- 1Arrhythmia Center, Ningbo First Hospital, The First Affiliated Hospital of Ningbo University, Ningbo, China
- 2Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, Ningbo First Hospital, The First Affiliated Hospital of Ningbo University, Ningbo, China
Background: Pericardial effusion (PE) is an uncommon but serious complication that occurs following percutaneous left atrial appendage closure (LAAC). There are few data regarding PE following implantation of the LAmbre device for LAAC.
Methods: Patients with nonvalvular atrial fibrillation (AF) undergoing percutaneous LAAC using the LAmbre device at the Arrhythmia Center of Ningbo First Hospital from October 2017 to March 2021 were retrospectively reviewed (n = 133). PE was defined as acute if diagnosed ≤7 days post LAAC (n = 3, 2.3%) or delayed if diagnosed >7 days post LAAC (n = 3, 2.3%). The clinical characteristics and procedural data were compared between patients with PE (PE group, n = 6) and without PE (non-PE group, n = 127). The predictors of PE were analyzed by logistic regression.
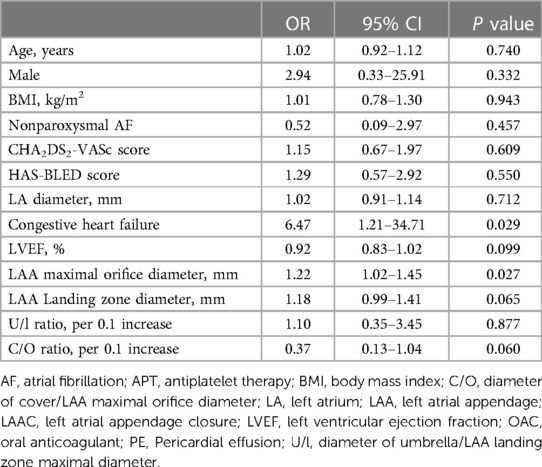
Results: All patients with PE recovered following treatment by pericardiocentesis. Patients with PE were found to have a higher incidence of congestive heart failure (50.0% vs. 13.4%, P = 0.044) and had larger measured LAA orifice diameters (33.5 mm ± 6.0 mm vs. 28.3 mm ± 5.2 mm, P = 0.018) and landing zone diameters (27.8 mm ± 4.8 mm vs. 23.9 mm ± 4.8 mm, P = 0.054) compared with those without PE. The diameters of the device umbrellas (31.7 mm ± 5.6 mm vs. 26.9 mm ± 5.0 mm, P = 0.026) and covers (36.3 mm ± 4.6 mm vs. 33.4 mm ± 4.0 mm, P = 0.075) implanted were larger in the PE group compared to the non-PE group. Univariate logistic regression revealed that congestive heart failure (OR = 6.47, 95% CI = 1.21–34.71, P = 0.029) and LAA maximal orifice diameter (OR = 1.22, 95% CI = 1.02–1.45, P = 0.027) were both associated with PE following LAmbre device implantation.
Conclusions: In this single-center experience, both acute and delayed PE were uncommon in patients with AF following LAmbre device implantation. Congestive heart failure and a larger LAA orifice were identified as predictors for the occurrence of PE.
1. Introduction
Percutaneous left atrial appendage closure (LAAC) is now considered a viable alternative to oral anticoagulation (OAC) for stroke prevention in patients with nonvalvular atrial fibrillation (AF) (1). Prior randomized controlled trials have demonstrated the safety and efficacy of LAAC (2, 3). Pericardial effusion (PE) requiring intervention is one of the most serious complications following LAAC. The reported rates of acute PE (≤7 days) in previous investigations range between 1.8% and 5% (4). Data regarding delayed PE (>7 days) following LAAC are limited. The incidence of delayed PE was reported as 0.8% in our recent article (5). In the Amulet IDE trial, the incidence of postprocedural PE was reported to be higher for procedures in which the Amplatzer Amulet occluder was implanted than when the Watchman device was used (6). Several factors associated with PE after LAAC have been reported, including older age, female sex, left ventricular function, paroxysmal AF, and higher CHA2DS2-VASc score (7, 8).
The majority of studies of PE were performed in patients with LAAC using either the Watchman or Amplatzer devices (8–11). The LAmbre device is a self-expanding, nitinol-based device consisting of an umbrella and a cover connected by a short central waist. It has been used in clinical practice since 2017 with a high success rate (12, 13). Previous investigations have shown that both acute and delayed PE can occur following LAAC with the LAmbre device (5, 12, 14). We retrospectively investigated the incidence and predictors of PE following the LAAC procedure with the LAmbre device in our institution.
2. Materials and methods
2.1. Study population
Patients with nonvalvular AF who underwent percutaneous LAAC using the LAmbre device at the Arrhythmia Center of Ningbo First Hospital from October 2017 to March 2021 were retrospectively studied. Periprocedural baseline data were collected to calculate the CHA2DS2-VASc score and HAS-BLED score. Preprocedural transthoracic echocardiography (TTE) was performed 24 h prior to the procedure to measure the left atrial (LA) diameter and the left ventricular ejection fraction (LVEF). The left atrial appendage (LAA) was visualized at 0°, 45°, 90°, and 135°, and the LAA orifice diameter and landing zone diameter were measured and recorded. The inclusion criteria for LAAC were as follows: age >18 years, CHA2DS2-VASc score ≥2 for males or ≥3 for females, presence of a contraindication to long-term OAC therapy, history of bleeding events while on OAC therapy, history of stroke while on long-term OAC therapy, and intolerance or refusal to take OAC. The exclusion criteria included presence of a thrombus in the LAA or LA detected by TEE, AF in the setting of moderate-to-severe mitral stenosis and/or in the presence of a mechanical heart valve, acute myocardial infarction or unstable angina, prior stroke or transient ischemic attack (TIA) within 30 days, and uncontrolled hemorrhagic disease. This study was conducted in compliance with the guidelines of the Helsinki Declaration. The study was approved by the Ethics Committee of Ningbo First Hospital. Written informed consent for the percutaneous LAAC procedure was obtained from all patients.
2.2. Percutaneous LAAC with the LAmbre device
All procedures were performed under local anesthesia guided by TEE or intracardiac echocardiography (ICE). The technique used for LAmbre device implantation have been published previously (12). To briefly summarize, intravenous heparin was administered to patients immediately after transseptal puncture aiming to achieve an activated clotting time of at least 250 s. The delivery sheath was then placed in the proximal part of the LAA. The device size was selected by the operator according to the measurements taken during intraprocedural echocardiography or LAA angiography. The distal umbrella was released into the LAA by pushing the device stepwise out of the delivery sheath. Subsequently, the sheath was withdrawn to expose the proximal cover, allowing it to expand in the LA and seal the LAA ostium. Echocardiography was performed to confirm satisfactory positioning of the device. A satisfactory device position was defined as absence of, or minimal contrast peridevice leakage (PDL) ≤3 mm into the LAA. A gentle tug test was performed to ensure device stability. Recapture or resizing of the device was attempted unless satisfactory device position was achieved. The C/O ratio was calculated as the diameter of the cover divided by the LAA maximal orifice diameter. The U/l ratio was calculated as the diameter of the umbrella divided by the maximum diameter of the LAA landing zone.
2.3. Follow-up
TTE was performed on the day following the procedure to detect the presence of PE. OAC was prescribed for 45 days after the procedure, followed by both aspirin and clopidogrel for 6 months, and subsequently aspirin or clopidogrel for the duration of the patient's lifetime. Repeat TEE was performed at 45 days, 6 months, and 12 months following the index procedure in order to evaluate the position of the device, PDL, and device-related thrombus (DRT). We considered major adverse events to include death, stroke/TIA, systemic embolism, device embolization, and major bleeding events. Major bleeding was defined according to the Bleeding Academic Research Consortium (BARC) criteria (type 3 or higher) (15).
2.4. Management of PE
Acute and delayed PE were defined according to the time of diagnosis following LAmbre device implantation (acute was defined as ≤7 days, and delayed defined as >7 days). Treatment of PE was dependent to the patient's symptoms, vital signs, and test results, as well as their physicians' judgment (5). If there were no signs of cardiac tamponade, intensive monitoring alone was performed. If cardiac tamponade was present, emergency pericardiocentesis was performed. If the vital signs were still unstable after pericardiocentesis, urgent cardiac surgery was recommended. Repeated TTE was also performed 1 or 2 weeks following discharge. The routine postimplantation follow-up strategy was then followed if the patient remained stable.
2.5. Statistical analysis
The study sample was divided into PE group and non-PE group based on the occurrence of PE. Normally distributed continuous variables are expressed as the mean (standard deviation), while the median (interquartile range) is used for variables with a skewed distribution. Categorical variables are expressed as absolute numbers (percentages). Continuous variables were compared using the t-test and Mann–Whitney U-test for normally and nonnormally distributed data, respectively. Categorical variables were compared using the Fisher's exact test. The predictors of PE were evaluated by logistic regression. According to a rule of thumb, logistic models should be used with a minimum of 10 events per predictor (16). Therefore, univariate logistic model would be run for the predictor of PE if the number of cases was not enough for the multivariate adjusted model. All analyses were performed by SPSS 19.0 (IBM, Armonk, NY, USA), and P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
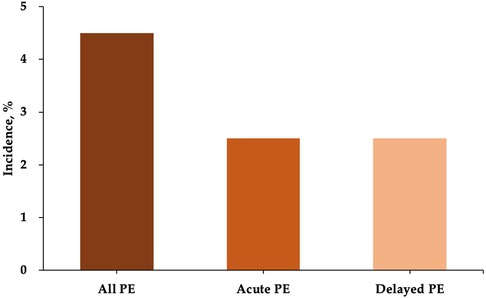
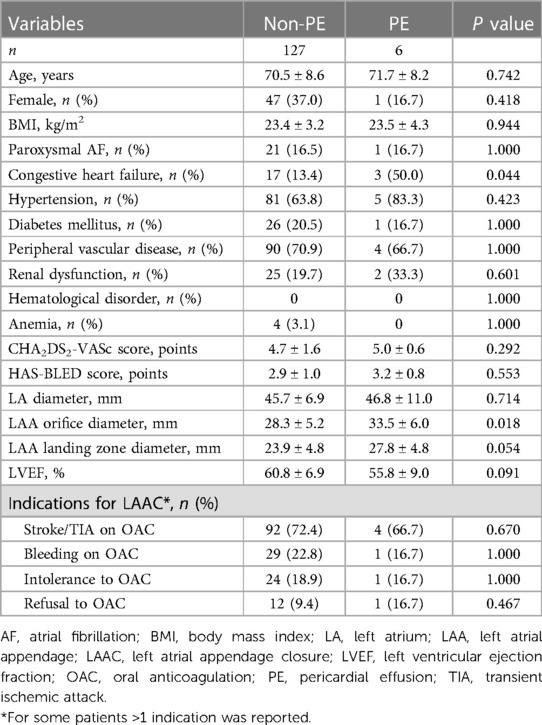
A total of 133 patients who underwent LAmbre device implantation were included in our analysis. Acute and delayed PE occurred in 3 patients each, resulting in a total incidence of 4.5% for all PE events (Figure 1). Baseline characteristics of the patients are shown in Table 1. Patients with PE had a higher percentage of congestive heart failure (50.0% vs. 13.4%, P = 0.044). The LAA orifice diameter (33.5 mm ± 6.0 mm vs. 28.3 mm ± 5.2 mm, P = 0.018) and landing zone diameter measured by TEE (27.8 mm ± 4.8 mm vs. 23.9 mm ± 4.8 mm, P = 0.054) were both greater in the PE group. The CHA2DS2-VASc score and HAS-BLED score were similar between the two groups.
3.2. Periprocedural data
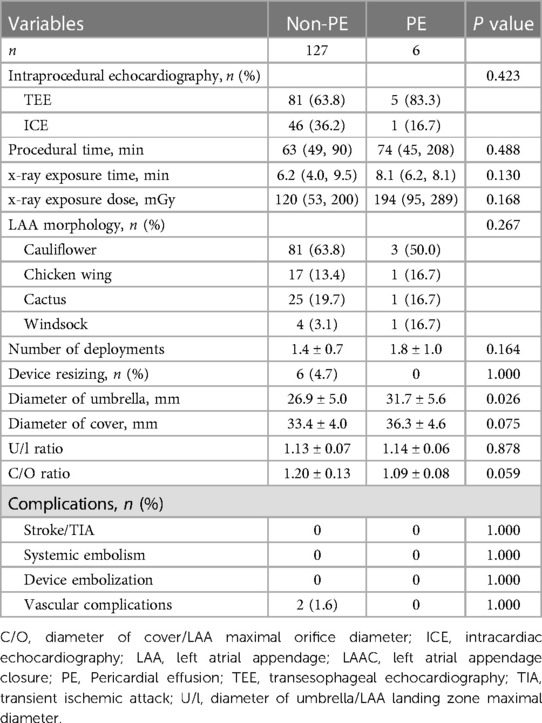
The type of intraprocedural echocardiography, procedural time, x-ray exposure time and dose was similar between the two groups. The number of deployments and device resizing in the non-PE group were similar to those in the PE group. The average diameters of the device umbrella (31.7 mm ± 5.6 mm vs. 26.9 mm ± 5.0 mm, P = 0.026) and cover (36.3 mm ± 4.6 mm vs. 33.4 mm ± 4.0 mm, P = 0.075) were larger in the PE group than in the non-PE group. The calculated C/O ratio (1.20 ± 0.13 vs. 1.09 ± 0.08) was larger in the non-PE group, while the calculated U/l ratio was similar between the two groups (Table 2).
3.3. Management of PE
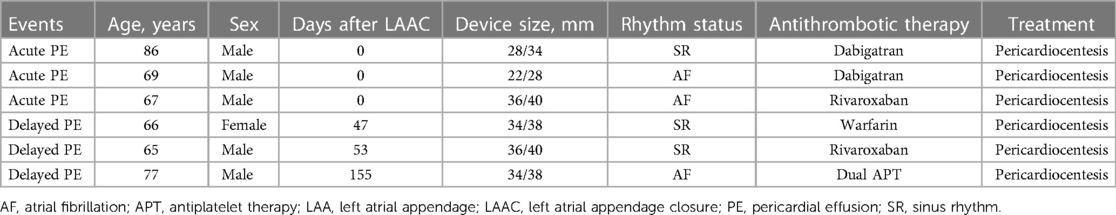
Three patients developed acute PE on the day of the LAmbre implantation. Two of the patients had taken dabigatran periprocedurally, with one patient having been in sinus rhythm (SR) and the other had been in AF rhythm. One patient took rivaroxaban and had been in AF rhythm. OAC was discontinued immediately and reinitiated before discharge. The following antithrombotic medication was prescribed as scheduled. Three individuals developed delayed PE following the procedure. PE was detected in one patient 47 days after device implantation while on warfarin and in SR. One patient developed PE 53 days following the procedure and was taking rivaroxaban and was in SR. The third patient experienced PE 155 days following device implantation and was taking both aspirin and clopidogrel and was in AF rhythm. All patients with acute and delayed PE were treated with pericardiocentesis, and none required blood transfusion (Table 3). All acute and delayed PE was hematic nature. Antithrombotic medication was switched to single antiplatelet therapy or none.
3.4. Predictors of PE
Univariate logistic regression analysis revealed that congestive heart failure (OR = 6.47, 95% CI = 1.21–34.71, P = 0.029) and LAA maximal orifice diameter (OR = 1.22, 95% CI = 1.02–1.45, P = 0.027) were both correlated with PE following LAmbre device implantation (Table 4).
3.5. Follow-up results
In the PE group, 1 patient underwent DRT 44 days after the procedure and totally dissolved after prolongation of OAC medication. No patients experienced stroke, systemic embolism, device embolization, or major bleeding and there were no deaths. None of the patients had recurrent PE following treatment. In the non-PE group, 1 patient died. Ischemic stroke and major bleeding occurred in 1 and 2 patients, respectively. DRT was detected in 4 patients and confirmed complete dissolution after prolonging OAC therapy. Antithrombotic medication was switched as scheduled of other patients in the non-PE group. No PDL >5 mm was detected in any patient during the follow-up TEE study.
4. Discussion
To the best of our knowledge, the present study was the first to investigate both acute and delayed PE following LAmbre device implantation. We found that PE was not uncommon after LAAC with the LAmbre device. Congestive heart failure and a large LAA orifice diameter were both correlated with the development of PE following LAmbre implantation.
4.1. Incidence of PE following LAAC
PE requiring intervention is one of the most serious complications that can occur following LAAC. The reported incidence of PE following Watchman implantation is relatively low. In a recent study from the NCDR Left Atrial Appendage Occlusion Registry, the incidence of PE requiring intervention was reported to be 1.4% (7). A real-world analysis of >17,000 recipients of the Watchman implant reported the incidence of PE requiring intervention to be 1.2% (8). The rate of PE requiring intervention following Watchman implantation was 1.1% in our previously reported study (17). In the Amulet IDE trial, the incidence of PE was higher in patients receiving the Amulet occluder (2.4% vs. 1.2%) (6). However, most of the previous studies focused on periprocedural PE, and the timing of the PE events was generally not specified. Two patients (0.2%) experienced PE within 30 days of the procedure in the EWOLUTION study (18). According to data reported to a US nationwide registry, 43 out of 13,309 subjects (0.3%) with Watchman implantation were readmitted with delayed PE/PT within 30 days (10).
There is only limited data currently reported regarding PE following LAmbre device implantation. PE occurred in 3 patients (2.0%) in a prospective multicenter clinical study of the use of the LAmbre device. Park et al. (13) reported an incidence of 3.3% (2/60) for PE in patients who underwent LAAC with the LAmbre device. The two cases were both delayed presentations (Days 8 and 33 postprocedure) and both required pericardiocentesis. Schnupp et al. (19) reported a rate of 5% (2/40) periprocedural PE requiring intervention following LAmbre device implantation. In the study of Xiao et al. (14), 4 of 224 (1.8%) patients who underwent LAmbre device implantation developed delayed PE/PT. In the present study, the incidence of acute and delayed PE was 2.5% each, which was similar to previous investigations.
4.2. Predictors of PE following LAAC
Most previous investigations reporting on the factors associated with the development of PE were conducted in patients who had implantation of the Watchman device implantation. Data on the predictors of PE after implantation of the LAmbre device are limited. Xiao et al. (14) reported that the only factor associated with delayed PE events was if the umbrella of the LAmbre device did not fully open. Of note, age, sex, presence of paroxysmal AF, and number of recaptures were not found to be correlated with the incidence of delayed PE. However, several other studies have demonstrated that the presence of SR at the time of implantation was correlated with PE (7, 11). It has been postulated that LAA contraction may exert force on the device, particularly at the site of anchors, leading to the development of PE. In our report, we found neither the presence of paroxysmal AF nor SR was correlated with the development of PE. The significance of these inconsistent findings across the reported studies are unclear and may be caused by the different study samples.
We found that larger LAA orifice diameter was correlated with the development of PE following LAmbre device implantation. A prior study showed that larger LAA orifice was independently correlated with PDL after Watchman device implantation (20). We postulate that the occluding devices may be relatively less stable with increasing LAA orifice size. Compared with LA myocardium, LAA myocardium demonstrates increased expansibility, and we feel that the discrepancy between the two may lead to slight movement of the device from the LAA wall, which may be more evident when the LAA opening is larger. The slight movement of the device may cause damage to the LAA wall and result in microperforation.
The presence of congestive heart failure was a predictor of occurrence of PE after LAAC in the present study. The NCDR Left Atrial Appendage Occlusion Registry also showed that left ventricular function was correlated with incidence of PE (7). Theoretically, patients with congestive heart failure may experience LA pressure overload, which may increase the risk of PE (21, 22). However, no data regarding the relationship between LA pressure and PE following LAAC have been reported. From a pathophysiology perspective, elevated LA pressure may cause alveolar-capillary network destruction and pulmonary vascular remodeling, subsequently leading to pulmonary arterial hypertension. Recently, Zou et al. (23) found that elevated pulmonary artery systolic pressure was correlated with postprocedure PE in patients with AF undergoing LAAC.
4.3. Potential mechanism
The majority of acute PE reported following LAAC have been bloody effusions, which suggests that they may result from injury at various points during the procedure, including transseptal puncture, manipulation of wires and sheaths, or occluders in the LA or LAA. Therefore, monitoring by the use of intraprocedural echocardiography and repeated echocardiography following the procedure are necessary to detect this complication. Previous reports have demonstrated that most delayed pericardial effusions were also bloody. The main cause of bloody delayed PE's is determined to be microperforation of the LAA caused by the occlude device. The literature suggests that patients who had nitinol plug devices (Amulet or LAmbre) implanted have a higher rate of delayed PE than those who had nitinol cage devices (Watchman) implanted. One possible explanation for this difference is that the anchor length of the nitinol plug devices are longer than that of the nitinol cage devices. Our findings may provide insight into factors associated with PE and may inform decision-making on both device selection and patient management. Comprehensive LAA evaluation should be performed to provide information to assist in device selection. Regular echocardiography follow-up examinations are recommended following LAmbre device implantation, especially in those patients with larger LAA orifices or congestive heart failure. In addition, chronic nonspecific pericardial inflammation may be another potential reason for delayed PE although this is relatively rare. We have previously reported a patient with delayed PE who responded well to administration of anti-inflammatory agents. A similar case has been reported in a patient who had undergone an atrial septal defect closure procedure. The underlying cause of this rare complication is hypothesized to be a reaction the nickel content of the device material.
4.4. Study limitations
Several limitations of this study should be noted. Firstly, the study was limited to a single-center's experience. Secondly, the number of patients with PE in our cohort was not sufficiently powered to conduct multivariable logistic regression. Therefore, we used a univariate model to identify predictive factors of the development of PE. Thirdly, acute and delayed PE were analyzed together due to the limited number of patients who developed PE. Further multicenter investigations with larger study samples are needed to confirm the findings of the current research.
5. Conclusion
In this single-center experience, acute and delayed PE were uncommon in patients with AF following the LAmbre device implantation. Congestive heart failure and larger LAA orifice were identified as the predictors of PE.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Ningbo First Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YY designed the study and drafted the manuscript. YY, BW, and GF collected patient data and edited the images. YY and BW did the statistical analysis. HC, BW, BH and GF critically revised the manuscript and approved the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Traditional Chinese Medical Science and Technology Project of Zhejiang Province (grant number 2023ZL157), the Medical Health Science and Technology Project of Zhejiang Province Health Commission (grant number 2020KY821 and 2021KY980), the Key Technology R&D Program of Ningbo (2022Z149), and the Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, China (grant number 2022E10026).
Acknowledgments
We wish to acknowledge all the patients in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
2. Reddy VY, Doshi SK, Kar S, Gibson DN, Price MJ, Huber K, et al. 5-Year Outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. (2017) 70:2964–75. doi: 10.1016/j.jacc.2017.10.021
3. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. (2020) 75:3122–35. doi: 10.1016/j.jacc.2020.04.067
4. Yerasi C, Lazkani M, Kolluru P, Miryala V, Kim J, Moole H, et al. An updated systematic review and meta-analysis of early outcomes after left atrial appendage occlusion. J Interv Cardiol. (2018) 31:197–206. doi: 10.1111/joic.12502
5. Fu G, He B, Chu H, Wang B, Pan Y, Xie B, et al. Delayed pericardial effusion or tamponade following left atrial appendage closure: a single-center experience. J Formos Med Assoc. (2022):S0929-6646(22)00431-4. doi: 10.1016/j.jfma.2022.11.010. [Epub ahead of print]
6. Lakkireddy D, Thaler D, Ellis CR, Swarup V, Sondergaard L, Carroll J, et al. Amplatzer amulet left atrial appendage occluder versus watchman device for stroke prophylaxis (amulet IDE): a randomized, controlled trial. Circulation. (2021) 144:1543–52. doi: 10.1161/CIRCULATIONAHA.121.057063
7. Price MJ, Valderrábano M, Zimmermann S, Friedman DJ, Kar S, Curtis JP, et al. Periprocedural pericardial effusion complicating transcatheter left atrial appendage occlusion: a report from the NCDR LAAO registry. Circ Cardiovasc Interventions. (2022) 15(5):e011718. doi: 10.1161/CIRCINTERVENTIONS.121.011718
8. Munir MB, Khan MZ, Darden D, Pasupula D, Balla S, Han FT, et al. Pericardial effusion requiring intervention in patients undergoing percutaneous left atrial appendage occlusion: prevalence, predictors, and associated in-hospital adverse events from 17,700 procedures in the United States. Heart Rhythm. (2021) 18(9):1508–15. doi: 10.1016/j.hrthm.2021.05.017
9. Mogalapalli A, Kumar S, Lobo T, Reed J, Dallan LAP, Yoon S-H, et al. Delayed pericardial effusion following left atrial appendage closure: a 5-year single-center experience. J Invasive Cardiol. (2023) 35:E1–6.36446576
10. Isogai T, Saad AM, Shekhar S, Kuroda S, Abushouk AI, Gad MM, et al. Incidence and outcomes of pericardial effusion/tamponade following percutaneous left atrial appendage closure. Am J Cardiol. (2021) 160:126–9. doi: 10.1016/j.amjcard.2021.08.028
11. Schmidt B, Betts TR, Sievert H, Bergmann MW, Kische S, Pokushalov E, et al. Incidence of pericardial effusion after left atrial appendage closure: the impact of underlying heart rhythm-data from the EWOLUTION study. J Cardiovasc Electrophysiol. (2018) 29:973–8. doi: 10.1111/jce.13626
12. Huang H, Liu Y, Xu Y, Wang Z, Li Y, Cao K, et al. Percutaneous left atrial appendage closure with the LAmbre device for stroke prevention in atrial fibrillation: a prospective, multicenter clinical study. JACC Cardiovasc Interv. (2017) 10:2188–94. doi: 10.1016/j.jcin.2017.06.072
13. Park JW, Sievert H, Kleinecke C, Vaskelyte L, Schnupp S, Sievert K, et al. Left atrial appendage occlusion with lambre in atrial fibrillation: initial European experience. Int J Cardiol. (2018) 265:97–102. doi: 10.1016/j.ijcard.2018.02.120
14. Xiao F, Chen Y, Chen Y, Zhou X, Wu X, Chen X, et al. Delayed pericardial effusion after left atrial appendage closure with the LAmbre device: importance of a fully open umbrella. J Cardiovasc Electrophysiol. (2021) 32:1646–54. doi: 10.1111/jce.15020
15. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
16. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. (1996) 49:1373–9. doi: 10.1016/s0895-4356(96)00236-3
17. Wang B, Wang Z, Chu H, He B, Fu G, Feng M, et al. Long-term safety and efficacy of left atrial appendage closure in patients with small appendage orifices measured with transesophageal echocardiography. Clin Cardiol. (2023) 46:134–41. doi: 10.1002/clc.23950
18. Boersma LV, Ince H, Kische S, Pokushalov E, Schmitz T, Schmidt B, et al. Evaluating real-world clinical outcomes in atrial fibrillation patients receiving the WATCHMAN left atrial appendage closure technology: final 2-year outcome data of the EWOLUTION trial focusing on history of stroke and hemorrhage. Circ Arrhythm Electrophysiol. (2019) 12:e006841. doi: 10.1161/CIRCEP.118.006841
19. Schnupp S, Liu X-X, Buffle E, Gloekler S, Mohrez Y, Cheikh-Ibrahim M, et al. Late clinical outcomes of lambre versus amplatzer occluders for left atrial appendage closure. J Cardiovasc Electrophysiol. (2020) 31:934–42. doi: 10.1111/jce.14398
20. Chen Y-Y, Zhang Y-H, Chen X, Huang W-P, Xu B, Su X, et al. Left atrial appendage orifice diameter measured with trans-esophageal echocardiography is independently related with peri-device leakage after Watchman device implantation. Int J Cardiovasc Imaging. (2019) 35:1831–9. doi: 10.1007/s10554-019-01625-6
21. Hoshida S, Watanabe T, Shinoda Y, Ikeoka K, Minamisaka T, Fukuoka H, et al. Sex-related differences in left ventricular diastolic function and arterial elastance during admission in patients with heart failure with preserved ejection fraction: the PURSUIT HfpEF study. Clin Cardiol. (2018) 41:1529–36. doi: 10.1002/clc.23073
22. Hoshida S, Tachibana K, Shinoda Y, Minamisaka T, Yamada T, Higuchi Y, et al. Left atrial pressure overload and prognosis in elderly patients with heart failure and preserved ejection fraction: a prospective multicenter observational study. BMJ Open. (2021) 11:e044605. doi: 10.1136/bmjopen-2020-044605
23. Zou T, Chen Q, Zhang L, Chen C, Ling Y, Liu G, et al. Pulmonary artery pressure is associated with mid-term major adverse cardiovascular events and postprocedure pericardial effusion in atrial fibrillation patients undergoing left atrial appendage occlusion. Ann Transl Med. (2021) 9:1324. doi: 10.21037/atm-21-3561
Keywords: pericardial effusion, predictor, LAmbre, left atrial appendage closure, atrial fibrillation
Citation: Yu Y, Chu H, Wang B, He B and Fu G (2023) Pericardial effusion following percutaneous left atrial appendage closure using the LAmbre device. Front. Cardiovasc. Med. 10:1188322. doi: 10.3389/fcvm.2023.1188322
Received: 17 March 2023; Accepted: 24 May 2023;
Published: 6 June 2023.
Edited by:
Ruiqin Xie, Second Hospital of Hebei Medical University, ChinaReviewed by:
Mario Matta, AOU Città della Salute e della Scienza, ItalyTong Liu, Tianjin Medical University, China
© 2023 Yu, Chu, Wang, He and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huimin Chu ZXBuYmhlYXJ0QDE2My5jb20=
 Yibo Yu
Yibo Yu Huimin Chu
Huimin Chu Binhao Wang
Binhao Wang Bin He
Bin He Guohua Fu
Guohua Fu