- Department of Cardiology, Chonnam National University Medical School/Hospital, Gwangju, Republic of Korea
Background and objectives: To investigate the clinical relevance of the timing of heart failure (HF) development on long-term outcome in patients with acute myocardial infarction (AMI).
Materials and methods: A total of 1,925 consecutive AMI patients were divided into 4 groups according to the timing of HF development; HF at admission (group I, n = 627), de novo HF during hospitalization (group II, n = 162), de novo HF after discharge (group III, n = 98), no HF (group IV, n = 1,038). Major adverse cardiac events (MACE) defined as the development of death, re-hospitalization, recurrent MI or revascularization were evaluated.
Results: HF was developed in 887 patients (46.1%) after an index AMI. HF was most common at the time of admission for AMI, but the development of de novo HF during hospitalization or after discharge was not uncommon. MACE was developed in 619 out of 1,925 AMI patients (31.7%). MACE was highest in group I, lowest in group IV, and significantly different among groups; 275 out of 627 patients (43.9%) in group I, 64 out of 192 patients (39.5%) in group II, 36 out of 98 patients (36.7%) in group III, and 235 out of 1,038 patients (22.6%) in group IV (P < 0.001). MACE free survival rates at 3 years were 56% in group I, 62% in group II, 64% in group III, and 77% in group IV (P < 0.001).
Conclusions: HF was not uncommon and can develop at any time after an index AMI, and the development of HF was associated with poor prognosis. The earlier the HF has occurred after AMI, the poorer the clinical outcome was. To initiate the guideline directed optimal medical therapy, therefore, the development of HF should be carefully monitored even after the discharge from an index AMI.
Introduction
Heart failure (HF) is a major public health problem with over 37.7 million cases reported worldwide, and its prevalence is increasing rapidly (1). The most common cause of HF is ischemic heart disease including acute myocardial infarction (AMI), which is also growing constantly. With the advances in both optimal revascularization and medical therapy, the survival rate after AMI have improved, on the other hand, the incidence of HF associated with MI also has been increased (2, 3). The development of HF after an index MI is known to be associated with poor clinical outcomes (4, 5).
After an index AMI, HF may develop at the time of hospitalization in association with the degree of myocardial injury with a varying incidence of 14%–36% (2). As a maladaptive process for myocardial injury, so called adverse left ventricular (LV) remodeling, HF may also develop at any time of post-discharge period (6). Conflicting evidences on the incidence and temporal trend of HF after MI arises from the different definition of HF and the timing and population of HF differ among the previous reports (2, 7–9). Regardless of the type of infarct-related artery (IRA), the size of the infarcted or ischemic myocardium supplied by the IRA is a critical determinant for favorable clinical outcomes including HF prevention. In patients with chronic coronary artery diseases, routine invasive therapy for the lesions with significant myocardial ischemia failed to reduce the overall cardiovascular mortality compared to optimal medical treatment in the recent ISCHEMIA trial. However, the rapid restoration of IRA patency by percutaneous coronary intervention (PCI) and subsequent optimal medical therapy would be essential to prevent or minimize the risk of progression to HF through myocardial damage in patients with AMI. Therefore, it is important to understand the natural course of HF development or progression after MI to introduce optimal management, thereby to provide better long-term prognosis.
Contrary to the HF at the time of hospitalization for AMI, the risk of development or clinical course of de novo HF during post-discharge period for AMI has been poorly studied. Therefore, we investigated the post-MI clinical course for HF development and the impacts of the clinical relevance of HF development on long-term prognosis in patients with AMI.
Materials and methods
Study subjects and design
This is a single center retrospective and observational study, and the study protocol was approved by the institutional review board (IRB) of Chonnam National University Hospital (IRB file No. CNUH-2011-172).
We evaluated patients with clinically diagnosed for AMI at Chonnam National University Hospital (Gwangju, Korea) between November 2011 and June 2015. The diagnosis of MI was based on the criteria for a universal definition of MI: (1) when there was a rise and/or fall in cardiac biomarker values (troponin I/T or creatine kinase-MB with at least one value above the 99th percentile upper reference limit) and (2) with at least one of the following (a) symptoms of myocardial ischemia, (b) changes on the electrocardiogram (ECG) including new or presumed new significant ST-segment-T wave changes, new left bundle branch block, or pathologic Q waves in 2 contiguous leads, and (c) imaging evidence of new loss of viable myocardium or a new regional wall motion abnormality (10). Exclusion criteria included subjects with complex structural or congenital heart disease, and any clinical instability or life-threatening disease.
The diagnosis of HF was based on the following conditions, which were predominantly established from the European Society of Cardiology (ESC) guideline for AMI-associated HF: (1) cardinal manifestations of HF (such as dyspnea or fatigue), (2) rales (Killip class II or higher), (3) pulmonary edema on chest x-ray, (4) elevated level of N-terminal prohormone of brain natriuretic peptide (NT-proBNP) (11).
Patients were categorized into 4 groups according to their development of HF and their onset time of HF after an index AMI: (1) HF development at the time of admission (group I), (2) de novo HF during hospitalization (group II), (3) de novo HF after discharge (group III), and (4) no HF development during follow-up (group IV).
Data collection
Baseline clinical, angiographic, and echocardiographic data were obtained retrospectively from medical record. Demographic and clinical data included age at diagnosis, sex, associated cardiovascular risk factors such as presence of diabetes mellitus, hypertension or smoking history, and initial presentations. Pulmonary edema on initial chest x-ray were used in the assessment of Killip class (12). Coronary angiography (CAG) and percutaneous coronary intervention (PCI) were performed according to the standard protocol (13). Decision of revascularization was made by the agreement of attending physician and interventional cardiologist. Findings of CAG were analyzed based on the ACC/AHA (American College of Cardiology/American Heart Association) classification system (14). All echocardiographic parameters including left ventricular ejection fraction (LVEF) and chamber sizes were measured according to the current recommendations for cardiac chamber quantification of American Society of Echocardiography (15, 16).
Primary endpoint
The primary outcome of this study was a composite of major adverse cardiac events (MACE) defined as all-cause death, re-hospitalization, recurrent MI, or any revascularization.
Statistical analysis
Data were analyzed using SPSS statistical software (version 25.0 for windows, SPSS, Inc., Chicago, IL). Categorical variables were presented as frequencies and percentages. The chi-square test or Fisher’s exact test was performed appropriately to test the difference of categorical variables among groups. In order to determine any statistical difference of continuous variables among groups, analysis of variances (ANOVA) or Kruskal-Wallis test was performed. A post hoc Bonferroni test for multiple comparisons was applied in order to further determine any significant differences between means among the individual groups, if any statistical differences were observed from the ANOVA or Kruskal-Wallis test. Continuous variables were presented as mean ± standard deviations. The probability of freedom from MACE of each group and survival rate were estimated by the Kaplan-Meier method and log-rank test. P-values <0.05 were considered as significant. Multivariable Cox proportional hazard regression analysis was performed for the adjustment of baseline characteristics.
Results
Baseline clinical characteristics
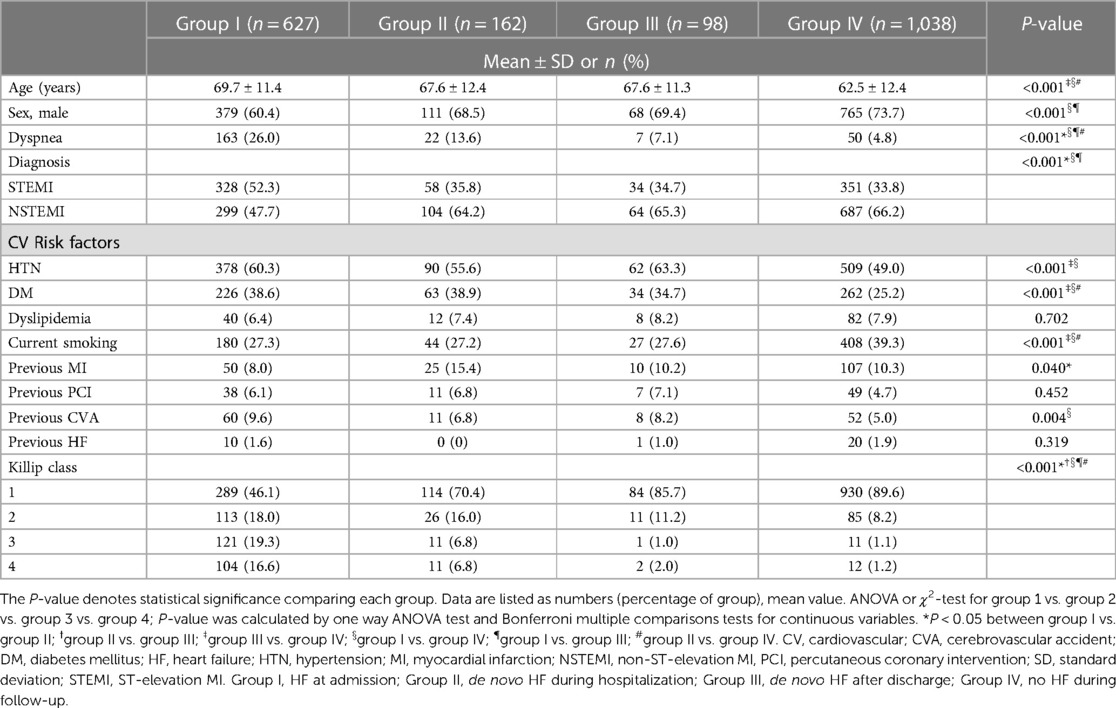
From November 2011 to June 2015, a total of 1,925 consecutive patients with AMI were included in this study (1,323 males, 65.7 ± 12.5 years), and HF was developed in 887 patients (46.1%) after an index AMI. HF was noted at the time of admission in 627 patients (group I: 32.6%), and de novo HF during hospitalization was developed in 162 patients (group II: 8.4%). Among 1,136 AMI patients who had no HF development during hospitalization, post-discharge de novo HF was developed in additional 98 patients (group III: 5.1%). HF was not developed in the remaining 1,038 AMI patients (group IV: 53.9%).
The clinical characteristics among the groups were statistically different in most variables, and the differences between group I and group IV were statistically significant, whereas the differences between group II and group III were not statistically significant in most variables in the post-hoc analysis.
Majority of clinical indicators, except the conventional cardiovascular risk factors, were most severe in group I and most favorable in group IV, and the values of group II and group III are allocated between the values in group I and group IV. Notably, the presence of past medical history of heart failure was not different among groups. The baseline characteristics of each group were described in detail in Table 1.
Laboratory, echocardiography, and CAG findings
The level of CK-MB (creatine kinase MB isoenzyme), troponin I and hsCRP (high sensitivity C-reactive protein) are greatest in group I and decreased in order of later onset of HF, and lowest in group IV.
Diminished LVEF at initial echocardiography were severe in group I and II and lesser severe in order of group III, and group IV (P < 0.001). In addition, there was a significant difference regarding the presence of multi-vessel disease on CAG, with the higher prevalence in group I, group II, and group III, while the lowest prevalence in group IV (P = 0.012). Laboratory, echocardiography, and CAG findings were summarized in Table 2.
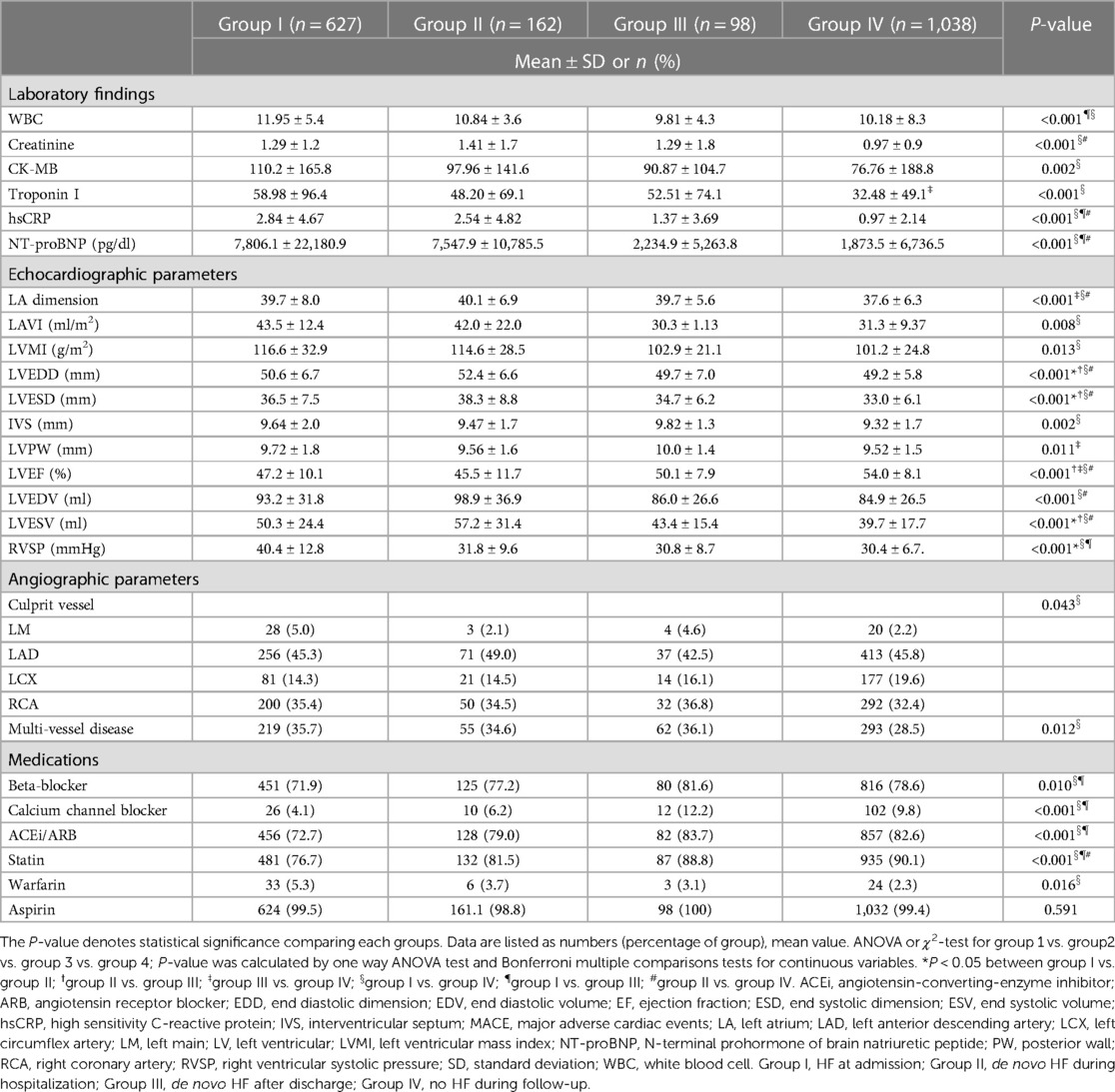
Table 2. Comparison of laboratory, echocardiography, angiography findings, and prescribed medications among groups.
Clinical outcomes
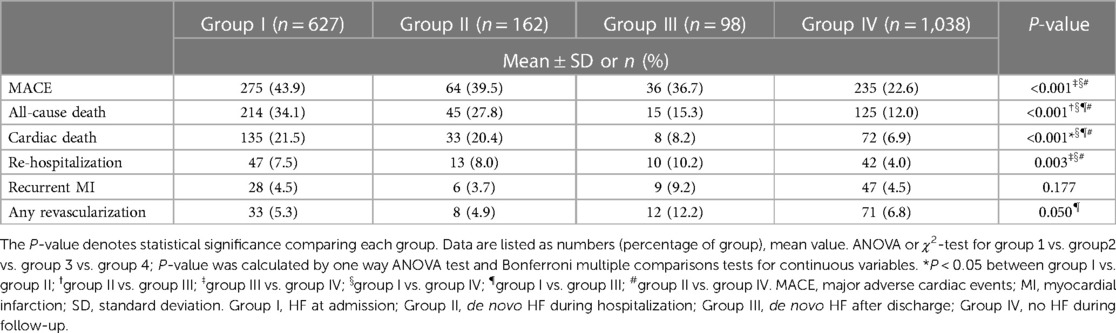
Clinical outcomes of the patients were summarized in Table 3. During the median 60 months (range, 0.03–93.4 months) of clinical follow-up, MACE occurred in 275 (43.9%) patients in group I, 64 (39.5%) patients in group II, 36 (36.7%) in group III, and 235 (22.6%) in group IV. MACE was significantly common in HF groups than in no HF group.
Overall MACE free survival was 80.8%, 73.7%, and 68.6% at 1, 2, and 3 years, respectively. Cumulative MACE free survival at 3 years were 56.2% in group I, 61.5% in group II, 63.7% in group III, and 77.5% in group IV. Cumulative MACE free survival was statistically lower in HF groups than in no HF group (P < 0.001) (Figure 1). However, no statistical difference in MACE free survival was demonstrated between group I and group II (P = 0.247), and between group II and group III (P = 0.375).
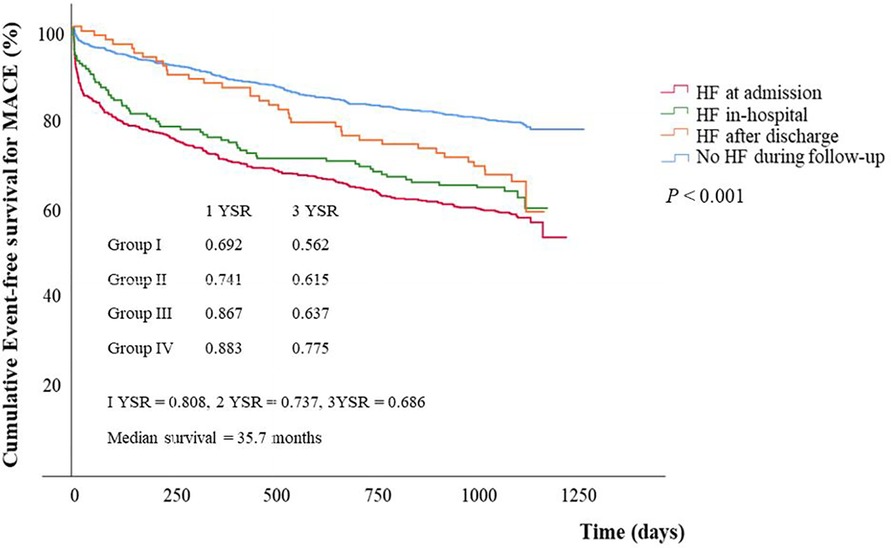
Figure 1. Event free survival curves for major adverse cardiac events stratified by groups. Event free survival for MACE was significantly different between group IV and any other groups (group I vs. group IV, P < 0.001; group II vs. group IV, P < 0.001; group III vs. group IV, P = 0.003) and between group III and group IV (P = 0.003), but it showed no significant difference between group I and group II (P = 0.247), and between group II and group III (P = 0.375). MACE, major adverse cardiac events; HF, heart failure; YSR, year survival rate.
All-cause mortality and cardiac death were occurred in 214 (34.1%) and 135 (21.5%) patients in group I, 45 (27.8%) and 33 (20.4%) in group II, 15 (15.3%) and 8 (8.2%) in group III, and 125 (12.0%) and 72 (6.9%) in group IV, with showing significant difference among groups (P < 0.001).
Overall cumulative survival for all-cause death and cardiac death were 86.0% and 90.1 at 1 year, 82.3% and 88.5% at 2 years, and 79.3% and 86.8% at 3 years of follow-up. Kaplan-Meier survival curves for all-cause mortality and cardiac death showed statistical differences among groups (P < 0.001, for all). However in the subgroup analysis, Kaplan-Meier survival curves for all-cause mortality demonstrated no statistical differences between group I and group II (P = 0.100) and between group III and group IV (P = 0.426) (Figure 2).
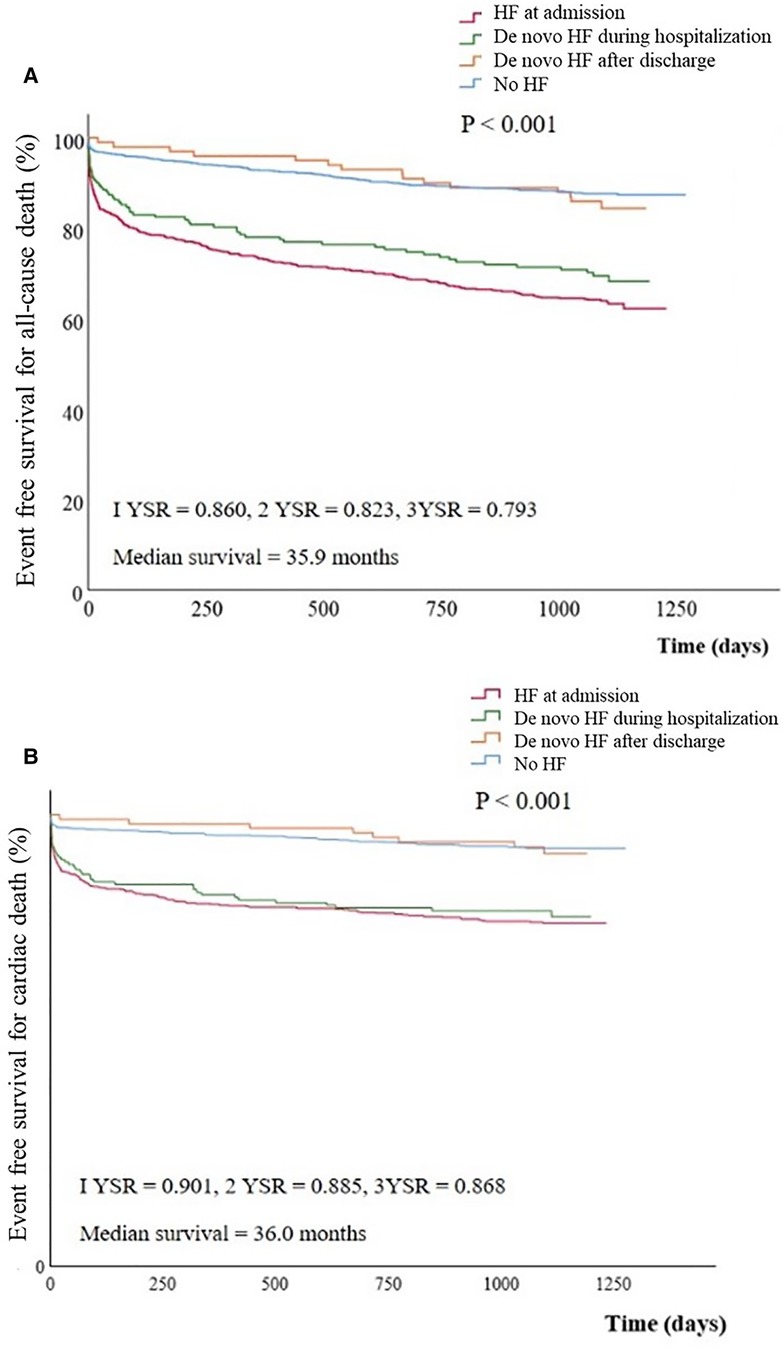
Figure 2. Event free survival curves for All-cause death (A) and cardiac death (B) stratified by groups. Cumulative all-cause (A) or cardiac death (B) free survival was significantly lower in group I or II than in group III or IV. (A) group I vs. group II, P = 0.100; group II vs. group III, P = 0.013; group III vs. group IV, P = 0.426; group I vs. group IV; P < 0.001 (B) group I vs. group II, P = 0.100; group II vs. group III, P = 0.007; group III vs. group IV, P = 0.713; group I vs. group IV, P < 0.001. HF, heart failure; YSR, year survival rate.
Predictors of MACE
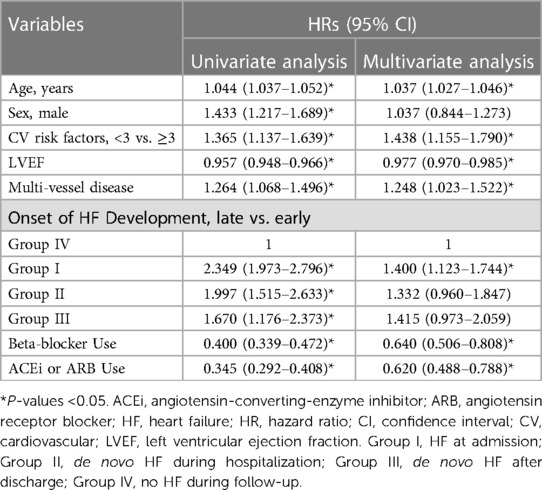
To identify independent predictors of MACE, multiple Cox regression analysis was performed, and the results were summarized in Table 4.
Older age (HR 1.037, 95% CI 1.027–1.046, P < 0.001), number of CV risk factors ≥3 (HR 1.438, 95% CI 1.155–1.790, P = 0.001), LVEF (HR 0.977, 95% CI 0.970–0.985, P < 0.001), multi-vessel disease (HR 1.248, 95% CI 1.023–1.522, P = 0.029), and earlier onset of HF development after index MI (P = 0.017), beta-blocker use (HR 0.640, 95% CI 0.506–0.808, P < 0.001), and ACEi or ARB use (HR 0.620, 95% CI 0.488–0.788, P < 0.001) were independent predictors for MACE.
A subgroup analysis on STEMI and NSTEMI
Among 771 STEMI patients, HF was developed in 328 patients at the time of hospitalization (group I, 42.5%), in 58 patients during hospitalization (group II, 7.5%), and in 34 patients after discharge (group III, 4.4%). HF was not developed in the remaining 351 STEMI patients (group IV: 45.5%). Whereas, HF was developed in 299 patients at the time of hospitalization (group I, 25.9%), in 104 patients during hospitalization (group II, 9.0%), and in 64 patients after discharge (group III, 5.5%) and HF was not developed in the remaining 687 patients (group IV: 59.5%) among a total of 1,154 NSTEMI patients. Kaplan-Meier survival curves for MACE showed significant difference among 4 groups in STEMI and NSTEMI patients (P < 0.001). However, in the results of z-test at 360, 720, and 1,080 days of STEMI patients, there were no statistical differences among the 4 groups at 1,080 days (P = 0.123). These findings are added in the Supplementary Figure S4. Multivariate analysis showed different results in several variables when STEMI and NSTEMI were analyzed separately. This finding is also added as a Supplementary Table S1.
Discussion
The present study investigated the timing of HF development and its relevance to clinical outcomes in patients with AMI and demonstrated several clinically important findings. First, HF was not uncommon and can develop at any time after an index AMI including post-discharge periods. Second, regardless of the timing, the development of HF in patients with AMI was significantly associated with poor clinical outcomes. Third, the pre-discharge development of HF (at admission or during hospitalization) was associated with poorer clinical outcomes including all-cause or cardiac death in patients with AMI as compared to those with the post-discharge or no HF development. Fourth, age, LV function, number of CV risk factors, multi-vessel disease, and earlier onset of HF development were significant predictors of MACE. To initiate optimal guideline directed medical therapy for HF and improve clinical outcomes, therefore, the development of acute de novo HF in AMI patients who had no HF at the time of admission should be carefully monitored not only in the initial hospitalized period, but also in post-discharge period, especially in AMI patients with older age, higher level of cardiac troponin, and lower LV EF (Supplementary File).
The current universal definition of myocardial infarction and heart failure were released in 2018 and 2021, respectively (10, 17). The current definition of myocardial infarction indicates the presence of acute myocardial injury detected by abnormal cardiac biomarkers in the setting of evidence of acute myocardial ischemia. The current universal definition represents HF as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion. In this study, we included patients enrolled before the release of the current guidelines, however there were no patients who were excluded from enrollment due to the changes of the definition.
HF can develop in any time after an index AMI, it occurred simultaneously with AMI in one-third of all cases, while it did not occur at all in a half of all cases in the present study. Among the baseline characteristics, it is demonstrated to be most favorable in the group IV and worst in the group I, in regards to the important clinical indicators such as older age, higher CV risk, diminished LVEF, multivessel disease. This means that patients with poor clinical indicators are more susceptible to HF development after MI. Meanwhile, previous history of HF was not significantly associated with the presence of HF or the onset of HF development after AMI.
There have been several studies reporting the outcomes and natural history of HF after AMI. A. Torabi et al. demonstrated that HF occurred in 62.7% of all subjects with MI, which was higher than our study results (18). In addition, almost one-third of patients who did not have HF at the time of discharge developed HF after discharge, which was also much higher than the result of our study. In the study, authors categorized HF groups according to early mortality, timing of onset, and persistence. Long-term prognosis of patients without HF at any time demonstrated most favorable, which is similar to our result. Another study demonstrated the increasing trend of HF development after MI over time; 5-year incidence of HF after MI was 27.6% in 1970s and rose up to 31.9% in 1990s (19). However in the recent study by Desta et al., the incidence of HF after MI declined from 46% to 28%, regardless of the onset of HF, between 1996 and 2008 (7). Limited and conflicting data may have contributed to the inconsistent results on the incidence and prognosis of HF after MI.
In the present study, event-free survival rates for MACE were significantly different according to the timing of HF occurrence. It is perceptible when considering that the baseline characteristics of groups were statistically different. Obviously, group I demonstrated the worst, and group IV demonstrated the most favorable prognosis. MACE is more likely to occur in patients who develop HF at least once, even after the discharge, compared to those who never develop HF during follow-up period. Unlikely, survival rates for all-cause mortality and cardiac death were favorable in patients who did not develop HF until discharge compared to those who developed during hospitalization. All-cause death and cardiac death were similar in patients who did not develop HF until discharge, whether HF developed or not after the index discharge. There were two different timing of delayed onset of HF after MI, in-hospitalization (group II) vs. after discharge (group III). Critical risk factors of HF including presence of pulmonary edema, higher Killip class, enlarged LV cavity size and diminished LV EF were significantly different between those two groups. The event-free survival for MACE was similar, and the overall survival for all-cause mortality and cardiac death were different between those two groups. This also supports that the earlier development of HF could be one of major risk factors of poor prognosis after MI. For better outcome, it is necessary to provide proper guideline-directed medical therapy for patients who have already experienced HF after MI, and to give close monitoring for patients who did not develop HF before their discharge as well.
There are several potential limitations in this study. First, the present study has inevitable limitations of retrospective study such as selection bias, inhomogeneity of the use of medications or percutaneous coronary intervention related parameters including reperfusion status, etc. These limitations of retrospective should be carefully considered in the interpretation of the results of this study. Second, number of group II and III were small and even unequal although relatively large number of study population, which could result in limited statistical power. Third, the diagnosis of HF was based on the symptoms and signs, which could be subjective and inaccurate indicators. However, criteria for the definition of HF also included chest x-ray and level of serum BNP to improve specificity. Fourth, the time interval of AMI onset to admission may differ for each patient, there remains a possibility of confounding bias on clinical outcomes. Further in this study, there is a possibility of immortal time bias on clinical outcomes of our study because of its retrospective design. Lastly in this study, not the timing of HF resolution but the timing of HF development was highlighted. It is likely that the temporal trends in HF after MI are associated with prognosis, not only with the development but also with its progression or improvement. In the future, the prognostic value of the temporal trends in HF after MI will be established more precisely in multicenter, prospective, larger population studies.
Conclusions
HF development after MI is a major cause of CV morbidity and mortality. The earlier development of HF after MI is one of the independent prognostic predictors in patients with MI. To provide better outcome, careful monitoring and the guideline-directed optimal medical therapy for HF should be provided earlier in patients with MI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HK and KK contributed to conception and design of the study. NL and HP organized the database. JC and HY performed the statistical analysis. HK wrote the first draft of the manuscript. YA, MJ and JGC wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was financially supported by Chonnam National University (grant number: 202233940001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1193973/full#supplementary-material
SUPPLEMENTARY FIGURE S1
Cumulative major adverse cardiac events free survival curves, No HF vs HF during hospitalization (P < 0.001).
SUPPLEMENTARY FIGURE S2
Cumulative major adverse cardiac events free survival curves, HF never vs HF ever (P < 0.001).
SUPPLEMENTARY FIGURE S3
A 30-day landmark analysis for event free survival curves for major adverse cardiac events (A) and all-cause death (B) stratified by groups. The MACE showed a difference between the 4 groups after 150 days. All-cause death was analyzed to have a difference between the 4 groups after 180 days. The prognostic analysis between the 4 groups showed no differences at 30 days.
SUPPLEMENTARY FIGURE S4
Event free survival curves for major adverse cardiac events (A) and all-cause death (B) stratified by groups in STEMI (A) and NSTEMI (B).
SUPPLEMENTARY TABLE S1
Predictors for major adverse cardiac events in STEMI and NSTEMI.
SUPPLEMENTARY TABLE S2
Analysis for proportional hazards for acute de novo HF in AMI patients without HF at the time of admission.
References
1. Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. (2021) 128(10):1421–34. doi: 10.1161/CIRCRESAHA.121.318172
2. Bahit MC, Kochar A, Granger CB. Post-Myocardial infarction heart failure. JACC Heart Fail. (2018) 6(3):179–86. doi: 10.1016/j.jchf.2017.09.015
3. Hamilton E, Desta L, Lundberg A, Alfredsson J, Christersson C, Erlinge D, et al. Prevalence and prognostic impact of left ventricular systolic dysfunction or pulmonary congestion after acute myocardial infarction. ESC Heart Fail. (2023) 10(2):1347–57. doi: 10.1002/ehf2.14301
4. Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, et al. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail. (2021) 8(1):222–37. doi: 10.1002/ehf2.13144
5. Hwang SY, Kim SH, Uhm IA, Shin JH, Lim YH. Prognostic implications for patients after myocardial infarction: an integrative literature review and in-depth interviews with patients and experts. BMC Cardiovasc Disord. (2022) 22(1):348. doi: 10.1186/s12872-022-02753-z
6. van der Bijl P, Abou R, Goedemans L, Gersh BJ, Holmes DR Jr., Ajmone Marsan N, et al. Left ventricular post-infarct remodeling: implications for systolic function improvement and outcomes in the modern era. JACC Heart Fail. (2020) 8(2):131–40. doi: 10.1016/j.jchf.2019.08.014
7. Desta L, Jernberg T, Löfman I, Hofman-Bang C, Hagerman I, Spaak J, et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART registry (Swedish web-system for enhancement and development of evidence-based care in heart disease evaluated according to recommended therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC Heart Fail. (2015) 3(3):234–42. doi: 10.1016/j.jchf.2014.10.007
8. Torabi A, Cleland JG, Rigby AS, Sherwi N. Development and course of heart failure after a myocardial infarction in younger and older people. J Geriatr Cardiol. (2014) 11(1):1–12. doi: 10.3969/j.issn.1671-5411.2014.01.002
9. Amon J, Wong GC, Lee T, Singer J, Cairns J, Shavadia JS, et al. Incidence and predictors of adverse events among initially stable ST-elevation myocardial infarction patients following primary percutaneous coronary intervention. J Am Heart Assoc. (2022) 11(17):e025572. doi: 10.1161/JAHA.122.025572
10. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138(20):e618–51. doi: 10.1161/CIR.0000000000000617
11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed). (2022) 75(6):523. doi: 10.1016/j.recesp.2021.11.027
12. Killip T 3rd, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. (1967) 20(4):457–64. doi: 10.1016/0002-9149(67)90023-9
13. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41(3):407–77. doi: 10.1093/eurheartj/ehz425
14. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(3):e4–e17. doi: 10.1161/CIR.0000000000001039
15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
16. Galderisi M, Cosyns B, Edvardsen T, Cardim N, Delgado V, Di Salvo G, et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: an expert consensus document of the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2017) 18(12):1301–10. doi: 10.1093/ehjci/jex244
17. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure. J Cardiac Fail. (2021) S1071-9164(21)00050-6. doi: 10.1016/j.cardfail.2021.01.022. [Epub ahead of print]
18. Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, et al. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J. (2008) 29(7):859–70. doi: 10.1093/eurheartj/ehn096
Keywords: heart failure, myocardial infarction, prognosis, acute myocardal infarct, death
Citation: Kim HY, Kim KH, Lee N, Park H, Cho JY, Yoon HJ, Ahn Y, Jeong MH and Cho JG (2023) Timing of heart failure development and clinical outcomes in patients with acute myocardial infarction. Front. Cardiovasc. Med. 10:1193973. doi: 10.3389/fcvm.2023.1193973
Received: 26 March 2023; Accepted: 19 June 2023;
Published: 30 June 2023.
Edited by:
Matteo Cameli, University of Siena, ItalyReviewed by:
Qingui Chen, The First Affiliated Hospital of Sun Yat-sen University, ChinaVyacheslav Ryabov, Russian Academy of Sciences, Russia
© 2023 Kim, Kim, Lee, Park, Cho, Yoon, Ahn, Jeong and Cho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kye Hun Kim Y2hyaXN0aWFua3llaHVuQGhhbm1haWwubmV0
 Hyung Yoon Kim
Hyung Yoon Kim Kye Hun Kim
Kye Hun Kim Nuri Lee
Nuri Lee Hyukjin Park
Hyukjin Park Jae Yeong Cho
Jae Yeong Cho Hyun Ju Yoon
Hyun Ju Yoon Youngkeun Ahn
Youngkeun Ahn