Abstract
Background:
The inflammatory process underlying atrial myopathy may affect the inflammatory response activated in acute ischemic stroke (AIS).
Objectives:
We aimed to assess whether left atrial enlargement (LAE) as a marker of atrial myopathy is associated with a different profile of circulating inflammatory markers in AIS patients.
Methods:
HIBISCUS-STROKE is a cohort study including anterior circulation AIS patients treated with mechanical thrombectomy following MRI. Ten circulating inflammatory markers were measured at admission and 6, 24, and 48 h after admission. LAE was defined as a left atrial volume index (LAVi) ≥34 ml/m2. A multiple logistic regression model was performed to detect an independent association between the area under the curve (AUC) of these markers and LAE.
Results:
We included 143 patients. Of them, 85 (59.4%) had LAE. On univariable analysis, we found that patients with LAE had higher soluble form suppression of tumorigenicity 2 (sST2), soluble tumor necrosis factor receptor I (sTNFR1), and vascular cellular adhesion molecule-1 (VCAM-1) AUC, were older, mostly female, had a higher National Institutes of Health Stroke Scale (NIHSS) score and blood glucose level at admission, had more often hypertension, and a cardioembolic source of AIS, such as atrial fibrillation, while they were less frequently current smokers and had a lower rate of tandem occlusion than patients without LAE. On multivariable analysis, we found that among circulating inflammatory markers, only high VCAM-1 (OR: 9.13, 95% CI: 3.21–25.9) and sST2 (OR: 3.40, 95% CI: 1.68–6.86) AUC remained associated with LAE.
Conclusions:
High VCAM-1 and sST2 levels within the first 48 h are associated with LAE in AIS patients.
Highlights
- •
Left atrial enlargement (LAE) was independently associated with higher vascular cellular adhesion molecule-1 (VCAM-1) and soluble form suppression of tumorigenicity 2 (sST2) within the first 48 h in acute ischemic stroke (AIS) patients.
- •
VCAM-1 levels were higher at baseline and remain increased within the first 48 h in AIS patients with LAE.
- •
sST2 levels were higher from 6 h after admission in AIS patients with LAE.
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and a major cause of stroke, heart failure, sudden death, and cardiovascular morbidity (1). Studies have found that left atrial enlargement (LAE) as a marker of atrial myopathy is associated with an increased risk of ischemic stroke and all-cause of death despite the controversy surrounding this (2, 3). The association between atrial myopathy and stroke may be unrelated to AF (4, 5). Experimental and clinical data indicate that inflammation is implicated in the pathophysiology of atrial remodeling. Inflammatory markers such as CRP, interleukin (IL)-6, -2, -8, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein (MCP-1), soluble form suppression of tumorigenicity 2 (sST2), vascular cellular adhesion molecule-1 (VCAM-1), and soluble intercellular adhesion molecule-1 (sICAM-1) have also been associated with AF, AF incidence, and recurrence (6–15). The inflammatory process is also accompanied by ischemia-reperfusion damage related to acute ischemic stroke (AIS) (16, 17). Indeed, ischemic insult elicits a strong neuroinflammatory response orchestrated by proinflammatory cytokines, which may offset the benefit of reperfusion (16, 18).
The question whether the inflammatory substrate underlying atrial myopathy may affect the overall inflammatory response in AIS has not yet been answered.
In the present study, we aimed to assess whether LAE is associated with a different profile of circulating inflammatory markers in AIS patients.
2. Methods
2.1. Study population
The design and methods of HIBISCUS-STROKE have been published previously (19). Briefly, from the year 2016, patients admitted to the Lyon Stroke Center for an anterior circulation AIS with large vessel occlusion (LVO) treated with mechanical thrombectomy (MT) using brain MRI were included in this cohort. Patients with active disease resulting in systemic inflammation were excluded. We collected peripheral blood samples from each patient at admission before performing any reperfusion therapy and at 6, 24, and 48 h after admission. Baseline data on medical history, risk factors, and demographic characteristics were collected at admission. Board-certified neurologists assessed the neurological status using the National Institutes of Health Stroke Scale (NIHSS) score at admission and the modified Rankin Scale (mRS) at 3 months during a face-to-face follow-up visit. Stroke subtype was classified using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria (20). The study was approved by the local ethics committee, and all subjects or their relatives signed an informed consent form (IRB number: 00009118).
2.2. Blood sampling protocol
Sera were prepared and stored at −80°C within a 3-h delay at the NeuroBioTec biobank (CRB-HCL: BB-0033-00046, France). All samples were thawed only once for study measurements. CRP, IL-6, IL-8, and IL-10 were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Affymetrix, eBioscience). Monocyte Chemoattractant Protein-1 (MCP-1), soluble tumor necrosis factor receptor I (sTNFR1), sST2, sP-selectin, matrix metalloproteinase-9 (MMP-9), and VCAM-1 were measured using the R&D systems ELISA kit (R&D Systems, Minneapolis, Minnesota).
2.3. Transthoracic echocardiography
Two-dimensional and 3D left atrial volume indices (LAVis) were assessed by transthoracic echocardiography. Left atrial enlargement (LAE) was defined as a LAVi ≥34 ml/m2.
2.4. Brain imaging
MRIs were conducted using 1.5-T Intera or 3-T Achieva scanners (Philips, Best, the Netherlands). The MRI protocol included fluid-attenuated inversion recovery (FLAIR), time-of-flight MR angiography (TOF-MRA), T2-gradient echo, and diffusion-weighted imaging (DWI) sequences. The DWI lesion was outlined using a semiautomated method (3D Slicer, https://www.slicer.org). The DWI-based Alberta Stroke Program Early CT Score (ASPECTS) was assessed (21). The Thrombolysis in Cerebral Infarction (TICI) score was used to assess angiographic reperfusion, and the treatment was considered successful if the score was 2b, 2c, or 3 (22). Hemorrhagic transformations were evaluated on day-1 CT using the European Co-operative Acute Stroke Study-II (ECASS II) classification (23). The follow-up MRI protocol at day 6 included FLAIR sequence.
2.5. Statistical analysis
Continuous variables are expressed as means [standard deviation (SD)] or medians [interquartile range (IQR)] depending on their distributions, and categorical variables are given as percentages. Medians were compared using the Mann–Whitney or Kruskall–Wallis test. Percentages were compared using Fisher's exact test. If data were missing for circulating inflammatory biomarkers, we employed imputation methods using the means between the previous and the next observation. If admission or 48 h data were missing, we performed the median slope to replace missing data. The normality of distributions was assessed graphically and by using the Shapiro–Wilk test. The association between each variable and LAE was measured by calculating the odds ratio (OR) and 95% confidence intervals (CI) using simple logistic regression. A multiple logistic regression model was then performed to assess the relationship between the area under the curve (AUC) of circulating inflammatory biomarkers within the first 48 h and LAE. Statistically significant covariates in univariate analyses (p < 0.05) that were supposed to be causal were implemented through a backward stepwise procedure with a removal criterion of p > 0.05. The multivariable logistic regression was, therefore, adjusted for age, sex, hypertension, current smoking, NIHSS score, glucose level on admission, atrial fibrillation, and tandem occlusion. Statistical testing used a two-tailed α level of 0.05. The data were analyzed using Stata Version 15™ (StataCorp, College Station, Texas 77845 USA) and GraphPad Prism (San Diego, California, USA).
3. Results
3.1. Study population
Among AIS patients treated with MT in our institution between October 2016 and April 2019, 143 were included (Figure 1). Excluded patients were older (71.6 ± 14.8 years vs. 68.1 ± 15.5 years, p = 0.016), were less likely male [234 (46.8%) vs. 88 (61.5%), p = 0.002], had a higher baseline National Institutes of Health Stroke Scale (NIHSS) score (16 [11–21] vs. 15 [9–19], p = 0.015), and were less likely to have an M1 segment middle cerebral artery [242 (48.4%) vs. 90 (62.9%), p = 0.003] or an intracranial internal carotid artery [140 (8.0%) vs. 27 (18.9%), p = 0.028] occlusion. The median LAVi was 36.9 [27.1–50.8] ml. Eighty-five (59.4%) patients had LAE. Patient characteristics are detailed in Table 1.
Figure 1

Study flowchart.
Table 1
| No LAE (n = 58) | LAE (n = 85) | p-value | |
|---|---|---|---|
| Age, years | 60.2 ± 15.4 | 73.5 ± 13.0 | <0.001 |
| Male | 42 (72.4) | 46 (54.1) | 0.04 |
| Hypertension | 20 (34.5) | 48 (56.5) | 0.01 |
| Diabetes | 8 (13.8) | 15 (17.7) | 0.65 |
| Hyperlipidemia | 12 (20.7) | 24 (28.2) | 0.33 |
| Current smoking | 20 (34.5) | 12 (14.1) | 0.01 |
| Coronary artery disease | 8 (13.8) | 15 (17.7) | 0.65 |
| NIHSS score | 13 [8–17] | 17 [11–20] | 0.03 |
| Glucose level, mmol/L | 5.86 [5.50–6.99] | 6.57 [5.72–8.20] | 0.01 |
| Atrial fibrillation | 7 (12.1) | 56 (65.9) | <0.001 |
| Etiology | <0.001 | ||
| Cardioembolism | 10 (17.2) | 65 (76.5) | |
| Large-artery atherosclerosis | 15 (25.9) | 6 (7.1) | |
| Other | 13 (22.4) | 2 (2.4) | |
| Undetermined | 20 (34.5) | 12 (14.1) | |
| Thrombus location | |||
| M1 MCA segment | 36 (62.1) | 54 (63.5) | 0.86 |
| M2 MCA segment | 11 (19.0) | 13 (15.3) | 0.65 |
| ICA terminus | 11 (19.0) | 16 (18.8) | 1.00 |
| Tandem occlusion | 18 (31.0) | 14 (16.5) | 0.04 |
| ASPECTS | 7 [6–8] | 7 [6–9] | 0.10 |
| Intravenous thrombolysis | 33 (56.9) | 41 (48.2) | 0.39 |
| Successful reperfusion | 43 (74.1) | 73 (85.9) | 0.09 |
| Stroke onset to groin puncture, minc | 216 [147–395] | 225 [165–373] | 0.91 |
| PH type 1 or 2 or SAH | 2 (3.5) | 5 (5.9) | 0.70 |
| LAVi, ml/m2 | 26.0 ± 4.8 | 51.4 ± 16.3 | <0.001 |
| LVEF, %d | 60.9 ± 5.5 | 56.5 ± 13.3 | 0.19 |
| LVEDVi, ml/m2e | 49.3 ± 11.9 | 54.2 ± 26.7 | 0.99 |
| IVS thickness, mmc | 10.2 ± 1.8 | 10.7 ± 2.5 | 0.27 |
Main characteristics of study patients according to the presence of left atrial enlargement (LAE).
NIHSS, National Institute of Health Stroke Scale; MCA, middle-cerebral-artery; ICA, internal carotid artery; ASPECTS, Alberta Stroke Program Early CT Score; PH, parenchymal hematoma; SAH, subarachnoid hemorrhage; LAVi, left atrial volume index; LVEF, left ventricular ejection fraction; LVEDVi, left ventricular end-diastolic volume index; IVS, interventricular septum. Variables are displayed as absolute number, mean ± SD, or median (25th–75th percentiles) as appropriate.
The bold values indicates a p value < 0.05.
Seventeen patients with missing data.
Thirty-five patients with missing data.
Fourteen patients with missing data.
Three with missing data.
Forty-seven with missing data.
3.2. Factors associated with LAE
On univariable analysis, patients with LAE were older, were mostly female, had a higher NIHSS score and blood glucose level at admission, had more frequent hypertension, and a cardioembolic source of AIS, such as AF, while they were less frequently current smokers and had a lower rate of tandem occlusion than patients without LAE (Figure 2). Patients with LAE had higher VCAM-1, sST2, and sTNFR1 AUC than patients without LAE (Table 2, Figure 3). VCAM-1 levels differed from admission and remained higher within the first 48 h in patients with LAE, whereas sST2 and sTNFR1 levels differed at 6 h and at 24 h, respectively (Figure 4). Following multivariable analysis, among circulating markers, only high VCAM-1 and sST2 AUC were associated with LAE, together with AF, in both models (Table 3).
Figure 2
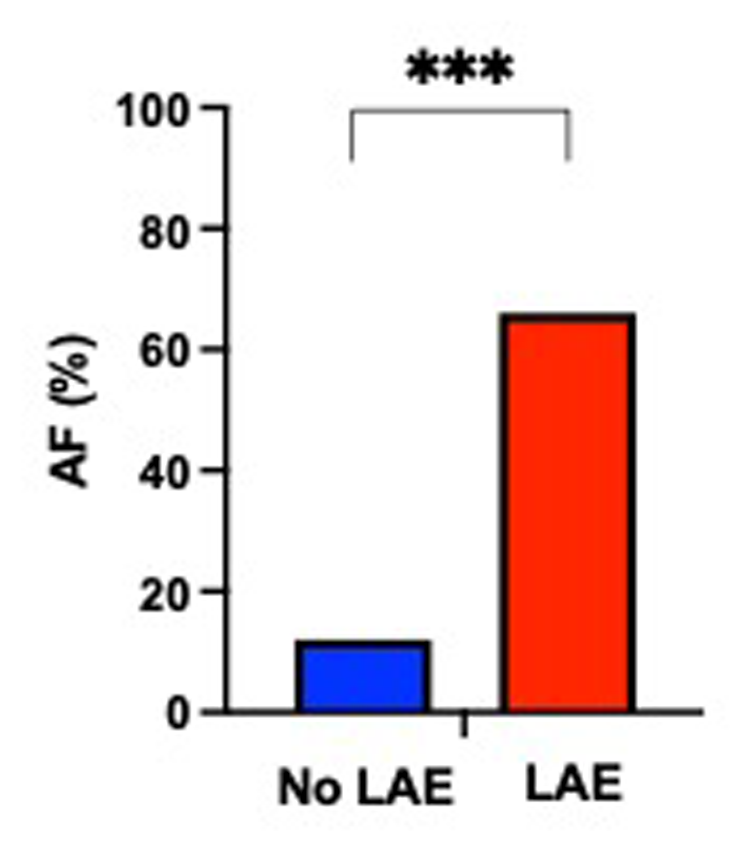
Proportion of patients with atrial fibrillation (AF) according to the presence of left atrial enlargement (LAE) (Fisher's exact test, ***p < 0.001).
Table 2
| AUC | No LAE (n = 58) | LAE (n = 85) | p-value |
|---|---|---|---|
| CRP (mg/L) | 1,189 [711–2,072] | 1,037 [736–1,436] | 0.23 |
| IL-6 (pg/ml) | 176.1 [82.1–288.1] | 189.8 [93.2–341.3] | 0.38 |
| IL-8 (pg/ml) | 67.5 [27.3–176.8] | 80.6 [41.3–201.4] | 0.35 |
| IL-10 (pg/ml) | 143.9 [79.1–227.6] | 158.2 [107.5–261.0] | 0.21 |
| MCP-1 (pg/ml) | 2,418 [1,340–3,063] | 2,475 [1,467–3,878] | 0.34 |
| sP-selectin (ng/ml) | 2,929 [2,395–3,894] | 2,817 [2,145–3,751] | 0.36 |
| sST2 (ng/ml) | 587.7 [396.0–952.4] | 840.1 [623.2–1,258.1] | 0.002 |
| sTNFR1 (pg/ml) | 24,392 [19,581–35,830] | 32,549 [22,980–50,060] | 0.01 |
| VCAM-1 (ng/ml) | 19,017 [15,031–22,409] | 27,087 [21,918–34,037] | <0.0001 |
| MMP-9 (ng/ml) | 38,866 [24,210–62,675] | 33,954 [26,271–48,071] | 0.27 |
Circulating inflammatory marker levels according to the presence of left atrial enlargement (LAE).
LAE, left atrial enlargement; AUC, area under the curve; CRP, C-reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; MCP-1, monocyte chemoattractant protein-1; sP-selectin, soluble P-selectin; sST2, soluble form suppression of tumorigenicity 2; sTNFR1, soluble tumor necrosis factor receptor I; VCAM-1, vascular cellular adhesion molecule-1; MMP-9, matrix metalloproteinase-9.
The bold values indicate a p-value < 0.05.
Figure 3
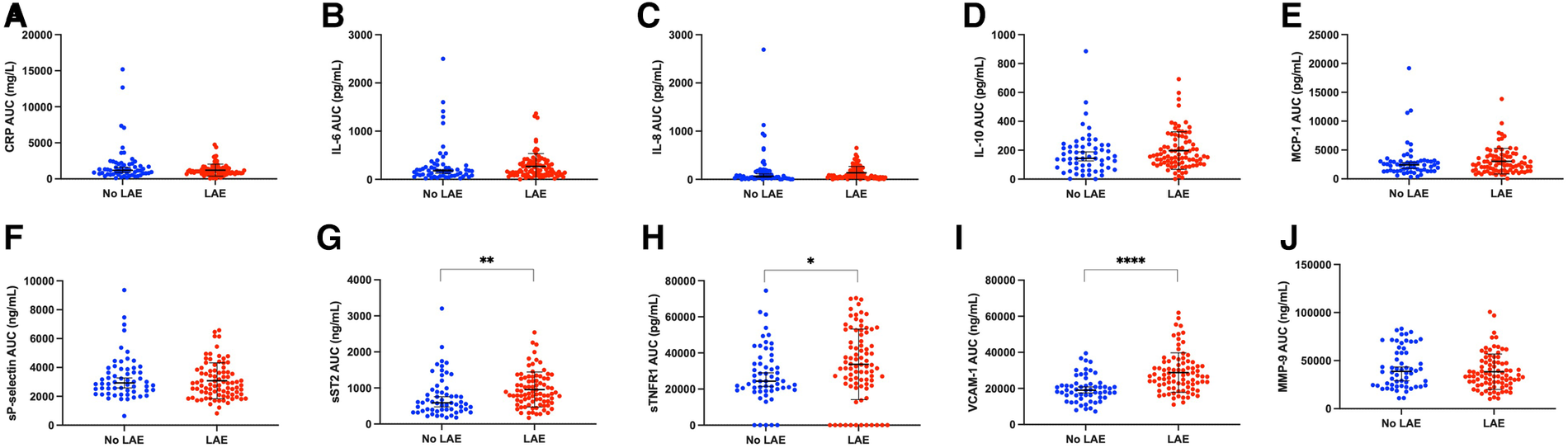
Scatter plot showing levels of circulating inflammatory markers according to the presence of left atrial enlargement (LAE): (A,C) reactive protein (CRP), (B) interleukin-6 (IL-6), (C) interleukin-8 (IL-8), (D) interleukin-10 (IL-10), (E) monocyte chemoattractant protein-1 (MCP-1), (F) soluble P-selectin (sP-selectin), (G) soluble form suppression of tumorigenicity 2 (sST2), (H) soluble tumor necrosis factor receptor I (sTNFR1), (I) vascular cellular adhesion molecule-1 (VCAM-1), (J) matrix metalloproteinase-9 [Whitney test, *p < 0.05, **p < 0.01, ****p < 0.0001, error bar (mean ± standard deviation) in black].
Figure 4
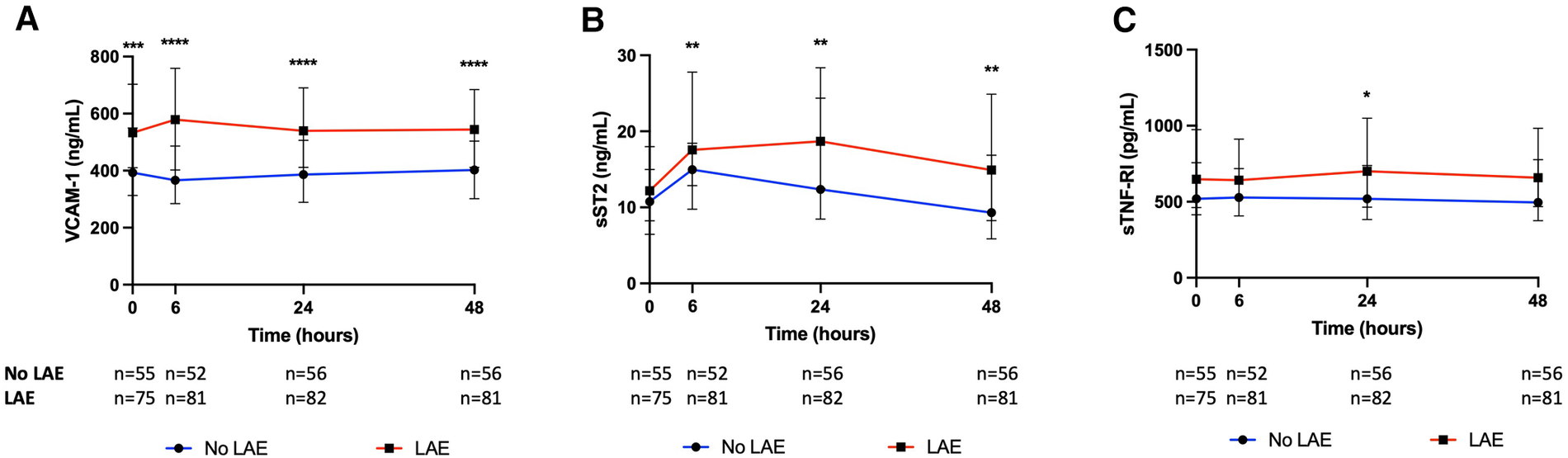
Kinetics of (A) vascular cellular adhesion molecule-1 (VCAM-1), (B) soluble form suppression of tumorigenicity 2 (sST2), and (C) soluble tumor necrosis factor receptor I (sTNFR1) according to the presence of left atrial enlargement (LAE) (Mann–Whitney test, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Table 3
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Multivariable analysis including VCAM-1 AUC | ||
| High VCAM-1 AUC | 9.13 (3.21–25.95) | <0.001 |
| Agea | 1.21 (0.83–1.77) | 0.31 |
| Male | 0.52 (0.19–1.42) | 0.20 |
| Hypertension | 2.43 (0.85–6.95) | 0.10 |
| NIHSS score | 1.06 (0.97–1.15) | 0.20 |
| Glucose level at admissionb | 1.18 (0.84–1.66) | 0.34 |
| Atrial fibrillation | 7.47 (2.37–23.60) | 0.001 |
| Tandem occlusion | 0.38 (0.11–1.28) | 0.12 |
| Multivariable analysis including sST2 AUC | ||
| High sST2 AUC | 3.40 (1.68–6.86) | 0.001 |
| Agea | 1.90 (1.46–2.48) | <0.001 |
| Male | 0.45 (0.22–0.92) | 0.03 |
| Hypertension | 2.46 (1.24–4.92) | 0.01 |
| Current smoking | 0.31 (0.14–0.71) | 0.01 |
| Glucose level at admissionb | 1.37 (1.07–1.75) | 0.01 |
| Atrial fibrillation | 14.07 (5.67–34.90) | <0.001 |
| Tandem occlusion | 0.44 (0.20–0.97) | 0.04 |
Factors associated with left atrial enlargement (LAE).
CI, confidence interval; VCAM-1, vascular cellular adhesion molecule-1; AUC, area under the curve; sST2, soluble form suppression of tumorigenicity 2.
Multivariable model including VCAM-1 AUC along with age, sex, hypertension, NIHSS score, glucose level on admission, atrial fibrillation, and tandem occlusion (current smoking not retained by the backward selection).
Multivariable model including sST2 AUC along with age, sex, hypertension, current smoking, glucose level on admission, atrial fibrillation, and tandem occlusion (NIHSS score not retained by the backward selection).
The bold values indicates a p value < 0.05.
Per 10-year increase.
Per a 1-mmol/L increase.
4. Discussion
Several studies have shown that the level of systemic markers of inflammation correlated with left atrial volume. However, the impact of the inflammatory substrate underlying atrial myopathy on the overall inflammatory response promoted by AIS remains poorly documented. We demonstrated that VCAM-1 and sST2 levels were higher within the first 48 in AIS patients with LAE (Figure 5).
Figure 5
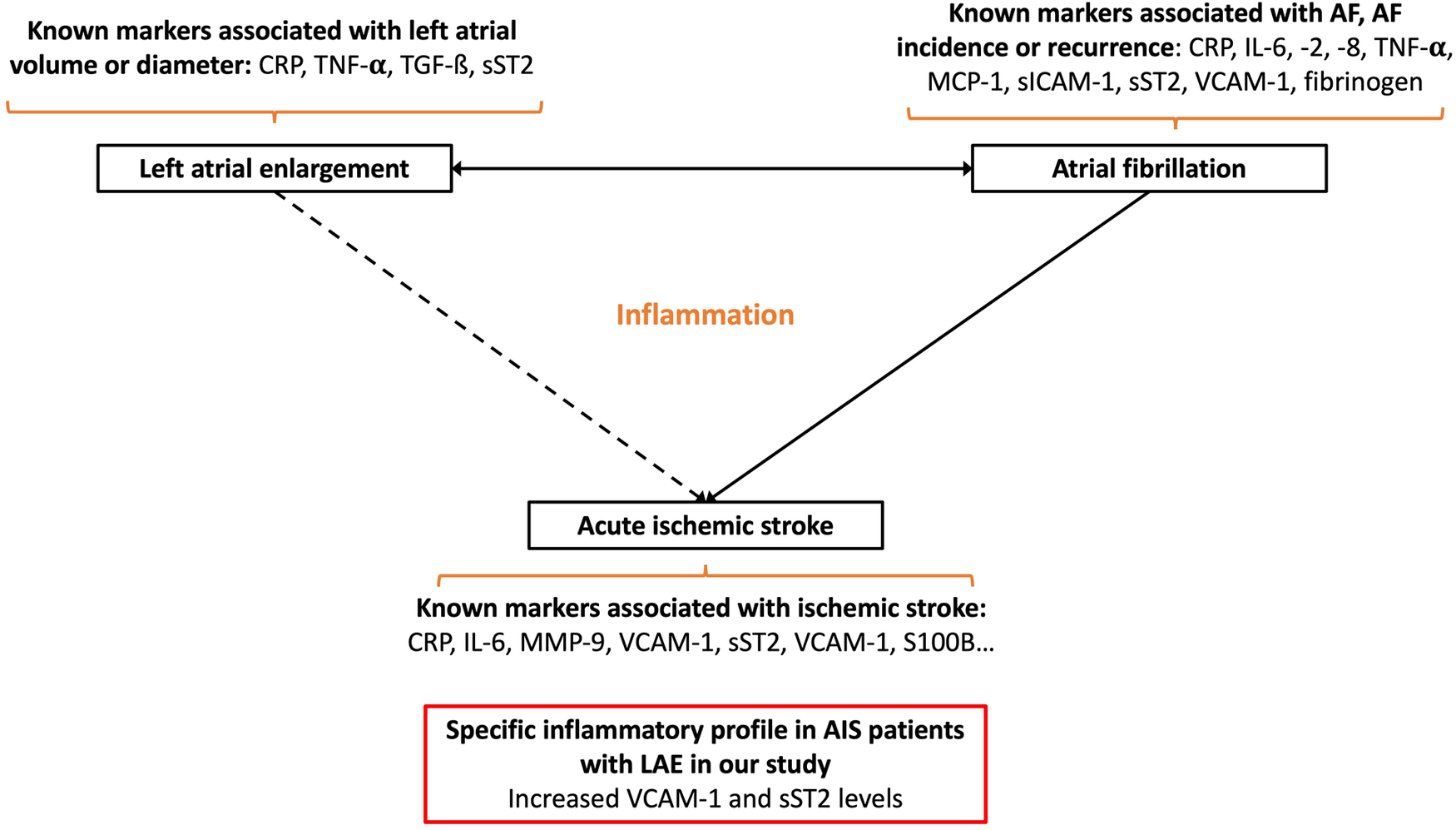
Schematic summarizing the main link between atrial fibrillation, left atrial enlargement, and acute ischemic stroke: known circulating inflammatory markers associated with these conditions and the main results of our study (CRP, C-reactive protein; sICAM-1, soluble intercellular adhesion molecule-1; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; MMP-9, matrix metalloproteinase-9; MCP-1, monocyte chemoattractant protein-1; S100B, S100 calcium binding protein B; sP-selectin, soluble P-selectin; sST2, soluble form suppression of tumorigenicity 2; sTNFR1, soluble tumor necrosis factor receptor I; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cellular adhesion molecule-1).
Higher baseline and persistently increased VCAM-1 levels in patients with LAE argue in favor of a chronic condition linked to LAE. VCAM-1 is involved in post-ischemic neuroinflammation as it permits extravasation of leukocytes through the vascular wall into brain tissue (24). Both systemic and intracranial levels of VCAM-1 during MT are associated with increased infarct and edema volumes and a worsened prognosis (25). Previous studies have also reported a link between VCAM-1 expression and AF. Two population-based cohort studies have shown an association between VCAM-1 levels and AF incidence (13, 14). In addition, patients with AF had higher VCAM-1 levels than those in sinus rhythm (26). Experimental studies have shown that rapid atrial pacing increases endocardial VCAM-1 expression, demonstrating that AF itself increases VCAM-1 expression (27).
With regard to sST2, higher levels from 6 h after admission in patients with LAE may suggest a differential postischemic inflammatory response. sST2 acts as a decoy receptor of IL-33 and therefore inhibits the M2 polarization effect of IL-33/ST2 signaling on microglial cells and macrophages (28). sST2 is associated with an expansion of the lesion within penumbra in AIS patients treated with MT and also with functional outcome and death (19, 29, 30). sST2 levels, which have proven their positive correlation with the left ventricular diameter and ejection fraction, and their diagnostic and prognostic value in heart failure, are also higher in patients with AF and are correlated with the left atrial diameter (15, 31). Our results would be helpful in further studies when interpreting the circulating levels of inflammatory markers in AIS patients.
Apart from circulating inflammatory markers, multivariable analysis demonstrated an association between LAE and higher age, female sex, hypertension, lack of current smoking, atrial fibrillation, higher glucose level on admission, and lack of tandem occlusion. These results are in line with previous studies conducted on non-stroke patients except for no current smoking and tandem occlusion (32–35). The latter two factors are more likely associated with large-artery atherosclerosis than with AF, which may explain why they are less likely associated with LAE in this specific population of AIS patients.
The strength of our study lies in its originality and the quality of the data based on a well-characterized and homogeneous cohort, which benefited from a sequential assessment of circulating inflammatory markers and MRI. Our study has some limitations, such as sample size, a monocentric design, and a narrow panel of markers. Indeed, other markers of endothelial dysfunction, such as von Willebrand's factor and soluble thrombomodulin, which correlate with left atrial volume in patients with lone permanent non-rheumatic AF, might have been of greater interest (36).
5. Summary/conclusion
These results call for a more differentiated analysis of the inflammatory response in AIS depending on the presence of atrial myopathy. Future studies should clarify whether this specific inflammatory profile affects the severity and ischemic recurrence in AIS patients with LAE.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the local ethics committee IRB 00009118. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the RHU MARVELOUS (ANR-16-RHUS-0009) of Université de Lyon, within the program “Investissements d'Avenir” operated by the French National Research Agency.
Acknowledgments
Human biological samples and associated data were obtained from NeuroBioTec (CRB HCL, Lyon France, BiobankBB-0033-00046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
WolfPAAbbottRDKannelWB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. (1991) 22(8):983–8. 10.1161/01.STR.22.8.983
2.
GardnerJDSkeltonWPKhouzamRN. Is it time to incorporate the left atrial size to the current stroke risk scoring systems for atrial fibrillation?Curr Probl Cardiol. (2016) 41(9–10):251–9. 10.1016/j.cpcardiol.2016.10.004
3.
BitekerMKayataşKBaşaranÖDoganVÖzlekEÖzlekB. The role of left atrial volume index in patients with a first-ever acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26(2):321–6. 10.1016/j.jstrokecerebrovasdis.2016.09.023
4.
HamataniYOgawaHTakabayashiKYamashitaYTakagiDEsatoMet alLeft atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. (2016) 6(1):31042. 10.1038/srep31042
5.
BarnesMEMiyasakaYSewardJBGershBJRosalesAGBaileyKRet alLeft atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. (2004) 79(8):1008–14. 10.4065/79.8.1008
6.
HammwöhnerMIttensonADierkesJBukowskaAKleinHULendeckelUet alPlatelet expression of CD40/CD40 ligand and its relation to inflammatory markers and adhesion molecules in patients with atrial fibrillation. Exp Biol Med (Maywood). (2007) 232(4):581–9.
7.
MarottSCWNordestgaardBGZachoJFribergJJensenGBTybjærg-HansenAet alDoes elevated C-reactive protein increase atrial fibrillation risk?J Am Coll Cardiol. (2010) 56(10):789–95. 10.1016/j.jacc.2010.02.066
8.
AvilesRJMartinDOApperson-HansenCHoughtalingPLRautaharjuPKronmalRAet alInflammation as a risk factor for atrial fibrillation. Circulation. (2003) 108(24):3006–10. 10.1161/01.CIR.0000103131.70301.4F
9.
LiuTLiGLiLKorantzopoulosP. Association between C-reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion. J Am Coll Cardiol. (2007) 49(15):1642–8. 10.1016/j.jacc.2006.12.042
10.
AmdurRLMukherjeeMGoABarrowsIRRamezaniAShojiJet alInterleukin-6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC study. Aguilera AI, editor. PLoS One. (2016) 11(2):e0148189. 10.1371/journal.pone.0148189
11.
LiJSolusJChenQRhoYHMilneGSteinCMet alRole of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. (2010) 7(4):438–44. 10.1016/j.hrthm.2009.12.009
12.
ConenDRidkerPMEverettBMTedrowUBRoseLCookNRet alA multimarker approach to assess the influence of inflammation on the incidence of atrial fibrillation in women. Eur Heart J. (2010) 31(14):1730–6. 10.1093/eurheartj/ehq146
13.
MendezIJManemannSMBellEJLarsonNBDeckerPAGuerreroMAet alAdhesion pathway proteins and risk of atrial fibrillation in the multi-ethnic study of atherosclerosis. BMC Cardiovasc Disord. (2021) 21(1):436. 10.1186/s12872-021-02241-w
14.
WilleitKPechlanerRWilleitPSkroblinPPaulweberBSchernthanerCet alAssociation between vascular cell adhesion molecule 1 and atrial fibrillation. JAMA Cardiol. (2017) 2(5):516. 10.1001/jamacardio.2017.0064
15.
ChenCQuXGaoZZhengGWangYChenXet alSoluble ST2 in patients with nonvalvular atrial fibrillation and prediction of heart failure. Int Heart J. (2018) 59(1):58–63. 10.1536/ihj.16-520
16.
ChamorroÁMeiselAPlanasAMUrraXvan de BeekDVeltkampR. The immunology of acute stroke. Nat Rev Neurol. (2012) 8(7):401–10. 10.1038/nrneurol.2012.98
17.
AcevedoMCorbalánRBraunSPereiraJGonzálezINavarreteC. Biochemical predictors of cardiac rhythm at 1 year follow-up in patients with non-valvular atrial fibrillation. J Thromb Thrombolysis. (2012) 33(4):383–8. 10.1007/s11239-012-0690-1
18.
DirnaglUIadecolaCMoskowitzMA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. (1999) 22(9):391–7. 10.1016/S0166-2236(99)01401-0
19.
MechtouffLPaccaletACrola Da SilvaCBuissonMMewtonNAmazCet alPrognosis value of serum soluble ST2 level in acute ischemic stroke and STEMI patients in the era of mechanical reperfusion therapy. J Neurol. (2022) 269(5):2641–8. Available at:https://link.springer.com/10.1007/s00415-021-10865-3(Cited October 29, 2021). 10.1007/s00415-021-10865-3
20.
AdamsHPBendixenBHKappelleLJBillerJLoveBBGordonDLet alClassification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24(1):35–41. 10.1161/01.STR.24.1.35
21.
BarberPADemchukAMZhangJBuchanAM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. (2000) 355(9216):1670–4. 10.1016/S0140-6736(00)02237-6
22.
HigashidaRTFurlanAJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003) 34(8):109–37. 10.1161/01.STR.0000082721.62796.09
23.
HackeWKasteMFieschiCvon KummerRDavalosAMeierDet alRandomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. (1998) 352(9136):1245–51. 10.1016/S0140-6736(98)08020-9
24.
KongDHKimYKimMJangJLeeS. Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. IJMS. (2018) 19(4):1057. 10.3390/ijms19041057
25.
MaglingerBSandsMFrankJAMcLouthCJTroutALRobertsJMet alIntracranial VCAM1 at time of mechanical thrombectomy predicts ischemic stroke severity. J Neuroinflammation. (2021) 18(1):109. 10.1186/s12974-021-02157-4
26.
GoetteABukowskaALendeckelUErxlebenMHammwöhnerMStrugalaDet alAngiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation. (2008) 117(6):732–42. 10.1161/CIRCULATIONAHA.107.730101
27.
GoetteABukowskaALendeckelUErxlebenMHammwöhnerMStrugalaDet alAngiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation. (2008) 117(6):732–42. 10.1161/CIRCULATIONAHA.107.730101
28.
XieDLiuHXuFSuWYeQYuFet alIL33 (interleukin 33)/ST2 (interleukin 1 receptor-like 1) axis drives protective microglial responses and promotes white matter integrity after stroke. Stroke. (2021) 52(6):2150–61. 10.1161/STROKEAHA.120.032444
29.
MechtouffLDebsNFrindelCBani-SadrABochatonTPaccaletAet alAssociation of blood biomarkers of inflammation with penumbra consumption after mechanical thrombectomy in acute ischemic stroke patients. Neurology. (2022). 10.1212/WNL.0000000000201038
30.
KorhonenPKanninenKMLehtonenŠLemarchantSPuttonenKAOksanenMet alImmunomodulation by interleukin-33 is protective in stroke through modulation of inflammation. Brain Behav Immun. (2015) 49:322–36. 10.1016/j.bbi.2015.06.013
31.
ChowSLMaiselASAnandIBozkurtBde BoerRAFelkerGMet alRole of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American Heart Association. Circulation. (2017) 135(22):1054–91. Available at:https://www.ahajournals.org/doi/10.1161/CIR.0000000000000490(Cited January 7, 2022). 10.1161/CIR.0000000000000490
32.
PsatyBMManolioTAKullerLHKronmalRACushmanMFriedLPet alIncidence of and risk factors for atrial fibrillation in older adults. Circulation. (1997) 96(7):2455–61. 10.1161/01.CIR.96.7.2455
33.
KatayamaTFujiwaraNTsuruyaY. Factors contributing to left atrial enlargement in adults with normal left ventricular systolic function. J Cardiol. (2010) 55(2):196–204. 10.1016/j.jjcc.2009.10.008
34.
SuGCaoHXuSLuYShuaiXSunYet alLeft atrial enlargement in the early stage of hypertensive heart disease: a common but ignored condition. J Clin Hypertens. (2014) 16(3):192–7. 10.1111/jch.12282
35.
AtasHKepezAAtasDBKanarBGDervisovaRKivrakTet alEffects of diabetes mellitus on left atrial volume and functions in normotensive patients without symptomatic cardiovascular disease. J Diabetes Complicat. (2014) 28(6):858–62. 10.1016/j.jdiacomp.2014.07.010
36.
MondilloSSabatiniLAgricolaEAmmaturoTGuerriniFBarbatiRet alCorrelation between left atrial size, prothrombotic state and markers of endothelial dysfunction in patients with lone chronic nonrheumatic atrial fibrillation. Int J Cardiol. (2000) 75(2–3):227–32. 10.1016/S0167-5273(00)00336-3
Summary
Keywords
inflammatory, left atrial enlargement, ischemic stroke, VCAM-1, SST2
Citation
Fontaine J, Leboube S, Bochaton T, Thibault H, Amaz C, Cho T-H, Paccalet A, Crola Da Silva C, Duhamel S, Buisson M, Rascle L, Bidaux G, Ovize M, Nighoghossian N and Mechtouff L (2023) Specific inflammatory profile of acute ischemic stroke patients with left atrial enlargement. Front. Cardiovasc. Med. 10:1190857. doi: 10.3389/fcvm.2023.1190857
Received
21 March 2023
Accepted
22 May 2023
Published
19 July 2023
Volume
10 - 2023
Edited by
Carole Sudre, University College London, United Kingdom
Reviewed by
Xiang Gu, Yangzhou University, China Roddy Hiram, Université de Montréal, Canada
Updates
Copyright
© 2023 Fontaine, Leboube, Bochaton, Thibault, Amaz, Cho, Paccalet, Crola Da Silva, Duhamel, Buisson, Rascle, Bidaux, Ovize, Nighoghossian and Mechtouff.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mechtouff laura.mechtouff@chu-lyon.fr
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.