- 1Department of Cardiology, Shandong Provincial Hospital, Shandong University, Jinan, China
- 2JiNan Key Laboratory of Cardiovascular Disease, Jinan, China
- 3Department of Biostatistics, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 4Institute for Medical Dataology, Cheeloo College of Medicine, Shandong University, Jinan, China
- 5Department of Cardiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: Hypertrophic cardiomyopathy (HCM) is the most common genetic heart disease. The purpose of this study was to evaluate the efficacy and safety of several medications and recommend better drug treatments for adults with HCM.
Methods: A review of PubMed, Embase, the Cochrane Controlled Register of Trials (CENTRAL), ClinicalTrials.gov and CNKI databases was conducted for studies on the efficacy and safety of drugs for adults with HCM. A frequentist random effects model was used in this network analysis.
Results: This network meta-analysis included 7 studies assessing seven medications, 6 studies evaluating monotherapy and 1 study evaluating combination therapy. Based on the network meta-analysis results, xiaoxinbi formula plus metoprolol (MD −56.50% [−72.43%, −40.57%]), metoprolol (MD −47.00% [−59.07%, −34.93%]) and mavacamten (MD −34.50% [−44.75%, −24.25%]) significantly reduced the resting left ventricular outflow tract gradient (LVOTG) in comparison with placebo. Resting LVOTG could also be reduced with N-acetylcysteine (NAC). The incidence of adverse drug reactions was not significantly different between the placebo group and the treatment group.
Conclusion: For adults with HCM, the top 4 treatments included xiaoxinbi formula plus metoprolol, metoprolol, mavacamten and NAC.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=374222], identifier [CRD42022374222].
1. Background
Hypertrophic cardiomyopathy (HCM) is a genetic disease which affects cardiac myocytes. HCM is characterized by cardiac hypertrophy unrelated to loading conditions, with a nondilated left ventricle and a normal or elevated ejection fraction (1). Over 1,400 mutations have been found in eleven genes that encode cardiac sarcomere proteins (2). Numerous previous studies have shown that approximately one in 500 adult subjects worldwide suffers from HCM (3, 4). Men are more likely to be affected than women, with a prevalence of approximately 60%. Even so, the mortality in females is higher than that in males (5). Importantly, HCM can result in sudden arrhythmic death, heart failure, and atrial fibrillation (AF) (6).
HCM can be treated with pharmacologic therapy, lifestyle intervention, surgical treatment and gene therapy. Currently, drug treatment mainly consists of beta-blockers, non-dihydropyridine calcium receptor antagonists, cardiac myosin inhibitors, ion channel inhibitors, angiotensin II receptor blockers and disopyramide (7). However, all of these drug treatments have side effects. As one of the most commonly used and effective drugs, non-vasodilating beta-blockers effectively reduce left ventricular outflow tract (LVOT) obstruction (LVOTO) and prolong diastole (8). If a patient has an intolerance or a contraindication to beta-blockers, then non-dihydropyridine calcium channel blockers may be a good alternative. As recommended by the guidelines, beta-blockers, non-dihydropyridine calcium channel blockers and disopyramide are considered drugs that reduce LVOTO and improve overall heart function (9). Through ion exchange, HCM produces enhanced late sodium current (INaL) activity owing to enzyme-mediated sodium-channel phosphorylation, which increases intracellular sodium (Na+) as well as calcium (Ca2+) overload (10). There may be an underlying mechanism for abnormal muscle contractions and diastolic dysfunction caused by prolonged intracellular Ca2+ transients and higher diastolic [Ca2+]i. And increased arrhythmogenicity can be attributed to longer action potentials and greater incidences of early afterdepolarizations (EADs) and delayed afterdepolarizations (DADs) (11). In theory, ion channel inhibitors, including ranolazine and eleclazine could counteract diastolic dysfunction, stimulation of microvascular function, and myocardial relaxation by inhibiting late sodium current activity (10). Studies on sarcomeric HCM mice have shown that hypertrophy and fibrosis are largely mediated by transforming growth factor-beta (TGF-b) (12). Angiotensin II receptor blockers (ARBs) are known to inhibit TGF-b activation and may slow the progression of HCM or even prevent its occurrence, according to a recent clinical trial (13). Recently, a new specific therapy, cardiac myosin inhibitors, has emerged for the treatment of HCM. Mavacamten (MYK-461) reduces cardiac contractility by reversible, selective inhibition of myosin ATPase (14). In the present study, mavacamten resulted in a greater increase in peak oxygen consumption (pVO2) (+1.4 ml/kg per min, p = 0.0006), greater reductions in post-exercise left ventricular outflow tract gradient (LVOTG) (−36 mmHg, p < 0.0001), and similar safety results compared to placebo (15). Surgical intervention may be required for patients who retain symptoms following guideline-guided management and treatment, including myectomy, septal ablation and cardiac resynchronization therapy. As these procedures are invasive, they need technical experience to be performed adequately (9, 16).
Since surgery is a complicated procedure, patients are more likely to be treated with medications to alleviate their symptoms than with surgery. Considering the increasing number of adults who have HCM, more effective medications should be given to reduce LVOTG, with fewer adverse reactions and complications. Thus, the aim of this systematic review and network meta-analysis was to provide an overview of the most commonly prescribed medications for the treatment of adults with HCM and provide suggestions for additional pharmaceutical management by assessing the safety and efficacy of several medications.
2. Material and methods
2.1. Search strategy
Our protocol was registered in PROSPERO (CRD42022374222), and our processes and outcomes were reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses extension statement for network meta-analyses) guidelines. A comprehensive search of the PubMed, Embase, Cochrane Library, ClinicalTrials.gov and CNKI databases was performed without language limitations from the date of inception until November 2022. After searching all randomized clinical trial (RCT) studies under the title of hypertrophic cardiomyopathy, studies were selected based on inclusion and exclusion criteria.
2.2. Selection criteria and eligibility criteria
The inclusion criteria were as follows: (1) Research type: RCTs were analyzed; (2) Population: studies enrolling participants with HCM aged 18 years or older were included; (3) Intervention: studies that compared at least two different medications (including placebo) were considered. Intervention could be a single medication or a combination of medications; (4) Comparisons: a placebo or other therapeutic agent. Direct and indirect comparisons formed a network between drugs; (5) Outcomes: studies were included if they reported changes in resting LVOTG compared with control groups after intervention. As part of the safety evaluation, the incidence of drug-related adverse events was also assessed.
Studies were independently searched and screened by two researchers (YM and WY). A discussion with another reviewer (HL) was held to resolve any disagreements.
2.3. Data extraction
For each of the included studies, the following variables were independently extracted by two investigators (Keying Mi and Sijia Wu): research characteristics (published year, country, duration of medication), research type (randomized controlled trials), participant characteristics (sample size, HCM diagnostic criteria, demographics), and outcomes (change in resting LVOTG from baseline, incidence of adverse events). For any disagreements, the reviewers discussed or consulted with a third reviewer (Jiangbing Li).
2.4. Risk of bias assessment
Using the risk of bias assessment tool from the Cochrane Collaboration, two researchers (Keying Mi and Sijia Wu) independently evaluated the included studies' risk of bias, taking into account factors such as random sequence generation, allocation concealment, blinding, missing outcome data and selective reporting of outcomes (17). Each domain was assigned a risk of bias rating of low, unclear, or high. A third reviewer (Jiangbing Li) resolved any disagreements.
2.5. Data synthesis
Using a frequentist network meta-analysis, the MD and 95% confidence intervals (CIs) were calculated for changes in resting LVOTG levels and the odds ratio (OR) and its 95% CIs for dichotomous outcomes. Cochran's Q statistic was examined as a method to quantify heterogeneity. The model to be used was determined based on Cochran's Q statistic. Due to the consistency between the fixed effects model and random effects model results, this analysis was conducted with a random effects model. In this study, our network consistency was evaluated both locally by comparing direct and indirect evidence, as well as globally by using the model of design-by-treatment interaction (18, 19). P-scores were based solely on network meta-analysis point estimates and standard errors were used to rank treatments. An average of all competitive treatments was used to determine whether one treatment was superior to another (20).
A subgroup analysis was also planned. Obstructive hypertrophic cardiomyopathy and non-obstructive hypertrophic cardiomyopathy are two types of HCM. For the subgroup analysis, all treatments were divided into an obstructive hypertrophic cardiomyopathy group and a non-obstructive HCM group, with the results compared to the previous results to determine consistency.
3. Results
3.1. Baseline characteristics
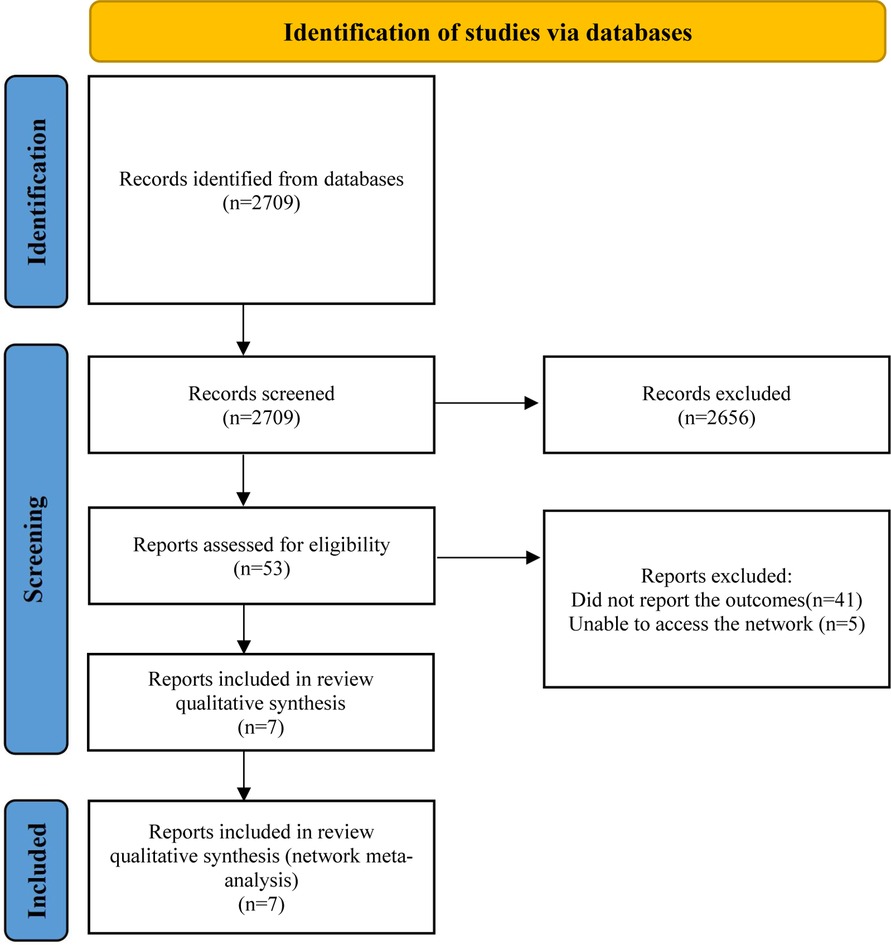
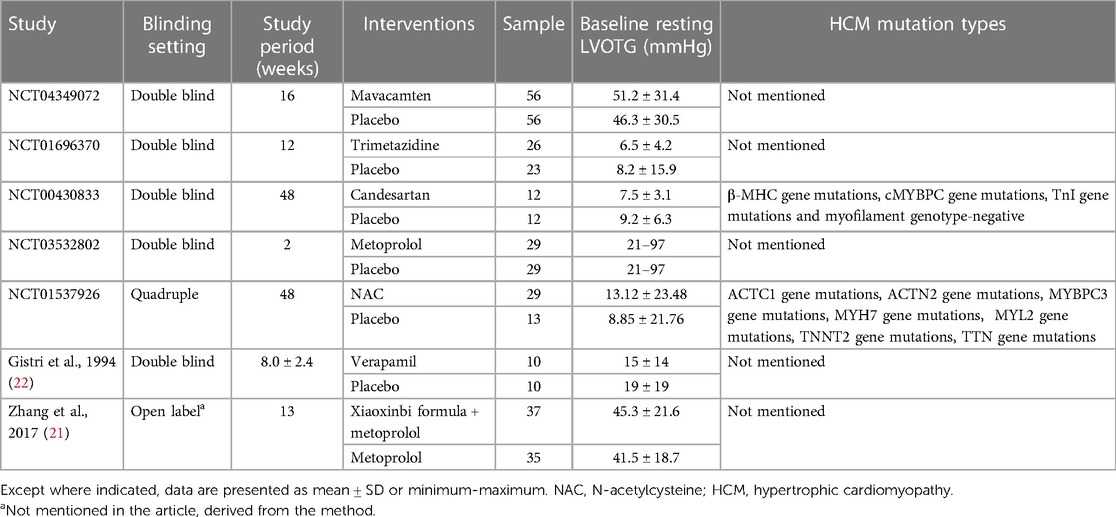
This systematic review and network meta-analysis included seven studies (377 patients), consisting of two published papers (21, 22) and five clinical trials (NCT04349072, NCT01696370, NCT00430833, NCT03532802, NCT01537926). A detailed description of the selection process is presented in Figure 1. Table 1 summarizes the characteristics of the included studies. There was less than a 12-month study period in all studies. Among the studies, five were double-blinded, one was quadruple-blinded and one may be open-label. In total, these studies examined seven drugs or drug combinations for the treatment of HCM, including mavacamten, trimetazidine, candesartan, metoprolol, N-acetylcysteine (NAC), verapamil, xiaoxinbi formula plus metoprolol.
3.2. Risk of bias assessment
Supplementary Figure S1 features an assessment of the risk of bias in the included studies. The biases of all included studies [(21, 22), NCT04349072, NCT01696370, NCT00430833, NCT03532802] were assessed as low risk.
3.3. Network meta-analysis
In our network meta-analysis, medications (mavacamten, trimetazidine, candesartan, metoprolol, NAC, verapamil, xiaoxinbi formula) and their combinations were evaluated (Figure 2). A random effects model was applied in this meta-analysis.
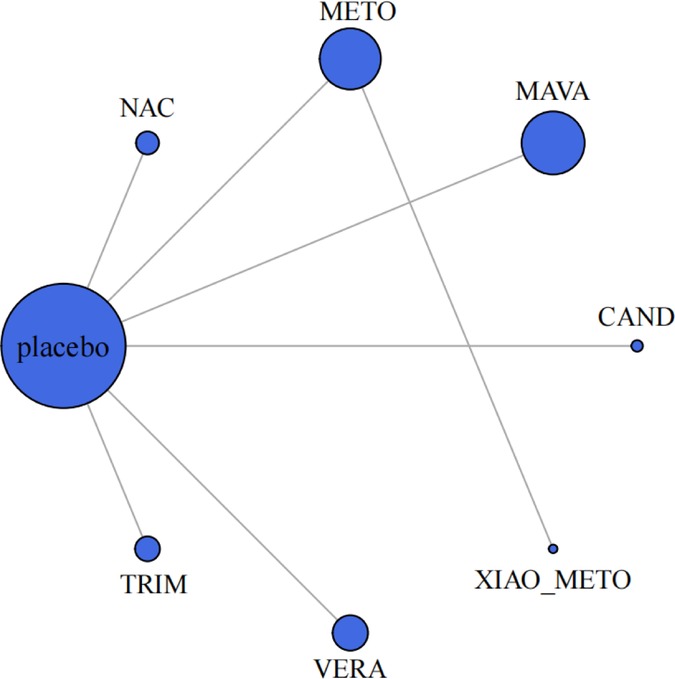
Figure 2. Meta-analysis networks for change in resting LVOTG level. Each circle indicates a treatment node. Lines connecting 2 nodes represent direct comparisons between 2 treatments. The size of the nodes is proportional to the number of trials evaluating each treatment. The thickness of the lines is proportional to the number of trials directly comparing the 2 connected treatments. NAC, N-Acetylcysteine; METO, metoprolol; MAVA, mavacamten; CAND, candesartan; XIAO_METO, xiaoxinbi formula + metoprolol; VERA, verapamil; TRIM, trimetazidine.
3.3.1. Primary outcomes
A total of 377 patients from seven studies were studied for changes in resting LVOTG from baseline. Based on a random effects model, compared with placebo, the diminution of resting LVOTG was obviously larger in xiaoxinbi formula + metoprolol (MD −56.50% [−72.43%, −40.57%]), metoprolol (MD −47.00% [−59.07%, −34.93%]), mavacamten (MD −34.50% [−44.75%, −24.25%]), NAC (MD −4.25% [−29.47%, 20.97%]), respectively (Figure 3A). Compared to placebo, in this model, three drugs did not show significant differences in resting LVOTG changes from baseline, including candesartan (MD 1.30% [−4.78%, 7.38%]), trimetazidine (MD 2.20% [−4.70%, 9.10%]) and verapamil (MD 7.00% [−11.03%, 25.03%]).
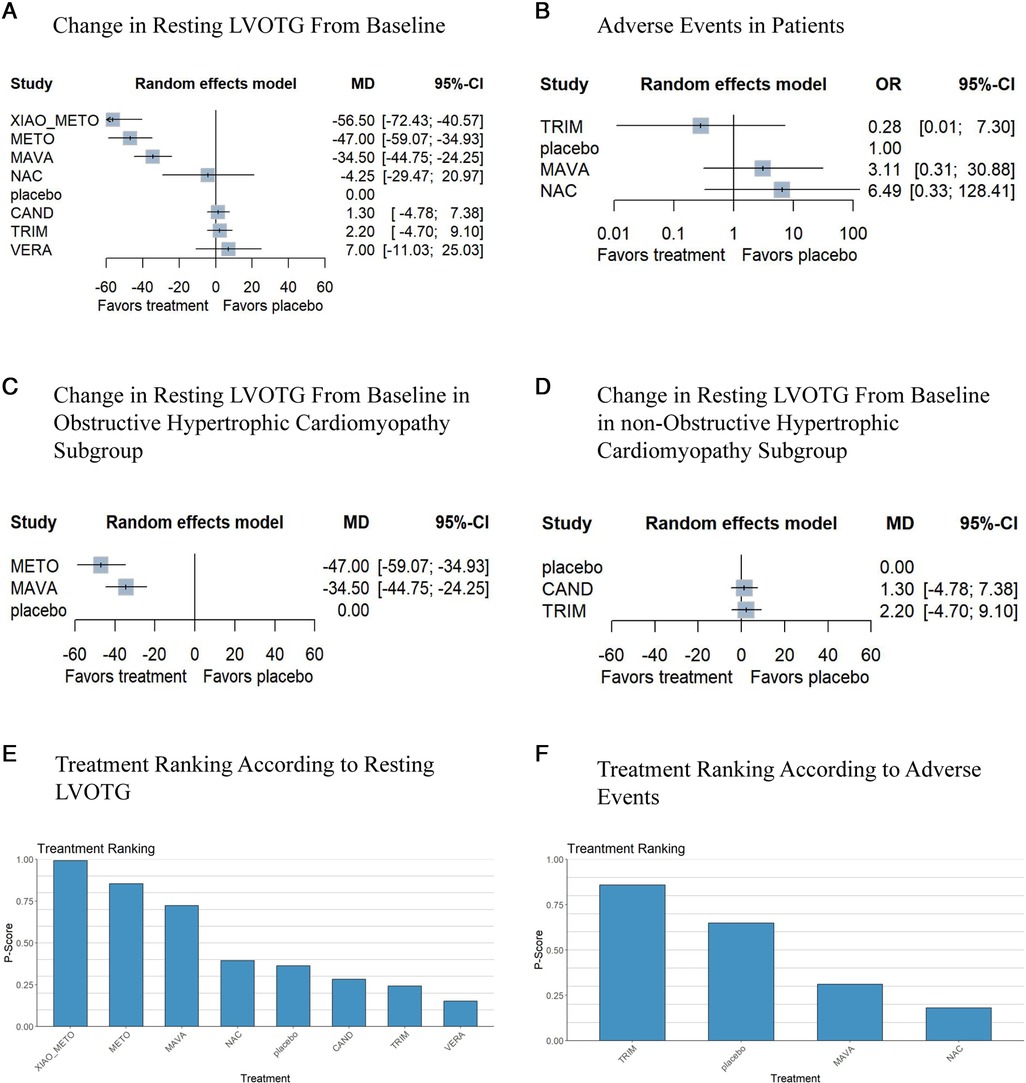
Figure 3. Network meta-analysis results for the primary outcomes compared with placebo. Treatments are presented according to their effect estimate compared with a placebo. Effects sizes are presented as MD or OR with 95% CIs. MD, mean difference; OR, odds ratio; NAC, N-Acetylcysteine; METO, metoprolol; MAVA, mavacamten; CAND, candesartan; XIAO_METO, xiaoxinbi formula + metoprolol; VERA, verapamil; TRIM, trimetazidine. (A) the change in resting LVOTG from the baseline of the random effects model; (B) adverse events of patients of the random effects model; (C) the change in resting LVOTG from the baseline of the random effects model in obstructive hypertrophic cardiomyopathy subgroup; (D) the change in resting LVOTG from baseline of random effects model in non-obstructive hypertrophic cardiomyopathy subgroup; (E) treatment ranking according to resting LVOTG of random effects model; (F) treatment ranking according to adverse events of random effects model.
In addition, xiaoxinbi formula + metoprolol was the most promising intervention for decreasing resting LVOTG, followed by metoprolol, and mavacamten (Figure 3E).
Based on the available three studies, three drugs were compared in terms of adverse events (Figure 3B). In comparison with placebo, trimetazidine, mavacamten and NAC showed no significant differences in the occurrence of adverse events (Figure 3F).
3.3.2. Secondary outcomes
3.3.2.1. Efficacy outcomes
The resting LVOTG of patients was reported to have changed from baseline in the seven studies. A significant reduction in resting LVOTG was observed using xiaoxinbi formula + metoprolol, metoprolol, mavacamten, and NAC in the random effects model. In contrast, trimetazidine, candesartan and verpamil yielded different results in this model. Additionally, xiaoxinbi formula + metoprolol was found to be the most effective drug treatment for decreasing resting LVOTG, while metoprolol was the second most effective intervention (Figure 3A).
3.3.2.2. Safety outcomes
Three different medications were compared to a placebo to determine their safety, as only three studies demonstrated the occurrence of adverse events. In this network meta-analysis, trimetazidine, mavacamten and NAC showed no significant difference from placebo (Figure 3B). As shown in Figure 3F, treatments were ranked based on their p-score for safety outcomes.
3.4. Subgroup analyses
In the subgroup analysis, to examine the efficacy of the different medications, the patients were divided into two groups: those with obstructive hypertrophic cardiomyopathy and those with non-obstructive hypertrophic cardiomyopathy. In the obstructive hypertrophic cardiomyopathy subgroup, metoprolol showed the best efficacy, followed by mavacamten (Figure 3C). However, trimetazidine and candesartan did not demonstrate a therapeutic benefit compared to placebo in the non-obstructive hypertrophic cardiomyopathy group (Figure 3D).
4. Discussion
Beta-blockers and non-dihydropyridine calcium channel blockers are the first recommended treatments for HCM. Recently, the FDA approved mavacamten for the targeted treatment of HCM. However, studies comparing the efficacy and safety of different drug therapies in adults with HCM are rare.
In this study, a random effects model was used to evaluate the effects of seven drugs or drug combinations on changes in resting LVOTG and the incidence of adverse events among patients with HCM. AF, palpitations, dizziness, and headache are some of the most common adverse effects of drugs used to treat HCM. In this study, three drugs for which adverse events had been reported were compared for their safety. Based on our network meta-analysis of the seven studies, significant reductions were found in resting LVOTG for xiaoxinbi formula + metoprolol, metoprolol, mavacamten and NAC. According to the results of the p-score ranking, xiaoxinbi formula plus metoprolol, metoprolol, mavacamten and NAC remained the top four treatments. Furthermore, no significant differences were observed between the comparison group and the placebo group with respect to adverse events. While xiaoxinbi formula + metoprolol and metoprolol had superior efficacy compared to other treatments in our study, their safety requires additional validation since adverse events were not compared in our study. In a subgroup analysis, metoprolol demonstrated the best efficacy in patients with obstructive hypertrophic cardiomyopathy. Additionally, there was no significant difference in efficacy between the drugs (candesartan and trimetazidine) and placebo in the non-obstructive hypertrophic cardiomyopathy subgroup.
Metoprolol treatment for HCM has been demonstrated to be safe and effective (23). In the 2020 AHA/ACC guidelines, metoprolol was recommended as a class 1 medication (9). Nonetheless, few studies have examined the occurrence of adverse events during metoprolol treatment. Through their negative inotropic and chronotropic effects, beta blockers reduce tachycardia and ventricular contractility, increasing passive ventricular filling and diastolic filling time (24). Our study indicated that metoprolol had the second-highest efficacy, which is consistent with the recommendations in the guidelines. In obstructive hypertrophic cardiomyopathy, an elevated LVOTG is often accompanied by high myocardial contractility and strong adrenergic stimulation (25). Additionally, due to microvascular ischaemia, diastolic dysfunction, and elevated filling pressures, non-obstructive hypertrophic cardiomyopathy patients experience angina and dyspnoea (9). Through the above pharmacological mechanisms, beta blockers are capable of reducing LVOTG and relieving symptoms. In traditional Chinese medicine, xiaoxinbi formula has been shown to significantly improve cardiac function in combination with Western medicine. Based on direct and indirect comparisons, xiaoxinbi formula plus metoprolol was most effective in treating HCM. Nevertheless, the mechanism of xiaoxinbi formula treatment for HCM is unclear and may be related to all-cause multitargeted therapy in traditional Chinese medicine, the mechanism of which needs further study (21). Apart from these medications, NAC was also effective in treating HCM compared with placebo in our study. Researchers found that NAC reversed myocardial hypertrophy and fibrosis in an animal model of HCM, and improved diastolic function indices. Marian AJ et al. found that NAC reduced left ventricular mass and LVOTG (26). The reduction in myocardial hypertrophy may reduce the degree of LVOTO, and LVOTG will be reduced as well. It might act by reducing oxidative stress, which is increased in patients with HCM (26). Conversely, metoprolol's target may play a greater role in disease development, which may explain its superiority over NAC. HCM can also be treated with mavacamten, a recently developed targeted therapy. The PIONEER-HCM (Pilot Study Evaluating MYK-461 in Subjects with Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction; NCT02842242) study, a phase 2, open-label study, showed significant reductions in post-exercise LVOT gradients in obstructive hypertrophic cardiomyopathy patients treated with mavacamten (27). A meta-analysis revealed that compared to placebo, mavacamten was associated with improved NYHA functional class and pVO2 (28). It reduces hypercontractility and improves myocardial energetics by reversibly inhibiting actin-myosin cross-bridging (29). There are three different functional states of myosin: the active cycling state, which involves actin-activated ATP hydrolysis with a rapid ATP turnover rate of a mere one second; the disordered relaxed (DRX) state, is the ATP turnover state of myosin without actin, in which ATP turnover times are extremely slow at 30s; the third state, the super-relaxed (SRX) state, has even longer ATP turnover times (30). Under resting muscle conditions, DRX state and SRX state are in dynamic equilibrium. The drug mavacamten significantly reduced DRX, enhancing SRX (31). Mavacamten treats HCM by altering the DRX↔SRX state equilibrium and influencing muscle contractility and energetics (30). In contrast to beta blockers, which improve symptoms but do not treat HCM specifically, mavacamten is emerging as a targeted treatment for this disease. Study results revealed that metoprolol was more effective than mavacamten since the LVOTG reduction was greater with metoprolol (−47.00% vs. −34.50%), and the potential mechanism may be that the two drugs regulate myocardial contractility to varying degrees. Metoprolol reduces heart rate and myocardial contractility by blocking adrenergic receptor, and mavacamten regulates myocardial contractility by increasing SRX and improving unbalanced DRX:SRX ratio. Future clinical studies directly comparing the two drugs may be required to further validate our results. And deeper basic research is needed to compare the extent to which the action mechanisms of the two drugs play a role in the occurrence and development of the disease, providing further evidence for better diagnosis and treatment of HCM in the future.
For other drugs, the drug candesartan is classified as an angiotensin II receptor (AT1) blocker. Both cardiomyocytes and fibroblasts express AT1 receptors on their membranes, which mediate hypertrophic and fibrotic responses in the cardiac chamber (4). The treatment may affect the hypertrophy of the left ventricle, thereby changing the degree of obstruction of the left ventricle outflow tract. In HCM patients, left ventricular fibrosis is an important pathological feature. However, it remains unclear what mechanisms lead to cardiac fibrosis when HCM mutations occur (4). This may account for the lack of a significant difference in the efficacy of candesartan compared to placebo. As a result, more basic research and cohort studies are needed to verify the efficacy and mechanism of candesartan in the treatment of HCM. Trimetazidine, an inhibitor of free fatty acid oxidation, causes glucose to be utilized by cardiac and muscle tissues to regulate myocardial metabolism (32). Cardiac energetics are abnormal in patients with HCM. Research shows that impaired inorganic phosphate metabolism is observed in patients carrying HCM-related sarcomeric mutations (33). This target is not responsive to trimetazidine, and therefore, the efficacy of trimetazidine is not significantly different from that of the placebo. Verapamil is a non-dihydropyridine calcium channel blocker. In cardiac myocytes, it inhibits L-type calcium channels, exerting a negative inotropic and chronotropic effect (4). Contrary to the guideline recommendations, verapamil was not more effective than the placebo in this study because the LVOTG was increased when verapamil was administered compared to the placebo (7.00% vs. 0%). This may have been due to the inclusion of patients with milder symptoms and the use of smaller doses of verapamil.
To assess the effectiveness of treatment for different types of HCM, a subgroup analysis was conducted. In the subgroup analysis, not all of the drugs were included, but the efficacy rankings did not change. Among the adverse events analyzed in this study were AF, palpitations, dizziness, and headaches. Based on this analysis, there were no significant differences between the comparison group and the placebo group in terms of adverse events. Moreover, Torsades de pointes (TdP) is also an important adverse event, but it was not analyzed in this study due to data limitations. And it is associated with a prolonged QT interval in the ECG (34). In an RCT study, no nonsustained ventricular tachycardia was observed in the mavacamten treatment group, but no data about the incidence of TdP was reported (35). Trimetazidine has been shown to shorten QT intervals in research (36). As a result, TdP is unlikely to occur. Also, verapamil is the first-line treatment for sc-TdP (37). There are other HCM treatments that can prolong the QTc interval, such as disopyramide and amiodarone, but these two treatments were not included in this network study due to data limitations. As TdP can have serious consequences, future research should also focus on the probability of TdP occurrence in order to better measure drug safety.
Currently, HCM targeted treatments are increasingly mutation-specific. Toepfer et al. found as HCM can be caused by different gene mutations, the central pathological mechanism differs (38). Toepfer et al. showed that alternate calcium regulation is the central pathomechanism of thin filament variants TNNT2R92Q/+ and TNNI3R21C/+ (38). Sarkar et al. reported SRX is decreased by mutations R403Q and R663H in the HCM (39). Individual specific treatments will be possible due to the different pathogenesis of HCM caused by different mutations. The efficacy of this therapy needs to be evaluated by further research.
Despite this, there were some limitations in our study. First, in our analysis, only seven studies were included, which constituted a relatively small sample size. As a result of the limited number of studies, it is not possible to choose a more common Bayesian model to analyze the data. The Bayesian model analysis is recommended if there will be future larger sample studies or therapeutic research targeting specific gene mutations. Therefore, more studies are needed to confirm our findings. Furthermore, as part of our analysis, only changes in resting LVOTG—the most representative indicator—as an indicator of efficacy was analysed, whereas several clinical studies chose changes in pVO2 as an observed endpoint; thus, additional studies are required to confirm our conclusions. Moreover, according to this research, xiaoxinbi formula plus metoprolol was found to be the most effective treatment for HCM. Due to the small sample size and the uncertainty concerning the mechanism by which the xiaoxinbi formula treats HCM, more clinical and basic researches are necessary to verify its effectiveness. In addition, limited data made it difficult to compare different populations and age groups. Last, the occurrence of adverse events was not reported for all drugs in our study, limiting our ability to determine the safety of the medications.
5. Conclusions
Our results indicated that the four most effective medications for HCM in adults are xiaoxinbi formula + metoprolol, metoprolol, mavacamten and NAC. In different subgroups, drug efficacy ranked the same. The use of candesartan, trimetazidine and verapamil had no effect on reducing LVOTG. Therefore, they cannot delay the progression of the disease and provide no benefit over a placebo in treating HCM. There is a need for further research to assess the efficacy of metoprolol versus mavacamten. What's more, research on basic and molecular levels can be complementary or informative for future statistical studies. In this way, they can reveal how drugs work and develop more targeted and effective treatment methods for HCM at the molecular level.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
KM, SW, and JL designed and monitored the whole analysis. YM and WY contributed to study selection. KM, SW, and CL contributed to data extraction. HL provided the methodological support. KM, SW, and JL contributed to the data analysis and paper writing. HY provided the project fund. HL and HY were responsible for the data review. All authors provided a critical review. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the 2021 Shandong Medical Association Clinical Research Fund—Qilu Special Project (grant numbers YXH2022DZX02008); XinXin Heart Foundation of SIP-China Cardiovascular Association ATTR-CM fund (grant numbers 2022-CCA-ATTR-CM-014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1190181/full#supplementary-material
Supplementary Figure S1
Summary of bias risk.
References
1. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. (2017) 121(7):749–70. doi: 10.1161/CIRCRESAHA.117.311059
2. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. (2013) 381(9862):242–55. doi: 10.1016/S0140-6736(12)60397-3
3. Moody WE, Elliott PM. Changing concepts in heart muscle disease: the evolving understanding of hypertrophic cardiomyopathy. Heart. (2022) 108(10):768–73. doi: 10.1136/heartjnl-2021-320145
4. Palandri C, Santini L, Argirò A, Margara F, Doste R, Bueno-Orovio A, et al. Pharmacological management of hypertrophic cardiomyopathy: from bench to bedside. Drugs. (2022) 82(8):889–912. doi: 10.1007/s40265-022-01728-w
5. Lorenzini M, Anastasiou Z, O’Mahony C, Guttman OP, Gimeno JR, Monserrat L, et al. Mortality among referral patients with hypertrophic cardiomyopathy vs the general European population. JAMA Cardiol. (2020) 5(1):73. doi: 10.1001/jamacardio.2019.4534
6. Maron BJ, Rowin EJ, Maron MS. Hypertrophic cardiomyopathy: new concepts and therapies. Annu Rev Med. (2022) 73(1):363–75. doi: 10.1146/annurev-med-042220-021539
7. Packard E, de Feria A, Peshin S, Reza N, Owens AT. Contemporary therapies and future directions in the management of hypertrophic cardiomyopathy. Cardiol Ther. (2022) 11(4):491–507. doi: 10.1007/s40119-022-00283-5
8. Zampieri M, Berteotti M, Ferrantini C, Tassetti L, Gabriele M, Tomberli B, et al. Pathophysiology and treatment of hypertrophic cardiomyopathy: new perspectives. Curr Heart Fail Rep. (2021) 18(4):169–79. doi: 10.1007/s11897-021-00523-0
9. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary. Circulation. (2020) 142(25): e533–e557. doi: 10.1161/CIR.0000000000000938
10. Tuohy CV, Kaul S, Song HK, Nazer B, Heitner SB. Hypertrophic cardiomyopathy: the future of treatment. Eur J Heart Fail. (2020) 22(2):228–40. doi: 10.1002/ejhf.1715
11. Coppini R, Poggesi C, Yao L, Fan P, Del Lungo M, Stillitano F, et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. (2013) 127(5):575–84. doi: 10.1161/CIRCULATIONAHA.112.134932
12. Kim JB, Porreca GJ, Song L, Greenway SC, Gorham JM, Church GM, et al. Polony multiplex analysis of gene expression (PMAGE) in mouse hypertrophic cardiomyopathy. Science. (2007) 316(5830):1481–4. doi: 10.1126/science.1137325
13. Ho CY, Day SM, Axelsson A, Russell MW, Zahka K, Lever HM, et al. Valsartan in early-stage hypertrophic cardiomyopathy: a randomized phase 2 trial. Nat Med. (2021) 27(10):1818–24. doi: 10.1038/s41591-021-01505-4
14. Argirò A, Zampieri M, Berteotti M, Marchi A, Tassetti L, Zocchi C, et al. Emerging medical treatment for hypertrophic cardiomyopathy. J Clin Med. (2021) 10(5):951. doi: 10.3390/jcm10050951
15. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P, et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2020) 396:759–69. doi: 10.1016/S0140-6736(20)31792-X.32871100
16. Kawana M, Spudich JA, Ruppel KM. Hypertrophic cardiomyopathy: mutations to mechanisms to therapies. Front Physiol. (2022) 13:975076. doi: 10.3389/fphys.2022.975076
17. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366(1):l4898. doi: 10.1136/bmj.l4898
18. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29(7-8):932–44. doi: 10.1002/sim.3767
19. Higgins JPT, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3(2):98–110. doi: 10.1002/jrsm.1044
20. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. (2015) 15(1):58. doi: 10.1186/s12874-015-0060-8
21. Zhang Z, Zhai J, Dai M, Zhu F, Han Y, Tian W, et al. Effects of Xiaoxinbi formula on left ventricular function and heart rate variability in patients with hypertrophic cardiomyopathy. World Chin Med. (2017) 12(8):1844–7. doi: 10.3969/j.issn.1673-7202.2017.08.030
22. Gistri R, Cecchi F, Choudhury L, Montereggi A, Sorace O, Salvadori PA, et al. Effect of verapamil on absolute myocardial blood flow in hypertrophic cardiomyopathy. Am J Cardiol. (1994) 74(4):363–8. doi: 10.1016/0002-9149(94)90404-9
23. Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. (2021) 78(25):2505–17. doi: 10.1016/j.jacc.2021.07.065
24. Dybro AM, Rasmussen TB, Nielsen RR, Ladefoged BT, Andersen MJ, Jensen MK, et al. Effects of metoprolol on exercise hemodynamics in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. (2022) 79(16):1565–75. doi: 10.1016/j.jacc.2022.02.024
25. Ammirati E, Contri R, Coppini R, Cecchi F, Frigerio M, Olivotto I. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. (2016) 18(9):1106–18. doi: 10.1002/ejhf.541
26. Marian AJ, Tan Y, Li L, Chang J, Syrris P, Hessabi M, et al. Hypertrophy regression with N-acetylcysteine in hypertrophic cardiomyopathy (HALT-HCM). Circ Res. (2018) 122(8):1109–18. doi: 10.1161/CIRCRESAHA.117.312647
27. Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy. Ann Intern Med. (2019) 170(11):741. doi: 10.7326/M18-3016
28. Ismayl M, Abbasi MA, Marar R, Geske JB, Gersh BJ, Anavekar NS. Mavacamten treatment for hypertrophic cardiomyopathy: a systematic review and meta-analysis of randomized controlled trials. Curr Probl Cardiol. (2023) 48(1):101429. doi: 10.1016/j.cpcardiol.2022.101429
29. Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H, et al. Deciphering the super relaxed state of human β-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A. (2018) 115(35):E8143–52. doi: 10.1073/pnas.1809540115
30. Nag S, Trivedi DV. To lie or not to lie: super-relaxing with myosins. eLife. (2021) 10:e63703. doi: 10.7554/eLife.63703
31. Pilagov M, Heling LWHJ, Walklate J, Geeves MA, Kad NM. Single-molecule imaging reveals how mavacamten and PKA modulate ATP turnover in skeletal muscle myofibrils. J Gen Physiol. (2022) 155(1):e202213087. doi: 10.1085/jgp.202213087.36394553
32. Marzilli M, Vinereanu D, Lopaschuk G, Chen Y, Dalal JJ, Danchin N, et al. Trimetazidine in cardiovascular medicine. Int J Cardiol. (2019) 293:39–44. doi: 10.1016/j.ijcard.2019.05.063
33. Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. (2003) 41(10):1776–82. doi: 10.1016/S0735-1097(02)03009-7
34. Trinkley KE, Lee Page R, Lien H, Yamanouye K, Tisdale JE. QT Interval prolongation and the risk of Torsades de pointes: essentials for clinicians. Curr Med Res Opin. (2013) 29(12):1719–26. doi: 10.1185/03007995.2013.840568
35. Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol. (2022) 80(2):95–108. doi: 10.1016/j.jacc.2022.04.048
36. Bubnova MG, Aronov DM. Efficacy of trimetazidine – an inhibitor of free fatty acids oxidation in the treatment of patients with stable angina pectoris and heart failure. Kardiologiia. (2021) 61(11):65–76. doi: 10.18087/cardio.2021.11.n1801
37. Steinfurt J, Nazer B, Aguilar M, Moss J, Higuchi S, Zarse M, et al. Catheter ablation of short-coupled variant of torsade de pointes. Clin Res Cardiol. (2021) 111(5):502–10. doi: 10.1007/s00392-021-01840-z
38. Margara F, Psaras Y, Wang ZJ, Schmid M, Doste R, Garfinkel AC, et al. Mechanism based therapies enable personalised treatment of hypertrophic cardiomyopathy. Sci Rep. (2022) 12(1):1–17. doi: 10.1038/s41598-022-26889-2
Keywords: hypertrophic cardiomyopathy, system review and meta-analysis, left ventricular outflow tract gradient, medications, frequentist network meta-analysis
Citation: Mi K, Wu S, Lv C, Meng Y, Yin W, Li H, Li J and Yuan H (2023) Comparing the efficacy and safety of medications in adults with hypertrophic cardiomyopathy: a systematic review and network meta-analysis. Front. Cardiovasc. Med. 10:1190181. doi: 10.3389/fcvm.2023.1190181
Received: 20 March 2023; Accepted: 2 August 2023;
Published: 14 August 2023.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Stefano Palermi, University of Naples Federico II, ItalyMohamadamin Forouzandehmehr, Tampere University, Finland
© 2023 Mi, Wu, Lv, Meng, Yin, Li, Li and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiangbing Li amlhbmdiaW5nODFAMTYzLmNvbQ== Haitao Yuan eXVhbmhhaXRhb0BzZGZtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Abbreviations HCM, hypertrophic cardiomyopathy; CENTRAL, the Cochrane Controlled Register of Trials; LVOTG, left ventricular outflow tract gradient; NAC, N-acetylcysteine; AF, atrial fibrillation; LVOT, left ventricular outflow tract; LVOTO, left ventricular outflow tract obstruction; TGF-b, transforming growth factor-beta; ARBs, angiotensin II receptor blockers; MYK-461, mavacamten; PRISMA, preferred reporting items for systematic reviews and meta-analyses extension statement for network meta-analyses; RCT, randomized clinical trial; CIs, confidence intervals; OR, odds ratio; PIONEER-HCM, pilot study evaluating MYK-461 in subjects with symptomatic hypertrophic cardiomyopathy and left ventricular outflow tract obstruction; pVO2, peak oxygen consumption; AT1, angiotensin II receptor; EADs, early afterdepolarizations; DADs, delayed afterdepolarizations; DRX, disordered relaxed; SRX, super-relaxed; TdP, Torsades de pointes.
 Keying Mi1,2,†
Keying Mi1,2,† Sijia Wu
Sijia Wu Chanyuan Lv
Chanyuan Lv Hongkai Li
Hongkai Li Haitao Yuan
Haitao Yuan