (2) serum miR-183-5p levels were positively correlated with the Gensini score and hs-CRP; (3) serum miR-183-5p levels could predict CAD presence and severity, with optimal cut-off values of 1.40 (sensitivity 82.6%, specificity 70.0%) and 3.65 (sensitivity 54.2%, specificity 88.3%), respectively; (4) the predictive value of serum miR-183-5p levels on CAD presence and severity were confirmed in multivariable analysis.
MiR-183 belongs to the miR-183 cluster, consisting of three microRNAs: miR-183, miR-96 and, miR-182. These homologous microRNAs are highly co-expressed in the murine retina, and their chromosomal loci are quite close (18). Increased miR-183 cluster members have been found in autoimmune diseases, neuronal and psychiatric disorders, and various malignancies (19). MiR-183 was identified in 2003 by cloning from the human Saos-2 cell line and mouse tissues (20). MiR-183-5p is known to overexpress in the peripheral blood mononuclear cells (PBMCs) (21) of breast cancer, in the tumor tissue of breast cancer (9), primary nasopharyngeal carcinoma (11), and hepatocellular carcinoma (10). In addition, elevated miR-183-5p is observed in PBMCs from patients with systemic lupus erythematosus (22), and in plasma-derived exosomes from patients with intestinal Behçet's syndrome (23).
The main underlying pathophysiology of CAD is atherosclerosis and/or thrombosis, which is a chronic inflammatory disease. Recently, Meerson et al. found that, compared to healthy individuals, plasma miR-183-5p levels were significantly increased in women with early diabetes (an important risk factor of atherosclerosis) (24). Meanwhile, overexpression of miR-183-5p was observed in the serum of patients with carotid atherosclerosis (12, 13), and positively correlated with carotid intima-media thickness (13). Similarly, elevated miR-183 levels were detected in the plasma exosome from patients with MI than in healthy individuals, which positively correlated with the degree of myocardial injury (14). Recently, Liu found that exosomal miR-183-5p from epicardial adipose tissue of patients with CAD were increased compared with those without CAD (25). Interestingly, Tong et al. found that plasma miR-183-5p levels were novel diagnostic markers only for NSTEMI, but not for STEMI (15). These discrepancies probably resulted from different pathophysiology of these two CAD subtypes, but also different sensitivity and specificity of miR-183-5p detecting methods (i.e., RNA-seq and qPCR).
Circulating miR-183-5p might be a promising marker of carotid atherosclerosis and ACS. However, less is known about its level in stable CAD patients. To fill the gap, we performed this cross-sectional study to investigate the relationship between serum miR-183-5p levels and CAD presence and severity. We found that serum miR-183-5p levels were increased in CAD patients with different severities than in non-CAD control. Serum miR-183-5p levels were positively correlated with the Gensini score, and hs-CRP, respectively. Interestingly, in patients with carotid atherosclerosis, serum miR-183-5p levels were positively correlated with CRP (13) and ox-LDL (12), which are well-known causes of atherosclerosis. Importantly, serum miR-183-5p levels had a relatively high power to diagnose the presence of CAD (AUC 0.82) and 3-vessel or LM disease (AUC 0.76). The optimal cut-off values of miR-183-5p predicting CAD presence and severity were 1.40 and 3.65, respectively, which were higher than its cut-off point predicting the presence of carotid atherosclerosis (0.91) in the study of Sun et al. (13). Moreover, this predictive value of serum miR-183-5p level was independent of age, sex, BMI, diabetes, and hs-CRP, which are well-established risk factors of CAD. Therefore, circulating miR-183-5p level is a marker of all categories of CAD presence, which could be used for early diagnosis of CAD; Meanwhile, serum miR-183-5p level could dynamically reflect CAD severity; More importantly, miR-183-5p might be a therapeutic target after fully elucidating the underlying mechanism between miR-183-5p and atherosclerosis/thrombosis.
Although the detrimental effects of miR-183-5p on vasculature have been reported, its exact mechanism leading to atherosclerosis is not fully understood. Zhang et al. showed that down-regulation of miR-183-5p in ox-LDL-treated human umbilical vascular endothelial cells could attenuate cell injury and inflammation by upregulation of insulin receptor substrate 1 (26). In subarachnoid hemorrhage rats, bone marrow mesenchymal stem cell-derived extracellular vesicles could alleviate endothelial dysfunction by regulating the KLF3-AS1/miR-183-5p/TCF7L2 signaling axis (27). In addition, Sun et al. found that overexpression of miR-183-5p accelerated the proliferation and migration of vascular smooth muscle cells (VSMCs) (13). Similarly, Fan et al. found, in VSMCs treated with ox-LDL, miR-183-5p was overexpressed, which may down-regulate FOXO1, leading to proliferation/apoptosis imbalance in VSMCs (12). Importantly, miR-183-5p might intervene the initiation and development of atherosclerosis by impacting not only vascular endothelial and VSMCs, but macrophages. In bone marrow-derived macrophages (BMDMs) transfected with a miR-183 inhibitor, the foam-cell formation was reduced, and cholesterol efflux increased (28). Furthermore, in BMDMs subjected to ox-LDL, miR-183 knockdown decreased the M1/M2 ratio with attenuated NF-kB activation, via targeting NR4A2 (28). However, exosomal miR-183-5p derived from bone marrow mesenchymal stem cell could protect ischemia/reperfusion injury in cardiomyocytes by targeting FOXO1 (29) or voltage-dependent anion channel 1 (30). These findings illustrated that miR-183-5p could potentially have multiple effects on the cardiovascular system.
The present study has several limitations. (1) study population was restricted to those without ASCVD, which may limit the extrapolation of the study results to a broader population; (2) number of the study population was relatively small, precluding us from finding a statistical difference of miR-183-5p between CAD patients with different numbers of diseased vessels; (3) given the cross-sectional design, a causal relation between miR-183-5p and CAD cannot be determined; (4) although the multivariate analysis was performed, the residual confounders affecting the correlation between miR-183-5p and CAD cannot be excluded; (5) CAD severity was judged by experienced physician visually, which may be improved using intracoronary imaging and functional examination.
In the population with chest pain who have no history of established ASCVD, serum miR-183-5p levels are independently and positively correlated with stable CAD presence and severity. Further studies are needed to elucidate the physiopathologic mechanism between miR-183-5p and atherosclerosis, as well as the outcome predictive value of miR-183-5p.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Beijing Renhe Hospital (RH20220103). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
The study was designed by GL. The clinical samples and information were collected by XL. The data were analyzed by DL. The experiments were performed by YG. The original manuscript was drafted by DL with comments by LZ. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70(1):1–25. doi: 10.1016/j.jacc.2017.04.052
2. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. (2021) 143(8):e254–743. doi: 10.1161/CIR.0000000000000950
3. Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. (2011) 124(9):827–33.e5. doi: 10.1016/j.amjmed.2011.04.010
4. Sugiyama T, Hasegawa K, Kobayashi Y, Takahashi O, Fukui T, Tsugawa Y. Differential time trends of outcomes and costs of care for acute myocardial infarction hospitalizations by ST elevation and type of intervention in the United States, 2001–2011. J Am Heart Assoc. (2015) 4(3):e001445. doi: 10.1161/JAHA.114.001445
5. Pasquinelli AE. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet. (2012) 13(4):271–82. doi: 10.1038/nrg3162
6. Bajan S, Hutvagner G. Regulation of miRNA processing and miRNA mediated gene repression in cancer. MicroRNA. (2014) 3(1):10–7. doi: 10.2174/2211536602666140110234046
7. Landskroner-Eiger S, Moneke I, Sessa WC. miRNAs as modulators of angiogenesis. Cold Spring Harbor Perspect Med. (2013) 3(2):a006643. doi: 10.1101/cshperspect.a006643
8. Melak T, Baynes HW. Circulating microRNAs as possible biomarkers for coronary artery disease: a narrative review. Ejifcc. (2019) 30(2):179–94.31263392
9. Cheng Y, Xiang G, Meng Y, Dong R. MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in human breast cancer by targeting the PDCD4. Reprod Biol. (2016) 16(3):225–33. doi: 10.1016/j.repbio.2016.07.002
10. Wojcicka A, Swierniak M, Kornasiewicz O, Gierlikowski W, Maciag M, Kolanowska M, et al. Next generation sequencing reveals microRNA isoforms in liver cirrhosis and hepatocellular carcinoma. Int J Biochem Cell Biol. (2014) 53:208–17. doi: 10.1016/j.biocel.2014.05.020
11. Tang JF, Yu ZH, Liu T, Lin ZY, Wang YH, Yang LW, et al. Five miRNAs as novel diagnostic biomarker candidates for primary nasopharyngeal carcinoma. Asian Pac J Cancer Prev. (2014) 15(18):7575–81. doi: 10.7314/APJCP.2014.15.18.7575
12. Fan M, Huang Y, Li K, Yang X, Bai J, Si Q, et al. ox-LDL regulates proliferation and apoptosis in VSMCs by controlling the miR-183-5p/FOXO1. Genes Genomics. (2022) 44(6):671–81. doi: 10.1007/s13258-022-01236-x
13. Sun B, Shan Z, Sun G, Wang X. Micro-RNA-183-5p acts as a potential diagnostic biomarker for atherosclerosis and regulates the growth of vascular smooth muscle cell. J Chin Med Assoc. (2021) 84(1):33–7. doi: 10.1097/JCMA.0000000000000433
14. Zhao X, Jia Y, Chen H, Yao H, Guo W. Plasma-derived exosomal miR-183 associates with protein kinase activity and may serve as a novel predictive biomarker of myocardial ischemic injury. Exp Ther Med. (2019) 18(1):179–87. doi: 10.3892/etm.2019.7555
15. Tong KL, Mahmood Zuhdi AS, Wan Ahmad WA, Vanhoutte PM, de Magalhaes JP, Mustafa MR, et al. Circulating MicroRNAs in young patients with acute coronary syndrome. Int J Mol Sci. (2018) 19(5):1467. doi: 10.3390/ijms19051467
16. Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS) developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J. (2014) 35(37):2541–619. doi: 10.1093/eurheartj/ehu278
17. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51(3):606. doi: 10.1016/S0002-9149(83)80105-2
18. Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science. (2003) 299(5612):1540. doi: 10.1126/science.1080372
19. Dambal S, Shah M, Mihelich B, Nonn L. The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res. (2015) 43(15):7173–88. doi: 10.1093/nar/gkv703
20. Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. RNA. (2003) 9(2):175–9. doi: 10.1261/rna.2146903
21. Chang CW, Wu HC, Terry MB, Santella RM. microRNA expression in prospectively collected blood as a potential biomarker of breast cancer risk in the BCFR. Anticancer Res. (2015) 35(7):3969–77.26124344
22. Zhou S, Zhang J, Luan P, Ma Z, Dang J, Zhu H, et al. miR-183-5p is a potential molecular marker of systemic lupus erythematosus. J Immunol Res. (2021) 2021:5547635. doi: 10.1155/2021/5547635
23. Hou CC, Bao HF, Shen Y, Ye JF, Ma HF, Guan JL. Expression of miRNAs derived from plasma exosomes in patients with intestinal Behçet's syndrome. Clin Exp Rheumatol. (2022) 40(8):1480–90. doi: 10.55563/clinexprheumatol/6xgxzk
24. Meerson A, Najjar A, Saad E, Sbeit W, Barhoum M, Assy N. Sex differences in plasma MicroRNA biomarkers of early and complicated diabetes mellitus in Israeli arab and Jewish patients. Noncoding RNA. (2019) 5(2):32. doi: 10.3390/ncrna5020032
25. Liu J, Gao A, Liu Y, Sun Y, Zhang D, Lin X, et al. MicroRNA expression profiles of epicardial adipose tissue-derived exosomes in patients with coronary atherosclerosis. Rev Cardiovasc Med. 23(6):206. doi: 10.31083/j.rcm2306206
26. Zhang Y, Zhan Y, Liu D, Yu B. Inhibition of microRNA-183 expression resists human umbilical vascular endothelial cells injury by upregulating expression of IRS1. Drug Deliv. (2019) 26(1):612–21. doi: 10.1080/10717544.2019.1628117
27. Cheng M, Liu L, Zhang T, Chen Y, Wang Q, Wu Y. Extracellular vesicles derived from bone marrow mesenchymal stem cells alleviate neurological deficit and endothelial cell dysfunction after subarachnoid hemorrhage via the KLF3-AS1/miR-83-5p/TCF7L2 axis. Exp Neurol. (2022) 356:114151. doi: 10.1016/j.expneurol.2022.114151
28. Gong FH, Long L, Yang YS, Shen DH, Zhang YS, Wang XS, et al. Attenuated macrophage activation mediated by microRNA-183 knockdown through targeting NR4A2. Exp Ther Med. (2021) 21(4):300. doi: 10.3892/etm.2021.9731
29. Mao S, Zhao J, Zhang ZJ, Zhao Q. MiR-183-5p overexpression in bone mesenchymal stem cell-derived exosomes protects against myocardial ischemia/reperfusion injury by targeting FOXO1. Immunobiology. (2022) 227(3):152204. doi: 10.1016/j.imbio.2022.152204
Keywords: miR-183-5p, coronary artery disease, severity, Gensini score, association
Citation: Lv D, Guo Y, Zhang L, Li X and Li G (2023) Circulating miR-183-5p levels are positively associated with the presence and severity of coronary artery disease. Front. Cardiovasc. Med. 10:1196348. doi: 10.3389/fcvm.2023.1196348
Received: 29 March 2023; Accepted: 10 May 2023;
Published: 15 June 2023.
Edited by:
Hongsong Zhang, Nanjing Medical University, ChinaReviewed by:
Yu Du, Capital Medical University, China© 2023 Lv, Guo, Zhang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangping Li dGljX3RqY2FyZGlvbEAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.