- 1Center for Rehabilitation Medicine, Department of Anesthesiology, Zhejiang Provincial People's Hospital, Affiliated People's Hospital, Hangzhou Medical College, Hangzhou, China
- 2Department of Anesthesiology, The Second XiangYa Hospital of Central South University, Hunan, China
Background: Parasternal intercostal nerve block has been increasingly used for postoperative analgesia and has shown that this technique can provide effective postoperative analgesia. This study aimed to investigate the effect of preemptive parasternal intercostal nerve block on the opioid and vasoactive drug dose required for intraoperative hemodynamic stability and postoperative analgesia in patients undergoing off-pump coronary artery bypass grafting.
Methods: In this prospective, randomized controlled study, 64 participants aged 45–75 years scheduled for off-pump coronary artery bypass grafting at The Second Xiangya Hospital of Central South University. Patients were randomized into two groups and preoperatively administered ropivacaine (group R) and saline (group S), in the parasternal intercostal spaces with ultrasound-guided bilateral nerve block.
Results: The primary outcome was intraoperative sufentanil and vasopressor dosage. The secondary outcomes were intraoperative hemodynamics, postoperative pain scores, and anesthesia recovery, postoperative use of rescue dezocine, stay in intensive care unit, and length of hospital stay. The consumption of intraoperative sufentanil and vasopressor was significantly lower in group R than in group S. The visual analog score in group R was significantly lower than that in group S up to 12 h postoperatively. The time to anesthesia recovery was significantly less in group R than in group S. Most patients in group S required rescue dezocine, whereas most patients in group R did not. The hemodynamic variables were stable in all patients.
Conclusions: A preemptive parasternal intercostal nerve block effectively reduced the required intraoperative sufentanil and norepinephrine dose and provided adequate analgesia for the first 12 h after surgery. Therefore, a preemptive parasternal intercostal nerve block is a good option for patients undergoing off-pump coronary artery bypass grafting.
Clinical trial registration: chictr.org.cn, identifier ChiCTR1800017210.
1. Introduction
Off-pump coronary artery bypass grafting (OPCABG) is a common surgical treatment for coronary heart diseases. Intraoperative hemodynamic stability and myocardial protection have been a hot topic in cardiac anesthesiology. Some operations of cardiac surgery, especially sternotomy including skin and subcutaneous tissue incision, sternum splitting and sternal retractor setup, as well as cannulation, may induce an increase in blood pressure and/or heart rate (HR) (1). These episodes are associated with an increase in myocardial oxygen consumption and possible myocardial ischemia. Usually, they are controlled by the administration of a high dose of anesthetic agents (2).
Adverse perioperative pain stimulates the neuroendocrine system and causes stress, which adversely affects the cardiovascular, respiratory, and digestive systems (3, 4). Effective postoperative pain control may be beneficial for early extubation, cost reduction, and rapid recovery (5–7). In addition, appropriate analgesia can reduce pain-related morbidity.
Recently, regional nerve block techniques have increasingly been used for analgesia during surgery. Epidural anesthesia and thoracic paravertebral nerve block provide effective analgesia after cardiac surgery and reduce postoperative mortality (6, 8–12). However, these techniques remain controversial owing to the risk of hematoma caused by inappropriate needle depth especially during antiplatelet or anticoagulant therapy, which is usually administered to elderly patients undergoing OPCABG (13, 14). Peripheral techniques offer safety and efficiency. The parasternal intercostals nerve block primarily anesthetizes the intercostal nerves close to the sternal border and anterior cutaneous branch of the intercostal nerve and is used as an adjuvant for pain management post-cardiac surgery. This modality is highly effective in patients experiencing sternal wound pain following cardiac surgery (15–19). However, preemptive parasternal intercostal nerve block in perioperative benefits are not well established. This study aimed to investigate the effect of preemptive parasternal nerve block on the opioid and vasoactive drug dose required for intraoperative hemodynamic stability and on postoperative analgesia in patients undergoing OPCABG.
2. Materials and methods
2.1. Study population
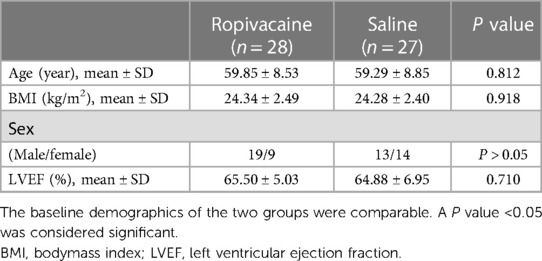
This randomized controlled trial was approved by the institutional ethics committee (The Second Xiangya Hospital of Central South University, Chairperson Jing Ping Zhao, ref: 2018-024) on March 29, 2018, and registered with ChiCTR (ref: ChiCTR1800017210). This study was conducted at the Second Xiangya Hospital between July 20, 2018, and September 30, 2018. All patients provided written informed consent before participating in this study. Patient data and postoperative evaluations were double-blinded in this prospective, single-center, randomized, placebo-controlled study. Coronary angiography revealed multivessel coronary artery diseases, and 64 patients planned to undergo OPCABG surgery were selected, regardless of sex. The inclusion criteria were as follows: age, 45–75 years; weight, 40–90 kg; American Society of Anaesthesiologists class III or IV status; and absence of intra-aortic balloon pump, and neuropsychiatric disorders included all patients with chronic pain or patients who take any type of pain medications.
The exclusion criteria were as follows: infection at the block site, left ventricular ejection fraction <50%, chronic liver or kidney disease, allergy to amide-type anesthetics, and low cardiac output syndrome with inotrope and/or intra-aortic balloon pump support. Other reasons for exclusion were as follows: change of treatment plan to cardiopulmonary bypass during the operation, development of postoperative complications requiring reoperation, requirement of intra-aortic balloon pump support intraoperatively or postoperatively, requirement of postoperative reintubation, and sedation for more than 48 h.
2.2. Study protocol
After obtaining written informed consent from the participants using the sealed envelope method, they were randomized into two groups: the participants were administered either ropivacaine (group R) or saline (group S). Medication administration and data collection were performed in a double-blinded manner: one anesthesiologist prepared the ropivacaine or saline and administered the block, another anesthesiologist administered anesthesia and collected the data.
For patients receiving the parasternal intercostal nerve block, 0.35% ropivacaine and 1 mg dexamethasone or 0.9% saline were administered in 20 ml aliquots injected into the space between the internal intercostal and the transvs. thoracis muscles on each side, 2 cm–2.5 cm lateral to the sternal edge, using ultrasound guidance (Figure 1). The block was administered by an anesthesiologist in a standardized manner before anesthesia induction.
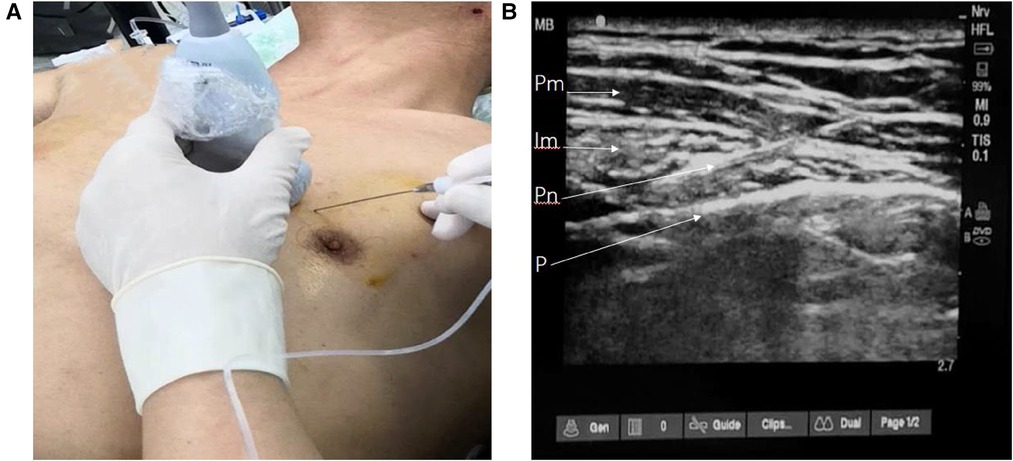
Figure 1. Parasternal intercostal nerve block procedure. (A) The needle and probe position, (B) ultrasonographic image of parasternal space. Pm, pectoralis major; Im,intercostal muscle; Pn, puncture needle; P, pleura.
A standardized anesthesia program was applied for all participants. Anesthesia was induced with midazolam (0.05–0.1 mg kg–1), sufentanil (0.3–0.7 ug kg–1), vecuronium (0.1–0.2 mg kg–1), and etomidate (0.03 mg kg–1) and maintained with a target controlled infusion of propofol (1.0–3.0 ugml–1) and remifentanil (1.0–4.0 ngml–1) and an infusion of cisatracurium (0.1 mg kg–1 h–1). Electrocardiography, oxygen saturation, HR, central venous pressure, bispectral index (BIS), and arterial blood pressure were monitored intraoperatively. The BIS was maintained between 40 and 55. The basal HR and systolic blood pressure (SBP) were the average values measured in the ward 3 days before surgery. Hypertension and/or tachycardia (the HR and SBP were >20% of the baseline value) were first treated by incremental increases in remifentanil, while the propofol dose was increased only if BIS >55. In these cases, the target concentrations of propofol and remifentanil were increased in steps of 1 ug ml–1 and 1 ng ml–1, respectively, every 15–20 s, until the appropriate level of anesthesia was achieved. If the effect-site concentration of remifentanil was up to 4.0 ng ml–1, sufentanil was added with a single intravenous push of 0.5 ug kg–1. Norepinephrine or fluid therapy was provided to treat hypotension [mean arterial pressure (MAP) <65 mmHg] and anisodamine to manage bradycardia (HR <45 bpm).
All patients were transferred to the ICU after surgery and underwent postoperative management. The postoperative analgesia protocol involved a single intravenous push of 5 mg dezocine as required.
The primary outcomes include consumption of sufentanil and intraoperative norepinephrine, intraoperative hemodynamics, pain scores for 48 h after surgery, and postoperative analgesia requirements, the time of anesthetic recovery, ICU stay, and length of hospital stay. The visual analog scale (VAS) was used to assess postoperative pain.
2.3. Statistical analyses
Descriptive statistics were used for all study variables, and a two-tailed Student’s t-test was performed to assess normally distributed data. Continuous variables are presented as means with standard deviations, and categorical variables are presented as the number of patients in each category and the corresponding percentages. Quantitative variables were compared using the Student’s t-test or Mann Whitney U-test according to the normality of distribution. Categorical variables were compared using the χ2 test. The intragroup comparison was performed using repeated measures analysis of variance. The tests were performed using the Statistical Analysis System software. Statistical significance was set to a P-value of <0.05.
3. Results
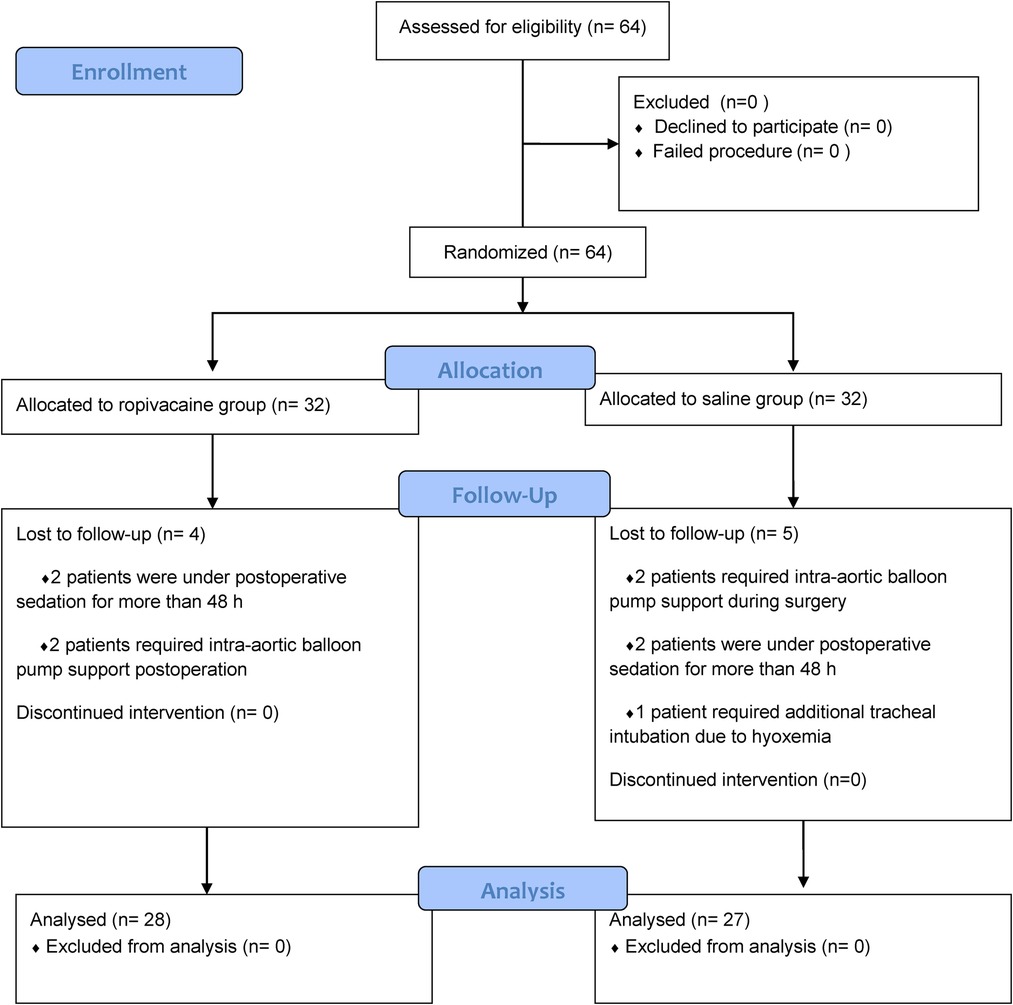
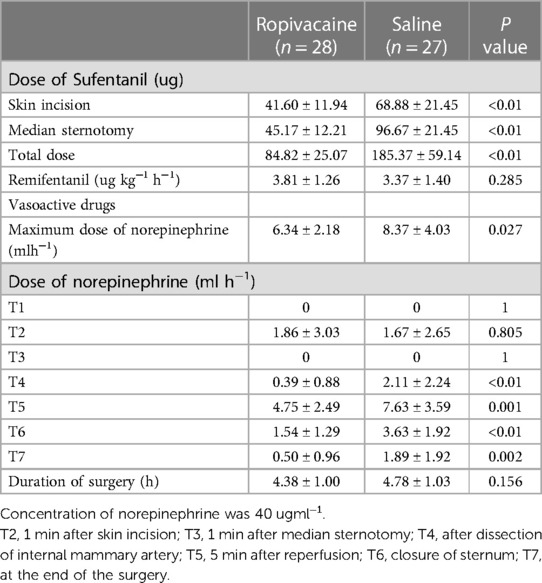
Sixty-four patients were randomized into group R and group S. However, four patients from group R and five from group S were excluded, leaving 28 patients in group R and 27 in group S (Figure 2). In group R, patients were excluded for the following reasons: two patients were under postoperative sedation for more than 48 h, and two patients required intra-aortic balloon pump support. Of the patients excluded from group S, two required intra-aortic balloon pump support, two were under postoperative sedation for more than 48 h, and one required additional tracheal intubation due to low oxygen in the blood. Patient demographics and baseline clinical characteristics were similar between the two groups (Table 1). Compared to patients who received saline, patients who received ropivacaine required significantly lower doses of sufentanil at the time of skin incision and especially at median sternotomy (Table 2). The total consumption of sufentanil was approximately 50% lower in the ropivacaine group than in the saline group (P < 0.001).
Hemodynamic data showed that the patients in the two groups were hemodynamically stable. Hemodynamics (HR and MAP) were not significantly different between both groups (Figure 3). However, at the time of dissection of the internal mammary artery, 5 min after reperfusion, closure of the sternum, and the end of the surgery, patients in group R required a significantly lower dose of norepinephrine than those in group S (P < 0.05) (Table 2).
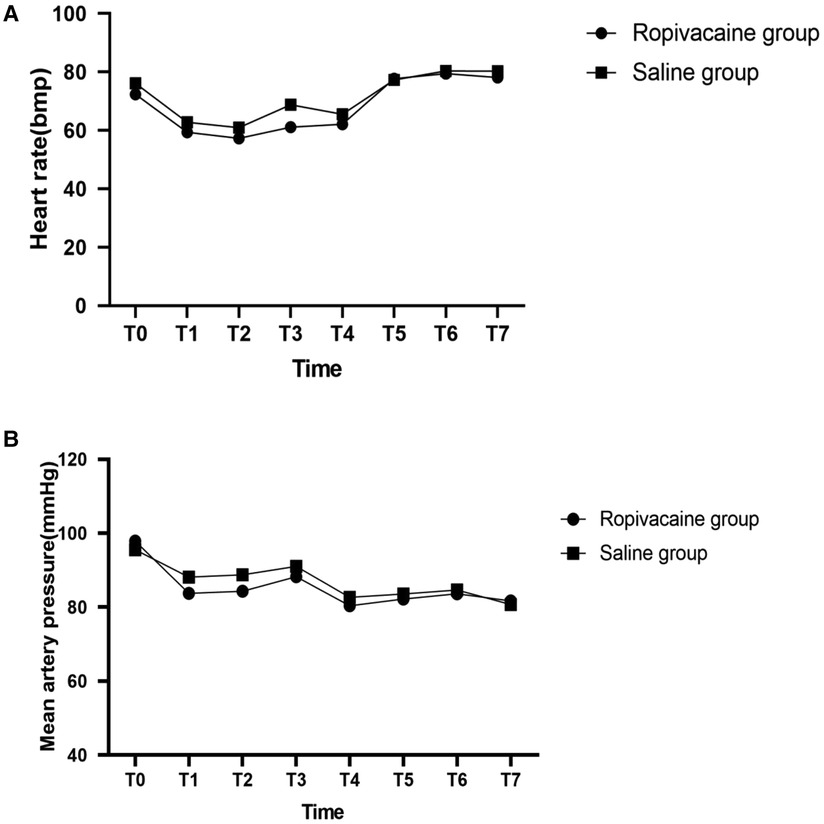
Figure 3. Heartrate (A) and mean artery pressure (B) with time in the two groups. T0, entering operation room; T1, before skin incision; T2, 1 min after skin incision; T3, 1 min after median sternotomy; T4, after dissection of internal mammary artery; T5, 5 min after reperfusion; T6, closure of sternum; T7, at the end of the surgery.
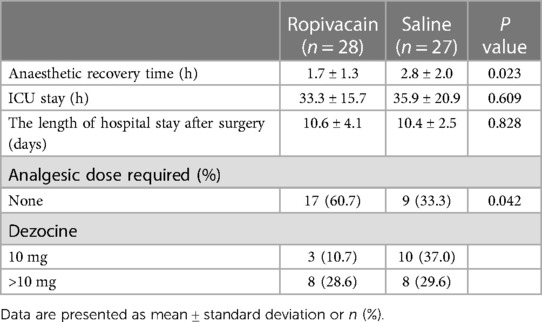
The pain scores (VAS) over a 48 h period are shown in Figure 4. Patients in the ropivacaine group experienced less pain during the first 12 h after surgery than those in the saline group. The VAS scores of the ropivacaine group were lower than those of the saline group 6, 8, and 12 h after the operation (P < 0.05). The difference in the VAS scores of the two groups at 18, 24, and 48 h after the operation was not statistically significant. Most patients in the ropivacaine group did not require rescue dezocine, whereas most patients in the saline group did (P < 0.05) (Table 3). The time to recovery from anesthesia was significantly lower in the ropivacaine group than in the saline group (P < 0.05). Nevertheless, no effect was observed on the length of stay in the ICU or hospital after surgery in the ropivacaine group (Table 3).
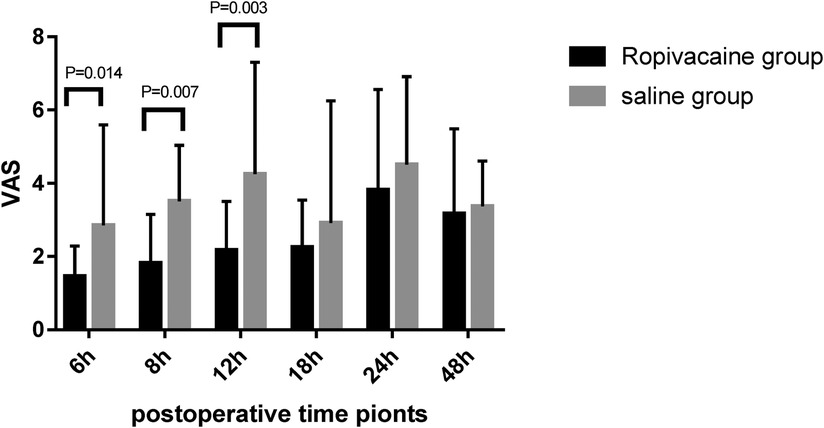
Figure 4. Visual analog scale scores for pain in the first 48 h after surgery (mean ± standard deviation).
4. Discussion
Hemodynamic stability is a significant concern in the intraoperative management of OPCABG. In the past, large doses of opioids were used in thoracotomy to inhibit the stress response caused by strong stimuli, such as skin incision, saw sternum, and chest closure (2). However, opioids are associated with side effects, such as inhibition of the cardiovascular system, making them unsuitable for maintenance of intraoperative hemodynamics. A preemptive parasternal intercostal nerve block could provide an effective level of anesthesia of the chest wall. Our study demonstrated that ropivacaine administration through a parasternal intercostal nerve block before anesthesia induction, helped decrease the required intraoperative opioid dose. There was no difference in the hemodynamic parameters between the ropivacaine and saline groups, but norepinephrine consumption was decreased in the ropivacaine group, this may owing to decreasing narcotic doses may contribute to more hemodynamic stability during the less stimulating portion of the surgery, which probably indicates that a parasternal nerve block can help maintain hemodynamic stability to a certain extent.
In recent years, combining general anesthesia with regional anesthesia has become a popular topic. Saad et al.showed that preemptive thoracic paravertebral and serratus anterior plane block provide comparable levels of adequate analgesia for the first 24 h after thoracotomy, with easier application and lower complication rates. The two procedures reduce intraoperative fentanyl and postoperative morphine consumption (20). In our study, we used the technique to compare ultrasound-guided parasternal intercostal nerve blocks with ropivacaine and saline. Local anesthetics can be injected into the internal intercostal muscle. The results of this study indicate the analgesic efficacy of the parasternal intercostal nerve block. Further, the parasternal intercostal nerve block is safer than the thoracic paravertebral block because of its lower rate of adverse events, especially hypotension and bradycardia (21).
Most studies on parasternal intercostal nerve block for postoperative analgesia have been conducted in patients requiring median sternotomy. Chen et al. studied the postoperative analgesia obtained with an ultrasound-guided parasternal intercostals nerve block in patients undergoing mediastinal mass resection by median sternotomy. They found that the parasternal intercostal nerve block effectively reduced postoperative pain and adjuvant analgesic requirement (22). Padaa et al.compared the effects of preincisional and postincisional parasternal intercostal blocks on postoperative pain in cardiac surgery and found that the intraoperative fentanyl requirement before cardiopulmonary bypass was significantly reduced and that both blocks provided comparable pain relief postoperatively (23). Abadi et al. evaluated the effectiveness of the parasternal intercostal nerve block in patients undergoing coronary artery bypass grafting with sternotomy as part of the enhanced recovery after surgery (ERAS) pain management protocol as compared with that in a non-ERAS patient group. They observed lower maximum pain scores and the requirement of a lower opioid dose for postoperative analgesia on administering the block. The data from our study also indicate that the parasternal intercostal block provided good postoperative analgesia. In addition, our study showed that the parasternal intercostal block reduced intraoperative opioid and norepinephrin use, which indirectly indicates that a preemptive parasternal intercostal block may help maintain intraoperative hemodynamic stability in patients with OPCABG.
The present study demonstrated that the parasternal intercostal nerve block provided adequate analgesia for the first 12 h after surgery. Pain intensity was significantly lower in the ropivacaine group than in the saline group after 12 h. One or more doses of dezocine were required during the first 48 h postoperatively in all patients, except in 17 patients in the ropivacaine group. The saline group required higher doses of dezocine for all except nine patients. Therefore, although a parasternal intercostal nerve block reduced dezocine consumption, it was not adequate as the only postoperative analgesic. In the current study, the effect of the local anesthetic in the ropivacaine group lasted up to 12 h after surgery. This may be related to the inclusion of dexamethasone to enhance the compatibility with local anesthetics. Studies have shown that local anesthetics with dexamethasone can effectively extend the action time of local anesthetics (24, 25).
In this study, we adopted the concept of preemptive analgesia through the application of regional blocks before surgical incisions. This approach focuses on postoperative analgesia in addition to the prevention of central sensitization and chronic neuropathic pain (26).
Poorly managed pain following cardiac surgery can lead to an increased risk of complications, such as lung collapse and chest infections, due to altered mechanical functions of the lungs and ventilation–perfusion mismatch. Pascarella et al. showed that parasternal block provided a better postoperative performance at spirometry compared to the control group (27). Effective relief of sternal pain is important for both patients and physicians. Increasing evidence suggests that severe pain in the sternum after surgery significantly affects the time of tracheal tube removal, hemodynamic stability, and subsequent postoperative recovery (4, 28, 29). Barr et al. and McDonald et al. found that a parasternal intercostal block significantly improves blood oxygen levels (16, 17). Our study did not monitor this parameter.
In this study, the time of recovery from anesthesia was lower in the ropivacaine group than in the saline group, which may be related to the lower intraoperative sufentanil dose. However, there was no difference in the ICU monitoring time and postoperative hospital stay between the groups, which may be attributable to strict criteria for ICU transfer. Apart from the parasternal nerve block, recovery of myocardial function, use of postoperative vasoactive drugs, and hemodynamic stability are also important factors that influence the duration of ICU stay.
5. Limitations
The outcomes of this study arelimited to the short-term effects of parasternal intercostal nerve block. Hence, its long-term effects require further study. In addition, we did not evaluate intraoperative and postoperative serological indicators, postoperative hemodynamic indicators, postoperative vasoactive drug use, and changes in myocardial enzymes. These aspects should therefore be explored in future studies.
6. Conclusion
This study demonstrates an effective and practical treatment for OPCABG. The parasternal intercostal block can help reduce opioid and vasopressors consumption during OPCABG and provide satisfactory analgesia in the earliest hours following surgery.
Author's note
This article was previously posted to research square. DOI: https://doi.org/10.21203/rs.2.1847/v1 is the submitted manuscript currently on a pre-print server.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of the Second XiangYa Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
This research was accomplished by four co-authors; the contribution of each author is as follows: MZ was involved in designing the study, accomplishing the work of postoperative follow-up, collecting the total of the data, and drafting the manuscript. JX instructed the design of the research protocol and performed the nerve block technique. WR participated in designing the intraoperative part of the research protocol, performed the general anesthesia processes and gave some instructions in designing and writing the manuscript. JL performed the statistical analysis. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank all study participants who were enrolled in this study. We would like to thank Editage for language editing and proofreading.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
OPCABG, off-pump coronary artery bypass grafting; BMI, body mass index; HR, heart rate; BIS, bispectral index; VAS, visual analog scale; IMA, internal mammary artery; T3-T4, third to fourth intercostal space; ERAS, enhanced recovery after surgery.
References
1. Elif BM, Suna G, Gulsen K, Gurkan T, Nur KF. Induction of anesthesia in coronary artery bypass graft surgery: the hemodynamic and analgesic effects of ketamine. Clinics (São Paulo, Brazil). (2010) 65(2):133–8. doi: 10.1590/S1807-59322010000200003
2. Philbin DM, Rosow CE, Schneider RC, Koski G, D'Ambra MN. Fentanyl and sufentanil anesthesia revisited: how much is enough? Anesthesiology. (1990) 73(1):5–11. doi: 10.1097/00000542-199007000-00002
3. Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg. (2007) 104(3):689. doi: 10.1213/01.ane.0000255040.71600.41
4. Zubrzycki M, Liebold A, Skrabal C, Reinelt H, Zubrzycka M. Assessment and pathophysiology of pain in cardiac surgery. J Pain Res. (2018) 11:1599–611. doi: 10.2147/JPR.S162067
5. Cheng DC, Karski J, Peniston C, Raveendran G, Asokumar B, Carroll J, et al. Early tracheal extubation after coronary artery bypass graft surgery reduces costs and improves resource use. A prospective, randomized, controlled trial. Anesthesiology. (1996) 85(6):1300–10. doi: 10.1097/00000542-199612000-00011
6. Scott NB, Turfrey DJ, Ray DA, Nzewi O, Sutcliffe NP, Lal AB, et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg. (2001) 93(3):528. doi: 10.1097/00000539-200109000-00003
7. Cheng DCH, Claus W, George D, Peragallo RA, Jo C, Cindy L, et al. Randomized assessment of resource use in fast-track cardiac surgery 1-year after hospital discharge. Anesthesiology. (2003) 98(3):651–7. doi: 10.1097/00000542-200303000-00013
8. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy—a systematic review and meta-analysis of randomized trials. Br J Anaesth. (2006) 96(4):418. doi: 10.1093/bja/ael020
9. Brandi AB, Stephen AE, Mark S-S. Pain management strategies for thoracotomy and thoracic pain syndromes. Semin Cardiothorac Vasc Anesth. (2014) 18(1):45–56. doi: 10.1177/1089253213514484
10. Bigeleisen PE, Nicholas G. Novel approaches in pain management in cardiac surgery. Curr Opin Anaesthesiol. (2015) 28(1):89–94. doi: 10.1097/ACO.0000000000000147
11. Amat-Santos IJ, Eric D, Jacques V, Daniel D, Michel R, Dominique L, et al. Effect of thoracic epidural analgesia on clinical outcomes following transapical transcatheter aortic valve implantation. Heart. (2013) 99(4):287. doi: 10.1136/heartjnl-2012-302185corr1
12. Poltak JM, Cobey FC, Augoustides JG, Connors CW. Paravertebral analgesia in transapical transcatheter aortic valve replacement. Heart Lung Vessels. (2015) 7(3):217–23. PMID:26495267; PMCID: PMC4593013.
13. Okitsu K, Iritakenishi T, Iwasaki M, Imada T, Fujino Y. Risk of hematoma in patients with a bleeding risk undergoing cardiovascular surgery with a paravertebral catheter. J Cardiothorac Vasc Anesth. (2016) 31(2):453–57. doi: 10.1053/j.jvca.2016.06.002
14. Mohsen Z, Rasoul A, Samad Ej G. A review of current analgesic techniques in cardiac surgery. Is epidural worth it? J Cardiovasc Thorac Res. (2014) 6(3):133–40. doi: 10.15171/jcvtr.2014.001
15. Saini K, Chauhan S, Kiran U, Bisoi AK, Choudhury M, Hasija S. Comparison of parasternal intercostal block using ropivacaine or bupivacaine for postoperative analgesia in patients undergoing cardiac surgery. World J Cardiovasc Surg. (2015) 05(6):49–57. doi: 10.4236/wjcs.2015.56009
16. Barr AM, Tutungi E, Almeida AA. Parasternal intercostal block with ropivacaine for pain management after cardiac surgery: a double-blind, randomized, controlled trial. J Cardiothorac Vasc Anesth. (2007) 21(4):547–53. doi: 10.1053/j.jvca.2006.09.003
17. Mcdonald SB, Jacobsohn E, Kopacz DJ, Desphande S, Helman JD, Salinas F, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesth Analg. (2005) 100(1):25–32. doi: 10.1213/01.ANE.0000139652.84897.BD
18. Vedat E, Christian D, Kasra A, Yvan S, Valérie S, Bruno P, et al. The analgesic effects of a bilateral sternal infusion of ropivacaine after cardiac surgery. Reg Anesth Pain Med. (2012) 37(2):166–74. doi: 10.1097/AAP.0b013e318240957f
19. Markham T, Wegner R, Hernandez N, Lee JW, Choi W, Eltzschig HK, et al. Assessment of a multimodal analgesia protocol to allow the implementation of enhanced recovery after cardiac surgery: retrospective analysis of patient outcomes. J Clin Anesth. (2019) 54:76–80. doi: 10.1016/j.jclinane.2018.10.035
20. Saad FS, El Baradie SY, Abdel Aliem MAW, Ali MM, Kotb TAM. Ultrasound-guided serratus anterior plane block versus thoracic paravertebral block for perioperative analgesia in thoracotomy. Saudi J Anaesth. (2018) 12(4):565–70. doi: 10.4103/sja.SJA_153_18
21. Coveney E, Weltz CR, Greengrass R, Iglehart JD, Leight GS, Steele SM, et al. Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases. Ann Surg. (1998) 227(4):496. doi: 10.1097/00000658-199804000-00008
22. Chen H, Song W, Wang W, Peng Y, Zhai C, Yao L, et al. Ultrasound-guided parasternal intercostal nerve block for postoperative analgesia in mediastinal mass resection by median sternotomy: a randomized, double-blind, placebo-controlled trial. BMC Anesthesiol. (2021) 21(1):98. doi: 10.1186/s12871-021-01291-z
23. Jha AK. Comparison of preincisional and postincisional parasternal intercostal block on postoperative pain in cardiac surgery. J Card Surg. (2020) 35(6): 1525–30. doi: 10.1111/jocs.14651
24. Albrecht E, Kern C, Kirkham KR. A systematic review and meta-analysis of perineural dexamethasone for peripheral nerve blocks. Anaesthesia. (2015) 70(1):71–83. doi: 10.1111/anae.12823
25. Mao Y, Zuo Y, Mei B, Chen L, Liu X, Zhang Z, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial. J Pain Res. (2018) 11:1811–9. doi: 10.2147/JPR.S164225
26. Vadivelu N, Mitra S, Schermer E, Kodumudi V, Kaye AD, Urman RD. Preventive analgesia for postoperative pain control: a broader concept. Local Reg Anesth. (2014) 7:17–22. doi: 10.2147/LRA.S62160
27. Pascarella G, Costa F, Nonnis G, Strumia A, Sarubbi D, Schiavoni L, et al. Ultrasound guided parasternal block for perioperative analgesia in cardiac surgery: a prospective study. J Clin Med. (2023) 12(5):2060. doi: 10.3390/jcm12052060
28. Pieczkoski SM, Agf M, Sbruzzi G. Noninvasive ventilation during immediate postoperative period in cardiac surgery patients: systematic review and meta-analysis. Braz J Cardiovasc Surg. (2017) 32(4):301. doi: 10.21470/1678-9741-2017-0032
Keywords: preemptive parasternal intercostal nerve block, analgesia, hemodynamics, enhanced recovery, off-pump coronary artery bypass grafting
Citation: Zou M, Ruan W, Liu J and Xu J (2023) Preemptive parasternal intercostal nerve block for patients undergoing off-pump coronary artery bypass grafting: a double-blind, randomized, controlled trial. Front. Cardiovasc. Med. 10:1188518. doi: 10.3389/fcvm.2023.1188518
Received: 17 March 2023; Accepted: 3 May 2023;
Published: 18 May 2023.
Edited by:
Enyi Shi, China Medical University, ChinaReviewed by:
Piero Farina, Agostino Gemelli University Polyclinic (IRCCS), ItalyZhengyuan Xia, The University of Hong Kong, Hong Kong SAR, China
© 2023 Zou, Ruan, Liu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junmei Xu MTU4MDI2NTA4ODJAMTM5LmNvbQ==
 Mengmeng Zou1
Mengmeng Zou1 Junmei Xu
Junmei Xu