- Department of Cardiology, Langfang People’s Hospital, Hebei Medical University, Langfang Core Laboratory of Precision Treatment of Coronary Artery Disease, Langfang, China
Background: This study aimed to systematically evaluate the effects of different types and doses of pretreatment with P2Y12 inhibitors in patients with non-ST-elevation acute coronary syndrome (NSTE-ACS) undergoing percutaneous coronary intervention (PCI).
Methods: Electronic databases were searched for studies comparing pretreatment with different types and doses of P2Y12 inhibitors or comparison between P2Y12 inhibitor pretreatment and nonpretreatment. Electronic databases included the Cochrane Library, PubMed, EMBASE, and Web of Science. Literature was obtained from the establishment of each database until June 2022. The patients included in the study had pretreatment with P2Y12 inhibitors with long-term oral or loading doses, or conventional aspirin treatment (non-pretreatment). The primary endpoint was major adverse cardiac and cerebrovascular events (MACCEs) during follow-up within 30 days after PCI, which included determining the composite endpoints of cardiac death, myocardial infarction, ischemia-driven revascularization, and stroke. The safety endpoint was a major bleeding event.
Results: A total of 119,014 patients from 21 studies were enrolled, including 13 RCTs and eight observational studies. A total of six types of interventions were included—nonpretreatment (placebo), clopidogrel pretreatment, ticagrelor pretreatment, prasugrel pretreatment, double loading pretreatment (double loading dose of clopidogrel, ticagrelor, prasugrel) and P2Y12 inhibitors pretreatment (the included studies did not distinguish the types of P2Y12 inhibitors, including clopidogrel, ticagrelor, and prasugrel). The network meta-analysis results showed that compared to patients without pretreatment, patients receiving clopidogrel pretreatment (RR = 0.78, 95% CI:0.66, 0.91, P < 0.05) and double-loading pretreatment (RR = 0.62, 95% CI:0.41, 0.95, P < 0.05) had a lower incidence of MACCEs. There was no statistically significant difference in the incidence of major bleeding events among the six pretreatments (P > 0.05).
Conclusions: In patients with NSTE-ACS, pretreatment with P2Y12 inhibitors before percutaneous intervention reduced the incidence of recurrent ischemic events without increasing the risk of major bleeding after PCI compared with nonpretreatment. Clopidogrel or double loading dose P2Y12 inhibitors can be considered for the selection of pretreatment drugs.
Introduction
In patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS), undergoing percutaneous coronary intervention (PCI), platelets are activated during the perioperative period, increasing the risk of stent thrombosis and coronary no-reflow (1). Early oral antiplatelet loading doses can improve prognosis with similar safety (2). In these patients, dual antiplatelet therapy with aspirin and P2Y12 inhibitors after PCI was effective in preventing the recurrence of thrombosis and ischemic events (3), also reducing the occurrence of major adverse cardiovascular events (MACEs) (4, 5). However, whether patients with NSTE-ACS should be pretreated with oral P2Y12 inhibitors before PCI remains controversial. With the widespread clinical application of new P2Y12 inhibitors, data from 20 years ago supported the use of pretreatment (4, 5). Therefore, the long-term efficacy of pretreatment with these drugs remains uncertain (6, 7). The 2020 European Society of Cardiology (ESC) guidelines recommend that pretreatment with a P2Y12 receptor inhibitor should be considered in patients with NSTE-ACS who do not plan to undergo an early invasive strategy and do not have a high bleeding risk (HBR) (IIb, C). Routine pretreatment with a P2Y12 receptor inhibitor is not recommended in patients whose coronary anatomy is unknown, and early invasive management is planned (III, A). The effects of pretreatment with P2Y12 inhibitors on bleeding and recurrent ischemic events in patients with NSTE-ACS undergoing PCI remain controversial. Therefore, we performed a network meta-analysis to assess the efficacy and safety of these therapies.
Methods
Inclusion and exclusion criteria
Studies were included for the following reasons: (1) randomized controlled trials and observational studies that compared P2Y12 inhibitor pretreatment (intervention group or control group) in patients with NSTE-ACS following PCI; (2) patients were clearly diagnosed with NSTE-ACS (8); and (3) patients were administered oral P2Y12 inhibitors before PCI. Additionally, the other treatments and nursing measures in the control group were the same as those in the intervention group and included conventional aspirin treatment.
Studies were excluded for the following reasons: (1) patients had other diseases that affected platelets and blood coagulation, such as severe hematological diseases or immune system diseases; (2) there was a combination of pretreatment and other interventions; and (3) the studies were duplicates.
Outcomes, definitions, and follow-up
The prognostic indicators synthesized in this study were the results of follow-up 30 days after PCI in patients with NSTE-ACS. The clinical endpoints analyzed included the following: The primary endpoint was major adverse cardiovascular and cerebrovascular event (MACCE) during follow-up within 30 days after PCI, and these MACCEs included the composite endpoints of cardiac death, myocardial infarction, ischemia-driven revascularization, and stroke. The MACCEs in this study were reported in a single study and were not recalculated. The safety endpoint was a major bleeding event.
Data sources and search strategy
Electronic databases were searched for studies related to pretreatment with P2Y12 inhibitors before PCI in patients with NSTE-ACS. The databases included the Cochrane Library, PubMed, EMBASE, and Web of Science. Literature retrieval, literature screening, data extraction, and data analysis were performed in accordance with the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Literatures were obtained from the establishment of each database until June 2022.
The search strategy was determined after multiple pre-searches using a combination of MeSH terms and free words, and fine adjustments were made based on a specific database. To reduce publication bias, manual queries were performed for relevant conference papers, academic reports, and dissertations to supplement the search results.
The search terms were as follows: “coronary artery disease”, “acute coronary syndromes”, “ACS”, “non-ST-elevation”, “NSTEMI”, “NSTE-ACS”, “percutaneous coronary intervention”, “PCI”, “angioplasty”, “balloon”, “coronary revascularization”, “high-dose”, “double-dose”, “loading dose”, “pretreatment”, “intensive”, “reload”, “load”, “preload”, “upstream”, “timing”, “antiplatelet”, “ticagrelor”, “clopidogrel”, “prasugrel”, “cangrelor”, and “elinogrel”.
The search strategy was as follows: (“coronary artery disease” OR “acute coronary syndromes” OR “ACS” OR “non-ST-elevation” OR “NSTEMI” OR “NSTE-ACS”) AND (“percutaneous coronary intervention” OR “PCI” OR “angioplasty” OR “balloon” OR “coronary revascularization”) AND (“high-dose” OR “double-dose” OR “loading dose” OR “pretreatment” OR “intensive” OR “reload” OR “load” OR “preload” OR “upstream” OR “timing”) AND (“antiplatelet” OR “ticagrelor” OR “clopidogrel” OR “prasugrel” OR “cangrelor” OR “elinogrel”).
Duplicate literatures were removed using the Endnote software. Literatures were excluded by reading the title and abstract, based on the inclusion and exclusion criteria. Finally, related literatures were excluded by reading the full text. The selected studies were independently checked and cross-checked by two researchers. If the opinions of the two researchers were inconsistent, a third researcher negotiated to reach a consensus.
Data extraction and assessment of quality
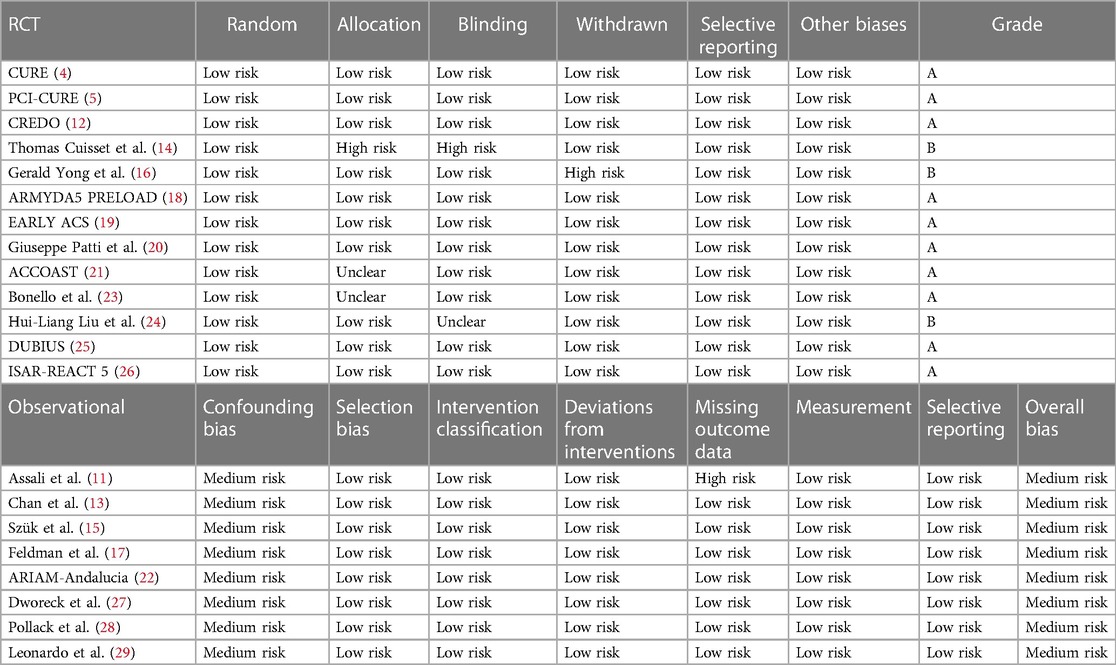
Data extraction and quality evaluation were independently completed by two researchers using the quality evaluation criteria, and a cross-check was performed. If any disagreement occurred about the inclusion of certain data, a third researcher was included to conduct the discussion and reach a consensus. The data extracted from the articles included basic information, inclusion and exclusion criteria, type of research, sample size, type and dosage of P2Y12 inhibitors, and outcome indicators. Quality was independently evaluated according to the method recommended in the Cochrane Handbook 5.1.0 (9). The evaluation included seven areas of the randomization method—allocation concealment, blindness of the participants and interveners, blindness of the measurement process, completeness of the outcome indicators (missing data), selective reporting, and other biases. The risk of bias for each area was judged as low, high, or unclear. Grade A included studies that fully met the abovementioned standards and had the lowest possibility of various deviations. Studies that partially met the above-mentioned criteria and had a moderate probability of bias were classified as grade B. Studies with a high probability of bias that did not meet the abovementioned criteria were grade C. The risk of bias in nonrandom studies of interventions (ROBINS-1) was used to evaluate the included cohort studies (10).
Statistical analysis
Stata software (version 17.0) was used for the data meta-analysis. Descriptive analyses were used for data that could not be quantitatively synthesized. Relative risks (RRs) and 95% confidence intervals (95% CIs) were calculated for categorical variables. In the presence of a closed loop, the consistency between the direct and indirect comparisons was judged using the node-splitting method. When P < 0.05, the inconsistency was evident. The area under the cumulative ranking (SUCRA) showed the possibility of each intervention being the best.
Results
Search results
A total of 5,844 articles were retrieved from the databases. Five related articles were obtained through manual retrieval of conference papers, academic reports, and dissertations. Duplicate articles were eliminated using the EndNote software, and 3,997 articles were obtained. On careful assessment of the titles and abstracts, a total of 3,874 articles which were not related to our topic were eliminated. The remaining number of articles was 123. Studies that did not meet the inclusion or exclusion criteria, or had unclear expressions were further eliminated by reading the full text, and 21 articles were obtained (4, 5, 11–29), including 13 RCTs (4, 5, 12, 14, 16, 18–21, 23–26) and eight observational studies (11, 13, 15, 17, 22, 27–29). Literature retrieval and screening processes were carried out in accordance with the PRISMA guidelines; the specific process of screening is shown in Figure 1. Six types of interventions were included—nonpretreatment (placebo), clopidogrel pretreatment, ticagrelor pretreatment, prasugrel pretreatment, double loading pretreatment, and P2Y12 pretreatment. Nonpretreatment refers to patients receiving only aspirin treatment before PCI without P2Y12 inhibitor treatment. Clopidogrel pretreatment refers to patients receiving clopidogrel (300 mg loading dose) treatment before PCI. Ticagrelor pretreatment refers to patients receiving ticagrelor (180 mg loading dose) before PCI. Prasugrel pre-treatment refers to patients receiving prasugrel treatment (30 mg prasugrel loading dose) before PCI. Double-loading pretreatment refers to treatment with a double-loading P2Y12 inhibitor (600 mg clopidogrel loading dose, 360 mg ticagrelor loading dose, and 60 mg prasugrel loading dose) before PCI. P2Y12 pretreatment refers to patients included in the original study who received P2Y12 inhibitor (clopidogrel, ticagrelor, or prasugrel) treatment before PCI, without distinguishing the types of P2Y12 inhibitors.
General features and methodological quality of the included studies
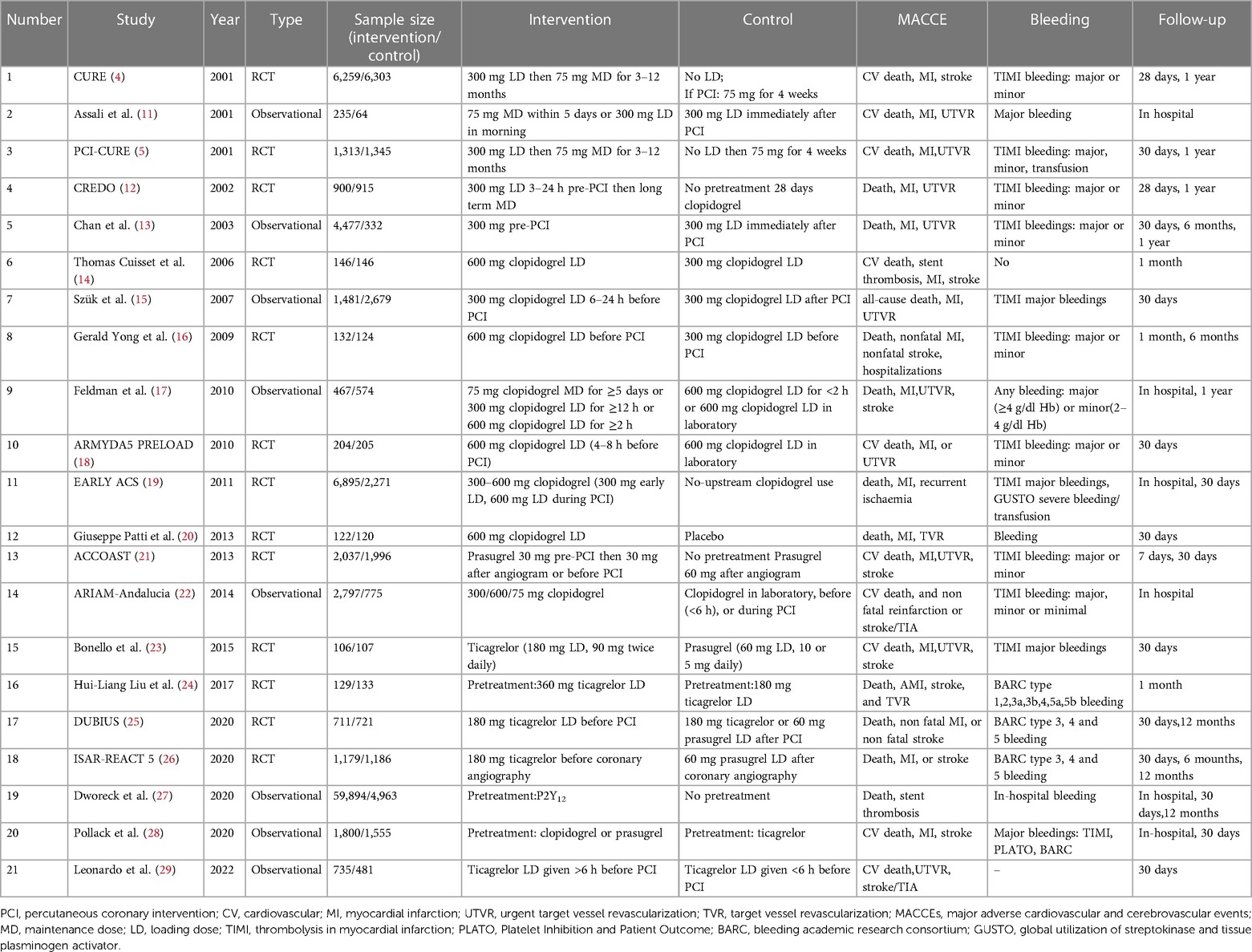
Twenty-one studies with 119,014 patients with NSTE-ACS were included in this study (4, 5, 11–29). The general characteristics of the included studies are presented in Table 1. The methodological qualities of the included studies are presented in Table 2.
MACCEs
Meta-analysis results for MACCEs
Twenty-one studies with MACCEs were included in the analysis (119,014 patients with NSTE-ACS) (4, 5, 11–29). The results of the inconsistency test showed that the inconsistency was not significant (I2 = 4.21, P = 0.239); therefore, the consistency model was used for the analysis. The network plots are shown in Figure 2. The results of the loop inconsistency test showed that loop inconsistency was not significant (P > 0.05). The results of the direct meta-analysis are shown in Figure 3A. The network meta-analysis results showed that compared with the patients without pretreatment, the patients receiving clopidogrel pretreatment (RR = 0.78, 95% CI: 0.66, 0.91, P < 0.05) and double loading pretreatment (RR = 0.62, 95% CI: 0.41, 0.95, P < 0.05) had a lower incidence of MACCEs; compared with the patients who received ticagrelor pretreatment, the patients who received double loading pretreatment (RR = 0.60, 95% CI: 0.36, 0.99, P < 0.05) had a lower incidence of MACCEs (Figure 3B).
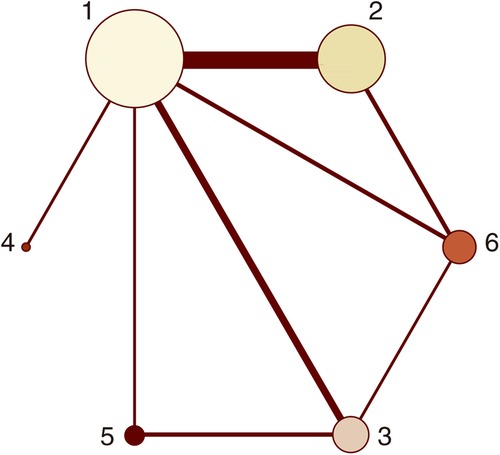
Figure 2. Network plots (MACCEs). 1 = placebo, 2 = clopidogrel pretreatment, 3 = ticagrelor pretreatment, 4 = prasugrel pretreatment, 5 = P2Y12 inhibitor pretreatment, 6 = double pretreatment.
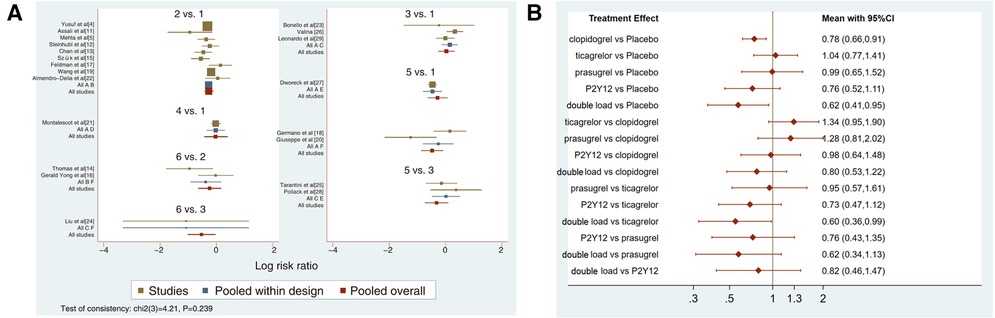
Figure 3. (A) direct meta-analysis results (MACCEs). 1 = placebo, 2 = clopidogrel pretreatment, 3 = ticagrelor pretreatment, 4 = prasugrel pretreatment, 5 = P2Y12 inhibitor pretreatment, 6 = double pretreatment. (B) Network meta-analysis results (MACCEs).
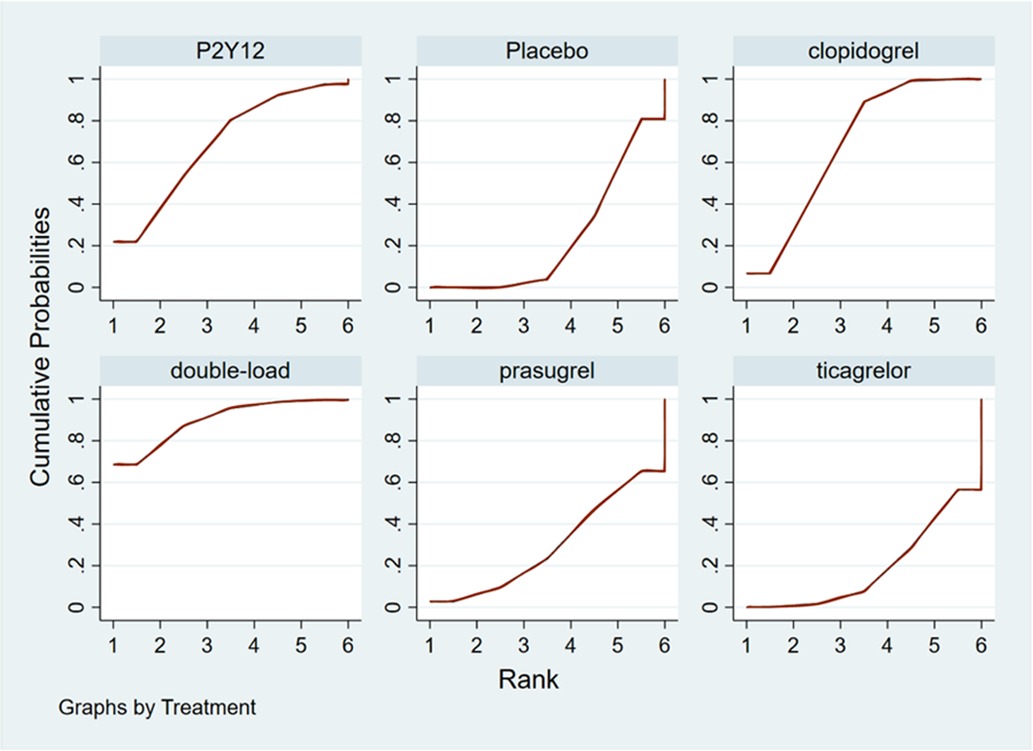
Ranking result of pretreatment for MACCEs
The higher the SUCRA value of the pretreatment method, the fewer the MACCEs that occurred in patients receiving the pretreatment method. The SUCRA values of the six pretreatment methods were ranked from highest to lowest as follows: double-loading pretreatment (SUCRA = 89.9), P2Y12 inhibitor pretreatment (SUCRA = 69.1), clopidogrel pretreatment (SUCRA = 68.5), prasugrel pretreatment (SUCRA = 29.7), placebo (SUCRA = 23.8), and ticagrelor pretreatment (SUCRA = 18.9). The SUCRA curves for the different pretreatment methods are shown in Figure 4. Compared to the patients who did not receive pretreatment, those who received clopidogrel pretreatment (RR = 0.78, 95% CI: 0.66, 0.91, P < 0.05) and double-loading pretreatment (RR = 0.62, 95% CI: 0.41, 0.95, P < 0.05) had a lower incidence of MACCEs. Compared to the patients who received ticagrelor pretreatment, those who received double-loading pretreatment (RR = 0.60, 95% CI: 0.36, 0.99, P < 0.05) had a lower incidence of MACCEs (Figure 3B). There were no significant differences in the other pairwise comparisons. Therefore, for MACCEs, it is better for patients to receive clopidogrel pretreatment and double-loading pretreatment than no pretreatment; it is better for patients to receive double-loading pretreatment than ticagrelor pretreatment.
Major bleeding events
Meta-analysis results for major bleeding events
Sixteen studies with major bleeding events were included in the subgroup analysis (52,056 patients with NSTE-ACS) (4, 5, 11–13, 15–17, 19, 21–26, 28). The results of the inconsistency test showed that the inconsistency was not significant (I2 = 0.04, P = 0.851); therefore, the consistency model was used for the analysis. The network plots are shown in Figure 5. The results of the loop inconsistency test showed that loop inconsistency was not significant (P > 0.05). The results of the direct meta-analysis are shown in Figure 6A. The network meta-analysis results showed no statistically significant differences in the incidence of major bleeding events among the six interventions (Figure 6B).
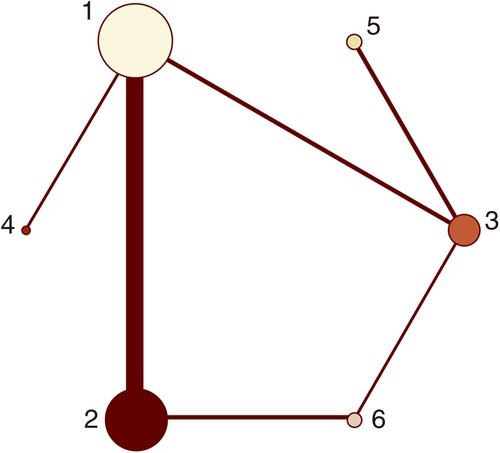
Figure 5. Network plots (major bleeding). 1 = placebo, 2 = clopidogrel pretreatment, 3 = ticagrelor pretreatment, 4 = prasugrel pretreatment, 5 = P2Y12 inhibitor pretreatment, 6 = double pretreatment.
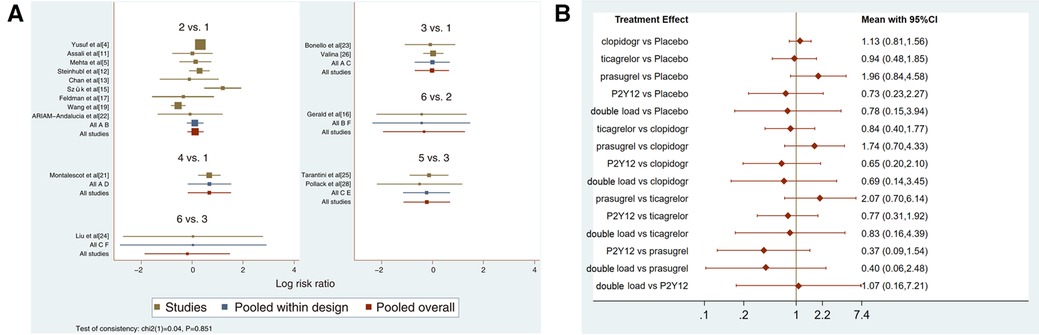
Figure 6. (A) direct meta-analysis results (major bleeding). 1 = placebo, 2 = clopidogrel pretreatment, 3 = ticagrelor pretreatment, 4 = prasugrel pretreatment, 5 = P2Y12 inhibitor pretreatment, 6 = double pretreatment. (B) Network meta-analysis results (major bleeding).
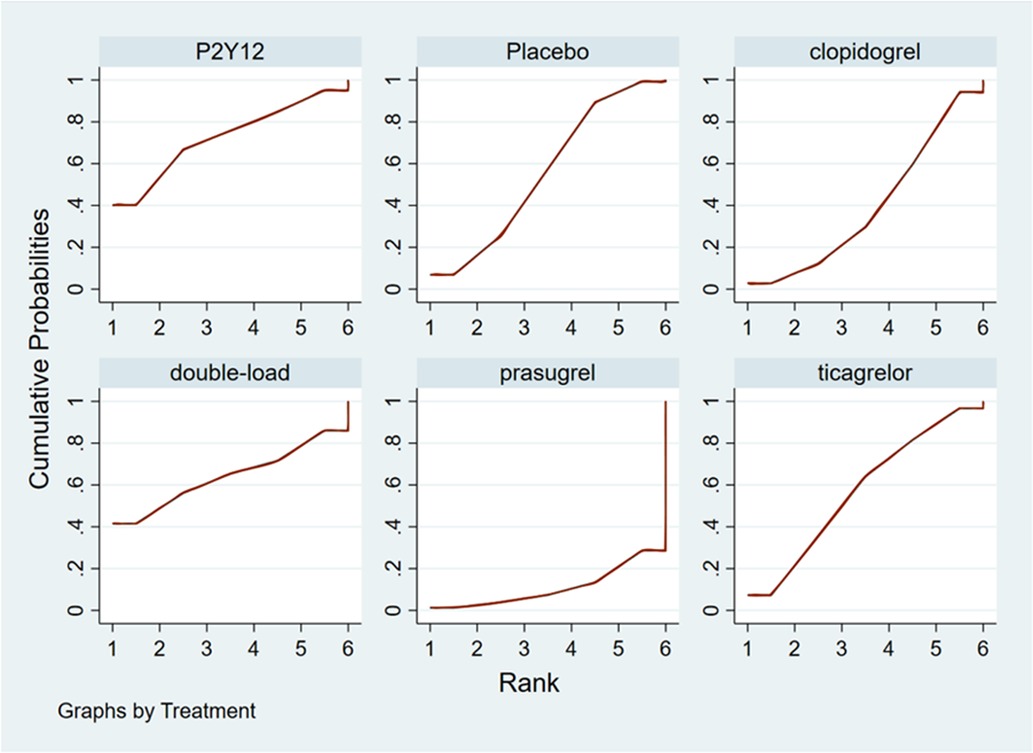
Ranking result of pretreatment for major bleeding events
The higher the SUCRA value of the pretreatment method, the fewer major bleeding events occurred in the patients who received the pretreatment method. The SUCRA values of the six pretreatment methods were ranked from highest to lowest as follows: P2Y12 inhibitor pretreatment (SUCRA = 72.0), double-loading pretreatment (SUCRA = 63.6), ticagrelor pretreatment (SUCRA = 56.9), placebo (SUCRA = 56.5), clopidogrel pretreatment (SUCRA = 40.4), and prasugrel pretreatment (SUCRA = 10.5). The SUCRA curves for different pretreatment methods are shown in Figure 7. There were no significant differences in the pairwise comparisons.
Publication bias and contribution plot
Funnel plots were constructed to test for publication bias (Figures 8A,B). Funnel plots showed that there was a publication bias in this study. A total of 21 studies were included in the meta-analysis. However, the number of studies included was insufficient for each comparison between the two groups. According to the Cochrane Handbook, funnel plots should only be used when the number of studies included is not less than 10 for each outcome indicator. With an extremely small number of included studies, the test efficiency was too low to distinguish between opportunity and true asymmetry. However, two retrieval methods were used to reduce publication bias. Duplicate published studies were excluded to avoid the use of the same data. A contribution plot was constructed for the network (Figures 9A,B).
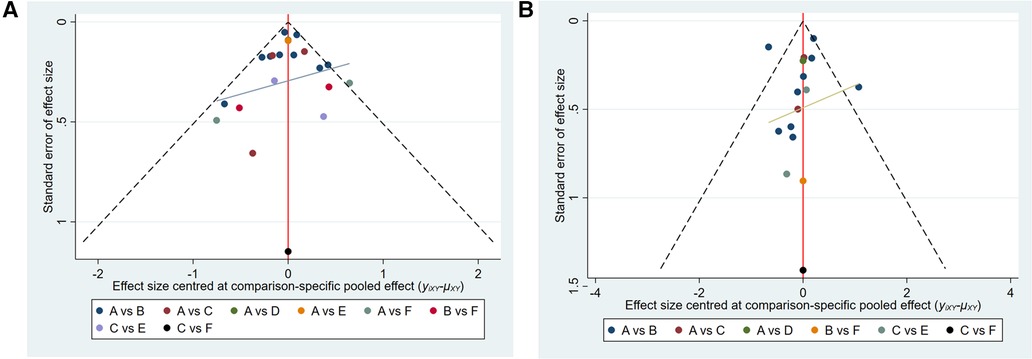
Figure 8. (A) publication bias (MACCEs). A = placebo, B = clopidogrel pretreatment, C = ticagrelor pretreatment, D = prasugrel pretreatment, E = P2Y12 inhibitor pretreatment, F = double pretreatment. (B) Publication bias (major bleeding). A = placebo, B = clopidogrel pretreatment, C = ticagrelor pretreatment, D = prasugrel pretreatment, E = P2Y12 inhibitor pretreatment, F = double pretreatment.
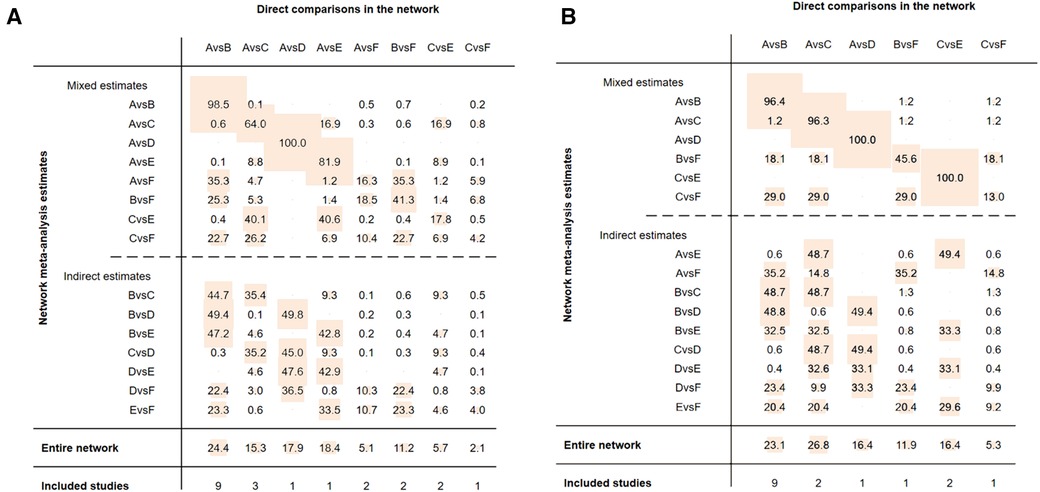
Figure 9. (A) contribution plot (MACCEs). Contribution plot for the network. The size of each square is proportional to the weight attached to each direct summary effect (horizontal axis) for the estimation of each network summary effect (vertical axis). The numbers re-express the weights as percentages. (A = placebo, B = clopidogrel pretreatment, C = ticagrelor pretreatment, D = prasugrel pretreatment, E = P2Y12 inhibitor pretreatment, F = double pretreatment). (B) Contribution plot (major bleeding). Contribution plot for the network. The size of each square is proportional to the weight attached to each direct summary effect (horizontal axis) for the estimation of each network summary effect (vertical axis). The numbers re-express the weights as percentages. (A = placebo, B = clopidogrel pretreatment, C = ticagrelor pretreatment, D = prasugrel pretreatment, E = P2Y12 inhibitor pretreatment, F = double pretreatment).
Discussion
Twenty-one studies involving 119,014 patients with NSTE-ACS were included in this network meta-analysis. We compared different pretreatment strategies for P2Y12 inhibitors to analyze the impact of P2Y12 inhibitor pretreatment on the incidence of MACCEs and major bleeding events in patients with NSTE-ACS. The results were as follows: 1) pretreatment with a P2Y12 inhibitor before PCI in patients with NSTE-ACS reduced the incidence of MACCEs without increasing the risk of major bleeding events; 2) clopidogrel or a P2Y12 inhibitor with a double-loading dose could be selected as the pretreatment scheme; and 3) compared with pretreatment with ticagrelor, a P2Y12 inhibitor with a double loading dose could reduce MACCEs.
Current guidelines recommend that patients with ST-elevation myocardial infarction be administered P2Y12 inhibitors orally before PCI (30). However, whether P2Y12 inhibitor pretreatment should be administered to patients with NSTE-ACS remains controversial (31). In addition, several meta-analyses have discussed P2Y12 inhibitor pretreatment (32–37), but there were differences in the types of objects in these meta-analyses. Some studies focused on patients with ACS or all patients receiving PCI (32–35) and did not distinguish between NSTE-ACS patients. Two meta-analyses discussed P2Y12 inhibitor pretreatment in patients with NSTE-ACS, and one meta-analysis included patients with NSTE-ACS (36). However, considering that there are many methods for P2Y12 inhibitor pretreatment (different drug types and timings), if the methods of P2Y12 inhibitor pretreatment are not differentiated, this may lead to some heterogeneity in the meta-analysis. Another network meta-analysis divided the interventions into six groups: early clopidogrel, delayed clopidogrel, early prasugrel, delayed prasugrel, early ticagrelor, and delayed ticagrelor (37). The duration of follow-up was between 1 and 12 months in the nine included studies, which may also lead to some heterogeneity in the meta-analysis. In addition, we believe that the risks and benefits of P2Y12 inhibitor pretreatment are mainly shown within 1-month of PCI. Therefore, we systematically reviewed all direct and indirect evidence from randomized controlled trials and observational cohort studies to establish this network meta-analysis, clarify the potential risks and benefits of P2Y12 inhibitor pretreatment in patients with NSTE-ACS, and compare the differences between pretreatment schemes. Based on the type, dosage, and timing of P2Y12 inhibitors, the interventions were divided into placebo, clopidogrel, ticagrelor, prasugrel, P2Y12 inhibitors, and double-loading pretreatment.
We ranked all potential combinations of P2Y12 inhibitor pretreatments for MACCEs and bleeding events in patients with NSTE-ACS. For MACCEs, the SUCRA values indicated that the priority of the selected pretreatment scheme was double-loading pretreatment, P2Y12 inhibitor pretreatment, clopidogrel pretreatment, prasugrel pretreatment, placebo, and ticagrelor pretreatment. The network meta-analysis results showed that, compared with patients without pretreatment, patients who received clopidogrel pretreatment (P < 0.05) and double-loading pretreatment (P < 0.05) had a lower incidence of MACCEs. Compared to patients who received ticagrelor pretreatment, those who received double-loading pretreatment (P < 0.05) had a lower incidence of MACCEs. For major bleeding events, the SUCRA values suggested that the priority of the selected pretreatment scheme was P2Y12 pretreatment, double-loading, ticagretor, placebo, clopidogrel, and prasugrel pretreatment. Therefore, pretreatment with P2Y12 inhibitors significantly reduced the number of recurrent ischemic events without increasing the occurrence of major bleeding events. Clopidogrel or P2Y12 inhibitors (clopidogrel, ticagrelor and prasugrel) with a double-loading dose should be selected as the pretreatment protocols for patients with NSTE-ACS.
Clopidogrel was the main pretreatment drug in the early studies. Clopidogrel was used as a pretreatment drug in 13 of the 21 studies included in our study. Compared with no pretreatment, the incidence of MACCEs within 30 days of PCI was lower. However, compared to a single loading dose (300 mg) of clopidogrel, a double-loading dose (600 mg) of clopidogrel did not show an advantage. The pretreatment method included a maintenance dose of 75 mg clopidogrel for 5 days or a loading dose of 300 mg clopidogrel before PCI. An early CURE study showed that the incidence of MACEs in patients with NSTE-ACS in the clopidogrel group was significantly reduced (9.3% vs. 11.4%, P < 0.001); however, the number of major bleeding events was significantly higher in patients with NSTE-ACS than in those in the control group (3.7% vs. 2.7%, P = 0.001) (4). Additionally, there was no significant difference in the number of fatal bleeding events between the two groups (2.1% vs. 1.8%, RR = 0.80, P = 0.13). Also, there were different research results regarding the timing of pretreatment. The onset of clopidogrel administration was relatively slow. Compared with patients who received clopidogrel pretreatment >6 h before PCI, those who received clopidogrel pretreatment <6 h before PCI had significantly fewer primary ischemic events (14). The pretreatment clopidogrel dose can be doubled in patients requiring rapid platelet inhibition within 6 h (18). With the gradual development and popularization of PCI, the emergence of new and more powerful antiplatelet drugs, and extensive application of drug-eluting stents in clinical practice, research on the application of new P2Y12 inhibitors in the pretreatment of patients with NSTE-ACS before PCI has gradually increased. This meta-analysis included eight studies on ticagrelor and prasugrel, but two studies supported double-loading dose P2Y12 inhibitors (24, 26), including ticagrelor and prasugrel, which were superior to pretreatment with ticagrelor in terms of the incidence of MACCEs, which may be related to the rapid inhibition of platelet activity. However, there are few studies, and the results may be inconsistent. Similarly, compared with a placebo, the use of a P2Y12 inhibitor with a double-loading dose can inhibit platelet activity more quickly and reduce the occurrence of MACCEs.
Our network meta-analysis has increased our understanding of the existing evidence related to P2Y12 receptor inhibitor pre-treatment and clarified that P2Y12 inhibitor pretreatment can reduce MACCEs within 30 days after PCI without increasing the number of bleeding events. However, more researches are needed to compare different P2Y12 receptor inhibitor pretreatment schemes and determine the optimal P2Y12 inhibitor pretreatment program for patients with NSTE-ACS. Compared with previous meta-analyses (32–37), our study showed some differences and advantages—this meta-analysis fully considered the differences in drug types, dosage, and administration timing of P2Y12 inhibitors to evaluate the different pretreatment schemes; it summarizes all available information, including direct evidence and indirect estimates and provides the potential benefits and risks of each pretreatment method, and the age range of the patients included in the study was wide, ensuring the external validity of the results.
In summary, this study supports the use of pretreatment with P2Y12 inhibitors in patients with NSTE-ACS. The suggested timing of pretreatment is when NSTE-ACS is diagnosed or before angiography. The pretreatment protocol is suggested to be clopidogrel [including 75 mg daily maintenance ≥5 days or loading dose (300 mg) before PCI] or a double-loading dose of a P2Y12 inhibitor (including 600 mg of clopidogrel, 360 mg of ticagrelor and 60 mg of prasugrel). Additionally, this study found that a double-loading dose of a P2Y12 inhibitor was superior to a single-loading dose (180 mg) of ticagrelor. The above-recommended pretreatment method can reduce the incidence of MACCEs without increasing the risk of major bleeding events.
Current guidelines do not recommend routine pretreatment before angiography because of the risk of intracranial hemorrhage and delayed CABG. If the patient needs to undergo coronary artery bypass grafting after angiography, ticagrelor should be discontinued for at least 3 days, clopidogrel for at least 5 days, and prasugrel for at least 1 week (38). Therefore, routine pre-treatment may cause high platelet inhibition and increase the risk of bleeding in some patients during the perioperative period. We observed that prasugrel alone increased the risk of major bleeding. In addition, pretreatment with clopidogrel and ticagrelor did not increase the risk of major bleeding (5, 24). Some patients refused coronary artery bypass therapy after coronary angiography because of socioeconomic factors. These patients require long-term antiplatelet therapy to improve ischemia; therefore, there is no need to discontinue P2Y12 inhibitors.
Limitation
However, this study has some limitations. First, the outcome indicators in this study were based on follow-up 30 days after PCI, which lacked laboratory-related indicators (platelet aggregation) and in-hospital bleeding. Future investigations of the perioperative period and related laboratory indicators should be performed. Second, the 21 studies included in our analysis comprised 13 randomized controlled trials and eight observational studies. In addition, the current research on P2Y12 inhibitor pretreatment in patients with NSTE-ACS before PCI mainly included clopidogrel pretreatment. Third, for the definition of some endpoints, there were differences between the studies, especially TIMI bleeding, GUSTO bleeding, and MACCEs, which may lead to heterogeneity in this indicator. Fourth, it included open-label randomized controlled trials because it is difficult to use double-blind P2Y12 inhibitor strategies and timing. Finally, the comparison between the use of a P2Y12 inhibitor with a double loading dose and ticagrelor included only three studies, with a confidence interval of 0.36–0.99. Therefore, the results of this study need to be interpreted carefully.
Conclusions
For patients with NSTE-ACS, pretreatment with P2Y12 inhibitors before PCI reduced the incidence of recurrent ischemic events without increasing the risk of major bleeding events after PCI compared with nonpretreatment. Clopidogrel or double loading dose P2Y12 inhibitors can be considered for the selection of pretreatment drugs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZX performed the main research. YL and ML conducted a literature search. ZZ and YY analysed the data. LA, JW, XS, and CL prepared the tables and figures. YL and ML wrote the main article. ZX critically reviewed and revised the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li A, Chen J, Liang ZH, Cai J, Cai HH, Chen M. Comparison of ultrastructural and nanomechanical signature of platelets from acute myocardial infarction and platelet activation. Biochem Biophys Res Commun. (2017) 486:245–51. doi: 10.1016/j.bbrc.2017.03.009
2. Komosa A, Lesiak M, Krasiński Z, Grygier M, Siniawski A, Skorupski W, et al. Optimal timing of P2Y12 inhibitor loading in patients undergoing PCI: a meta-analysis. J Thromb Haemost. (2019) 119:1000–20. doi: 10.1055/s-0039-1683421
3. Verdoia M, Khedi E, Ceccon C, Suryapranata H, De Luca G. Duration of dual antiplatelet therapy and outcome in patients with acute coronary syndrome undergoing percutaneous revascularization: a meta-analysis of 11 randomized trials. Int J Cardiol. (2018) 264:30–8. doi: 10.1016/j.ijcard.2018.02.095
4. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. (2001) 345:494–502. doi: 10.1056/NEJMoa010746
5. Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. (2001) 358:527–33. doi: 10.1016/S0140-6736(01)05701-4
6. Golwala H, Bhatt DL. The timing of P2Y(12) inhibitor initiation in the treatment of ACS? Does the evidence exist in this era? Prog Cardiovasc Dis. (2018) 60:471–7. doi: 10.1016/j.pcad.2018.01.001
7. De Caterina R, Veneri AD. Do we need P2Y(12) inhibitor pretreatment in non-ST elevation acute coronary syndrome? Rev Port Cardiol. (2016) 35:319–21. doi: 10.1016/j.repc.2016.05.002
8. Rubini Gimenez M, Reiter M, Twerenbold R, Reichlin T, Wildi K, Haaf P, et al. Sex-specific chest pain characteristics in the early diagnosis of acute myocardial infarction. JAMA Intern Med. (2014) 174:241–9. doi: 10.1001/jamainternmed.2013.12199
9. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. New Jersey: Wiley-Blackwell (2011). p. 102–8.
10. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
11. Assali AR, Salloum J, Sdringola S, Moustapha A, Ghani M, Hale S, et al. Effects of clopidogrel pretreatment before percutaneous coronary intervention in patients treated with glycoprotein IIb/IIIa inhibitors (Abciximab or tirofiban). Am J Cardiol. (2001) 88:884–6, A6. doi: 10.1016/S0002-9149(01)01897-5
12. Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. (2002) 288:2411–20. doi: 10.1001/jama.288.19.2411
13. Chan AW, Moliterno DJ, Berger PB, Stone GW, DiBattiste PM, Yakubov SL, et al. Triple antiplatelet therapy during percutaneous coronary intervention is associated with improved outcomes including one-year survival: results from the do tirofiban and ReoProGive similar efficacy outcome trial (TARGET). J Am Coll Cardiol. (2003) 42:1188–95. doi: 10.1016/S0735-1097(03)00944-6
14. Cuisset T, Frere C, Quilici J, Morange PE, Nait-Saidi L, Carvajal J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol. (2006) 48:1339–45. doi: 10.1016/j.jacc.2006.06.049
15. Szük T, Gyöngyösi M, Homorodi N, Kristóf E, Király C, Edes IF, et al. Effect of timing of clopidogrel administration on 30-day clinical outcomes: 300-mg loading dose immediately after coronary stenting versus pretreatment 6 to 24 h before stenting in a large unselected patient cohort. Am Heart J. (2007) 153:289–95. doi: 10.1016/j.ahj.2006.10.030
16. Yong G, Rankin J, Ferguson L, Thom J, French J, Brieger D, et al. Randomized trial comparing 600- with 300-mg loading dose of clopidogrel in patients with non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: results of the platelet responsiveness to aspirin and clopidogrel and troponin increment after coronary intervention in acute coronary lesions (PRACTICAL) trial. Am Heart J. (2009) 157:e1–e9. doi: 10.1016/j.ahj.2008.09.024
17. Feldman DN, Fakorede F, Minutello RM, Bergman G, Moussa I, Wong SC. Efficacy of high-dose clopidogrel treatment (600 mg) less than two hours before percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes. Am J Cardiol. (2010) 105:323–32. doi: 10.1016/j.amjcard.2009.09.034
18. Di Sciascio G, Patti G, Pasceri V, Gatto L, Colonna G, Montinaro A, et al. Effectiveness of in-laboratory high-dose clopidogrel loading versus routine pre-load in patients undergoing percutaneous coronary intervention: results of the ARMYDA-5 PRELOAD (antiplatelet therapy for reduction of MYocardial damage during angioplasty) randomized trial. J Am Coll Cardiol. (2010 ) 56:550–7. doi: 10.1016/j.jacc.2010.01.067
19. Wang TY, White JA, Tricoci P, Giugliano RP, Zeymer U, Harrington RA, et al. Upstream clopidogrel use and the efficacy and safety of early eptifibatide treatment in patients with acute coronary syndrome: an analysis from the early glycoprotein IIb/IIIa inhibition in patients with non-ST-segment elevation acute coronary syndrome (EARLY ACS) trial. Circulation. (2011) 123:722–30. doi: 10.1161/CIRCULATIONAHA.110.958041
20. Patti G, Pasceri V, Mangiacapra F, Colonna G, Vizzi V, Ricottini E, et al. Efficacy of clopidogrel reloading in patients with acute coronary syndrome undergoing percutaneous coronary intervention during chronic clopidogrel therapy (from the antiplatelet therapy for reduction of MYocardial damage during angioplasty [ARMYDA-8 RELOAD-ACS] trial). Am J Cardiol. (2013) 112:162–8. doi: 10.1016/j.amjcard.2013.03.008
21. Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay JF, et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med. (2013) 369:999–1010. doi: 10.1056/NEJMoa1308075
22. Almendro-Delia M, Gonzalez-Torres L, Garcia-Alcantara Á, Reina-Toral A, Arboleda Sánchez JA, Rodríguez Yañez JC, et al. Prognostic impact of clopidogrel pretreatment in patients with acute coronary syndrome managed invasively. Am J Cardiol. (2015) 115:1019–26. doi: 10.1016/j.amjcard.2015.01.531
23. Bonello L, Laine M, Cluzel M, Frere C, Mancini J, Hasan A, et al. Comparison of ticagrelor versus prasugrel to prevent periprocedural myonecrosis in acute coronary syndromes. Am J Cardiol. (2015) 116:339–43. doi: 10.1016/j.amjcard.2015.04.050
24. Liu HL, Wei YJ, Ding P, Zhang J, Li TC, Wang B, et al. Antiplatelet effect of different loading doses of ticagrelor in patients with non-ST-elevation acute coronary syndrome undergoing percutaneous coronary intervention: the APELOT trial. Can J Cardiol. (2017) 33:1675–82. doi: 10.1016/j.cjca.2017.09.002
25. Tarantini G, Mojoli M, Varbella F, Caporale R, Rigattieri S, Andò G, et al. Timing of oral P2Y12 inhibitor administration in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. (2020 ) 76:2450–9. doi: 10.1016/j.jacc.2020.08.053
26. Valina C, Neumann FJ, Menichelli M, Mayer K, Wöhrle J, Bernlochner I, et al. Ticagrelor or prasugrel in patients with non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. (2020) 76:2436–46. doi: 10.1016/j.jacc.2020.09.584
27. Dworeck C, Redfors B, Angerås O, Haraldsson I, Odenstedt J, Ioanes D, et al. Association of pretreatment with P2Y12 receptor antagonists preceding percutaneous coronary intervention in non-ST-segment elevation acute coronary syndromes with outcomes. JAMA Netw Open. (2020) 3:e2018735. doi: 10.1001/jamanetworkopen.2020.18735
28. Pollack CV Jr, Peacock WF, Bhandary DD, Silber SH, Bhalla N, Rao SV, et al. Oral antiplatelet therapy administered upstream to patients with NSTEMI. Crit Pathw Cardiol. (2020) 19:166–72. doi: 10.1097/HPC.0000000000000243
29. De Luca L, Pugliese M, Putini RL, Natale E, Piazza V, Biffani E, et al. Periprocedural myocardial injury in high-risk patients with NSTEMI pretreated with ticagrelor for less or more than 6 hours before PCI. J Clin Pharmacol. (2022) 62:770–6. doi: 10.1002/jcph.2014
30. Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. (2012) 33:2569–619. doi: 10.1093/eurheartj/ehs215
31. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
32. Nairooz R, Valgimigli M, Rochlani Y, Pothineni NV, Raina S, Sardar P, et al. Meta-analysis of clopidogrel pretreatment in acute coronary syndrome patients undergoing invasive strategy. Int J Cardiol. (2017) 229:82–9. doi: 10.1016/j.ijcard.2016.11.226
33. Bellemain-Appaix A, O’Connor SA, Silvain J, Cucherat M, Beygui F, Barthélémy O, et al. Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. JAMA. (2012) 308:2507–16. doi: 10.1001/jama.2012.50788
34. Steiner S, Moertl D, Chen L, Coyle D, Wells GA. Network meta-analysis of prasugrel, ticagrelor, high- and standard-dose clopidogrel in patients scheduled for percutaneous coronary interventions. Thromb Haemost. (2012) 108:318–27. doi: 10.1160/TH11-08-0586
35. Salih M, Ayan M, Mlatoum H, Smer A, Traina M. To preload or not to preload: a meta-analysis of randomized controlled trials evaluating preloading thienopyridines prior to percutaneous coronary intervention. Catheter Cardiovasc Interv. (2015) 85:S17.
36. Bellemain-Appaix A, Kerneis M, O’Connor SA, Silvain J, Cucherat M, Beygui F, et al. Reappraisal of thienopyridine pretreatment in patients with non-ST elevation acute coronary syndrome: a systematic review and meta-analysis. Br Med J. (2014) 349:g6269. doi: 10.1136/bmj.g6269
37. Vicent L, Diaz-Arocutipa C, Tarantini G, Mojoli M, Hernandez AV, Bueno H. Early vs. delayed initiation of treatment with P2Y12 inhibitors in patients with non-ST-segment elevation acute coronary syndrome: a systematic review and network meta-analysis of randomized controlled trials. Front Cardiovasc Med. (2022) 9:862452. doi: 10.3389/fcvm.2022.862452
Keywords: non-ST-segment elevation acute coronary syndrome, antiplatelet, P2Y12 inhibitor, pretreatment, meta-analysis
Citation: Li Y, Lei M, Zhao Z, Yang Y, An L, Wang J, Sun X, Li C and Xue Z (2023) Effect of pretreatment with a P2Y12 inhibitor in patients with non-ST-elevation acute coronary syndrome: a systematic review and network meta-analysis. Front. Cardiovasc. Med. 10:1191777. doi: 10.3389/fcvm.2023.1191777
Received: 22 March 2023; Accepted: 3 July 2023;
Published: 19 July 2023.
Edited by:
Liqiu Yan, Guangdong Medical University, ChinaReviewed by:
Marco Ferlini, San Matteo Hospital Foundation (IRCCS), ItalyXiao Wang, Capital Medical University, China
© 2023 Li, Lei, Zhao, Yang, An, Wang, Sun, Li and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengming Xue eHVlemVuZ21pbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yachao Li†
Yachao Li† Zengming Xue
Zengming Xue