
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 June 2023
Sec. General Cardiovascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1185595
This article is part of the Research Topic Frontiers in Cardiovascular Medicine: Rising Stars 2022 View all 75 articles
 Lu Ren1,2
Lu Ren1,2 Fengjuan Li1,2
Fengjuan Li1,2 Xin Tan1,2
Xin Tan1,2 Yangkai Fan1,2
Yangkai Fan1,2 Bingbing Ke3
Bingbing Ke3 Yixin Zhang4
Yixin Zhang4 Hongfeng Jiang1,2
Hongfeng Jiang1,2 Lixin Jia1,2,5
Lixin Jia1,2,5 Yuan Wang1,2*
Yuan Wang1,2* Jie Du1,2*
Jie Du1,2*
Background: Worsening heart failure (WHF) is a heterogeneous clinical syndrome with poor prognosis. More effective risk stratification tools are required to identify high-risk patients. Evidence suggest that aberrant ceramide accumulation can be affected by heart failure risk factors and as a driver of tissue damage. We hypothesized that specific ceramide lengths and ratios serve as biomarkers for risk stratification in WHF patients by reflecting pathological changes of distinct organ dysfunctions.
Medthods: We measured seven plasma ceramides using liquid chromatography-mass spectrometry (LC-MS) in 1,558 patients, including 1,262 participants in retrospective discovery set and 296 WHF patients in prospective validation set in BIOMS-HF study (Registry Study of Biomarkers in Heart Failure). Univariable and multivariable logistic regression models were constructed to identify associations of ceramides with organ dysfunctions.
Results: We constructed three ceramide-based scores linked independently to heart, liver, and kidney dysfunction, with ceramides and ratios included in each score specifying systemic inflammation, chronic metabolic disorder, and water-sodium retention. The combined ceramide heart failure score (CHFS) was independently associated with adverse outcomes [Hazard Ratio, 2.80 (95% CI: 1.78–4.40; P < 0.001); 2.68 995% CI: 1.12–6.46; P = 0.028)] and improved the predictive value of Acute Decompensated Heart Failure National Registry score and BNP [net reclassification index, 0.34 (95% confidence interval, CI: 0.19–0.50); 0.42 (95% CI: 0.13–0.70)] in the discovery and validation set, respectively. Lower BNP levels, but higher CHFS had the highest hazard of future adverse events in WHF patients.
Conclusion: Abnormal plasma ceramides, associated with heart and peripheral organ dysfunctions, provide incremental prognostic information over the ADHERE score and brain natriuretic peptide concentration for risk stratification in WHF patients. This may facilitate the reclassification of high-risk patients in need of aggressive therapeutic interventions.
Worsening heart failure (WHF) is a life-threatening disorder with a 1-year event rate of 40% (1). The current treatment for WHF is mostly symptomatic; at best, it is tailored according to the initial hemodynamic status. However, some patients experience high mortality and hospital readmission rates (2).
Patients with WHF often have coexisting multi-organ injury/dysfunction (heart, kidney, and liver) upon admission (3–6). Maladaptive crosstalk between the heart and injured peripheral organs leads to insufficient peripheral perfusion, a persistent congestive state, and abnormal changes in cardiac systolic or diastolic function, resulting in pathological ventricular remodeling and adverse outcomes (7). Dysfunction or injury to the peripheral organs is a common result of different etiological and risk factors, as suggested by the severity and complex pathological heterogeneity of WHF (8). Several clinical markers have been recommended in the European Heart Failure (HF) guidelines for the identification of organ dysfunction, including alanine transaminase (ALT), aspartate transaminase (AST), creatine kinase-MB, and estimated glomerular filtration rate (9). The natriuretic peptide concentration and Acute Decompensated Heart Failure National Registry (ADHERE) score, which are derived from the patient's blood urea nitrogen concentration, systolic blood pressure, and creatinine concentration, provide a means of assessing an individual patient's risk of inpatient mortality due to HF (10–12). Despite the availability of these measures, we have not been able to reduce adverse clinical outcomes in patients with WHF. Therefore, identifying the underlying pathological changes linked to different types of organ dysfunction could help improve the risk stratification and treatment of patients with WHF.
Various underlying pathophysiological changes, such as proinflammatory activation, oxidative stress, persistent ischemia, unresolved hyperemia, unhealthy metabolic status, and hypoperfusion, often coexist in patients with WHF who have organ injury/dysfunction (13). Several studies have shown that lipid metabolism reflects the underlying pathological processes in peripheral organs and aggravates the deterioration of cardiac function (14–16). The bioactive sphingolipid ceramide is both a structural component of the cell membrane and a signaling molecule that regulates endoplasmic reticulum stress, apoptosis, mitochondrial energy metabolism, and insulin resistance (17). Evidence suggests that distinct ceramides are closely related to several cardiometabolic diseases such as diabetes, hypertension, and coronary heart disease, and act as prognostic biomarkers for cardiovascular diseases (14, 18–20). A recent study conducted using an animal model of fatty liver disease showed that plasma Cer(d18:1/24:1) and Cer(d18:1/24:1) levels were both affected by the activation of ceramide synthase 2, which strongly indicates metabolic disorders in the liver tissue. Ahmad et al. showed that tissue-specific ceramide synthase 6 in glomerular podocytes produces Cer(d18:1/16:0), which affects renal perfusion by participating in oxidative stress and inflammatory reactions during acute/chronic kidney injury (21). Thus, it is becoming evident that distinct plasma ceramides are probably tissue-specific and have different physiological functions. Exploring the relationship between distinct organ dysfunctions and specific ceramide lengths in patients with WHF may provide evidence to further elucidate how these bioactive sphingolipids affect the occurrence and development of HF. Therefore, we hypothesized that specific ceramide lengths and ratios could serve as biomarkers for risk stratification in patients with WHF by reflecting pathological changes in distinct organ dysfunction.
In the present study, we identified a correlation between distinct ceramides and adverse outcomes in patients with WHF. We assessed the association between ceramide molecules and organ dysfunction. Finally, we established a ceramide heart failure score (CHFS) and verified its feasibility for risk stratification to provide greater insight into the potential role of ceramide in WHF.
The overall study design is shown in Supplementary Figure S1. This study comprised a cross-sectional discovery set, a retrospective discovery set, and a prospective validation cohort. Data supporting the findings of this study are available from the corresponding author upon reasonable request.
One cross-sectional study was established to evaluate whether plasma ceramides are associated with heart failure progression. The cross-sectional set (Supplementary Figure S1A) included 447 patients in 3 heart failure stages: healthy controls, preclinical patients, and patients with worsening heart failure. All the participants were age- and sex-matched. Patients with worsening heart failure were identified from the Registry Study of Biomarkers in Heart Failure (BIOMS-HF) cohort (22). The inclusion criteria were as follows: A total of 149 healthy controls were recruited from the population participating in the physical examination center at Anzhen Hospital. The inclusion criterion was a healthy clinical status with a potential risk of HF but without structural heart disease. The 149 participants with preclinical HF had a history of structural heart disease but no symptoms of HF.
Discovery and validation sets were obtained from BIOMS-HF, a retrospective and prospective cohort study (Supplementary Figure S1B). The retrospective discovery cohort included 964 patients with WHF who visited the Beijing Anzhen Hospital, Capital Medical University, between August 2017 and March 2019 (BIOMS-HF study registration number: NCT03784833 in ClinicalTrials.gov). The prospective validation set included 296 patients with WHF who visited Beijing Anzhen Hospital, Capital Medical University, between April 2019 and June 2019. This study was based on the inclusion criteria of BIOMS-HF: age of ≥18 years, HF symptoms and signs (dyspnea or minimal exertion at rest, dry and wet oral, pleural and ascites, peripheral edema, or pulmonary congestion on x-ray films), and brain natriuretic peptide (BNP) and N-terminal pro-BNP (NT-pro-BNP) concentrations of ≥35 and ≥125 pg/ml, respectively (23). For a diagnosis of WHF, patients were required to have a relevant history of HF symptoms and signs and a diagnosis of structural heart disease. Structural heart disease included any one of the following criteria: an increased left ventricular end-diastolic size as measured by echocardiography (≥55 mm); a left ventricular ejection fraction (LVEF) of ≤50%; ventricular septal thickening (>12 mm) or left ventricular posterior wall thickening (>12 mm) as measured by echocardiography; severe valve stenosis/dysfunction; or significant myocardial abnormality (cardiomyopathy), congenital heart disease, or previous cardiac surgery (24). The detailed study design is presented in Supplementary Figure S1B. A total of 964 patients in the discovery set and 296 in the validation set were analyzed, including 972 patients without composite events and 288 patients with composite events.
This study was approved by the local ethics committee. The study was performed in accordance with the requirements of the Declaration of Helsinki. All the participants provided written informed consent.
The primary endpoints were all-cause mortality and the following events: repeated hospitalization for HF, recommendation for heart transplantation by physicians, implantation of a cardiac resynchronization therapy defibrillator (25), and follow-up with a New York Heart Association functional classification of IV. Unplanned emergency visits or hospitalizations leading to HF deterioration were defined as those due to HF. Data on events were obtained from telephone or electronic medical records. The primary endpoints and adverse events were reviewed and confirmed by certified physicians to ensure accuracy. There were 315 (33%) patients who suffered from HF event/hospitalization, and 152 (16%) patients who died in the experimental period in the discovery set. The validation set included 97 (33%) patients with HF event/hospitalization and 41 (14%) patients died in the experimental period. During the median follow-up of 325.5 days in the discovery set, 129 patients were lost to follow-up (follow-up rate: 86.6%). During a median follow-up of 208 days in the validation set, 13 patients were lost to follow-up (follow-up rate: 95.6%).
Fasting blood samples were collected in ethylenediaminetetraacetic acid tubes, centrifuged, aliquoted, and stored at −80°C until analysis. Ultra-performance liquid chromatography-tandem mass spectrometry was performed to quantitatively detect several plasma ceramides [Cer(d18:1/14:0), Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:0), Cer(d18:1/20:0), Cer(d18:1/22:0), Cer(d18:1/24:0), and Cer(d18:1/24:1)] using a Thermo TSQ Quantum mass spectrometer equipped with an electrospray ionization probe and interfaced with the Agilent 1,290 Infinity LC system (Agilent, Palo Alto, CA, United States). The injection volume of the extracted samples was 10 µl. The details of the ceramide tests were elaborated from previous research conducted by our team (26).
Organ dysfunction/injury was defined based on baseline measurements. A troponin I concentration greater than the upper reference limit (URL) (>0.056 ng/ml) was considered myocardial injury (27). Renal insufficiency was defined as an estimated glomerular filtration rate of <60 ml/min/1.73 m2, calculated using the Modification of Diet in Renal Disease Study equation (28, 29). The presence of at least one of these abnormal liver function test results indicates liver injury or dysfunction: ALT and AST levels beyond the upper limit reference range for liver function (male: 9–50 U/L, female: 7–40 U/L; AST: male:15–40 U/L; female: 13–35 U/L), bilirubin concentration higher than the URL (>1.3 mg/ml), and albumin concentration lower than the lower reference limit (<3.5 mg/dl) (30, 31).
Continuous variables with a normal distribution are expressed as means with standard deviations. The statistical significance of the differences between the groups was tested using analysis of variance, Student's t-test, or the χ2 test, as appropriate. Statistical significance was set at P < 0.05. Logistic regression was used to estimate the odds ratios (OR) per standard deviation. Cox proportional hazards were used to calculate hazard ratios (HR). Proportional hazard assumptions were tested using Schoenfeld residuals. The Spearman correlation coefficient was used to evaluate association for continuous variables. Logistic regression analysis was used to evaluate the correlation between scores with categorical clinical outcomes (e.g., diabetes, ascites, ortopnea). Excluding cases with missing values may bias the results (32); thus, imputation proceeded in two steps: first, continuous variables were imputed using the EM algorithm to create a monotone missingness pattern, and then categorical variables were imputed using the logistic regression method. Statistical analyses were performed using Stata 15.0 statistical software and R.
Univariate and multivariate logistic regression models were constructed to determine the relationship between ceramide length and distinct organ dysfunction (heart, liver, and kidney). To assess the robustness of the final model, we performed a 1,000-repeat boot analysis (using Stata's “swboot” package). Variables selected more than 700 times were considered as robust predictors. The Hosmer–Lemeshow test was performed to test for the model's goodness-of-fit. A restricted cubic spline (three nodes of all variables) was constructed to display the relationship between the Ceramide lengths and ratios and organ injuries.
The ceramide heart, liver, and kidney scores were constructed based on the logistic model coefficient for each given ceramide concentration and the corresponding ratio:
where βk is the coefficient of the multivariable logistic model for each ceramide length or ratio concentration and b is the constant term of the model. We decided ceramide heart, liver, and kidney scores after comparing models through the Akaike information criteria (AIC) and the Bayesian information criteria (BIC). Because dysfunction/injury to more than one organ (heart, kidney, or liver) is a well-recognized independent predictor of poor outcomes, we constructed the CHFS as the sum of the ceramide heart, liver, and kidney scores. A multicollinearity test was conducted to detect the multicollinearity between key variables of CHFS (ceramide heart score, ceramide liver score, and ceramide kidney score). We identified the effect modification on the BNP level using multiplicative interaction terms.
Among the 964 patients with WHF in the discovery set, the baseline characteristics of the patients with and without composite events are shown in Table 1. Patients with composite events were older and had a higher incidence of diabetes mellitus. During laboratory examinations, patients with composite events had higher BNP, lower high-density lipoprotein, higher high-sensitivity C-reactive protein, and higher creatine kinase concentrations. There were no other statistically significant differences in baseline characteristics. The characteristics of the validation set are presented in Supplementary Table S4. The baseline levels of Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1), and Cer(d18:1/24:0) and the distinct ratios of Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/18:0)/Cer(d18:1/24:0), and Cer(d18:1/24:1)/Cer(d18:1/24:0) were significantly different between event and no-event groups in the discovery cohort. No significant differences were found in other chain species [Cer(d18:1/14:0), Cer(d18:1/20:0), and Cer(d18:1/22:0)] (Supplementary Table S1). We also observed that the Cer(d18:1/16:0), Cer(d18:1/18:0), and Cer(d18:1/24:1) levels increased, whereas the Cer(d18:1/24:0) levels decreased. The results are presented in Supplementary Figure S2. The remaining chain lengths [Cer(d18:1/14:0), Cer(d18:1/20:0), and Cer(d18:1/22:0)] showed no trends or significant differences.
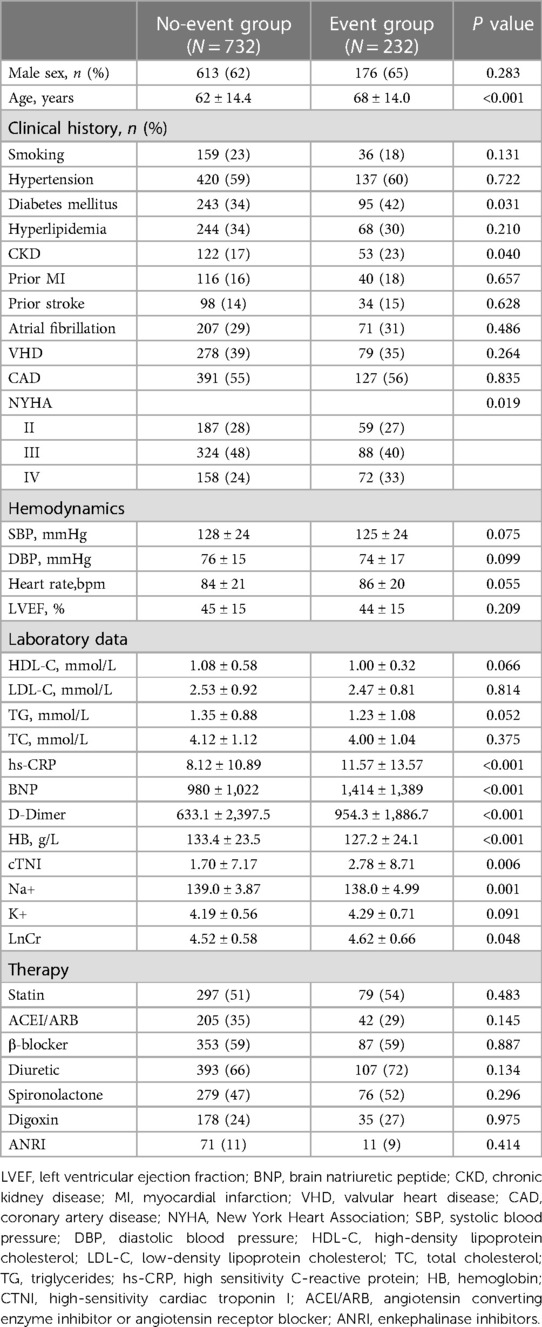
Table 1. Baseline characteristics of event and non-event groups of patients with worsening heart failure in the discovery cohort.
In the multivariate Cox regression analyses, Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1), and Cer(d18:1/24:0) accumulation was positively associated with the primary endpoint after adjustment for age and sex; the HR was 1.85 [95% confidence interval (CI): 1.38–2.74], 1.34 (1.08–1.65), 1.33 (1.02–1.76), and 0.69 (0.53–0.91), respectively; Supplementary Table S2]. After adjusting for age, sex, and clinical risk factors (Model 1, including sex, age, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, diabetes, hypertension, hyperlipidemia, chronic renal disease, atrial fibrillation, coronary heart disease, and smoking), the ratios Cer(d18:1/16:0)/Cer(d18:1/24:0), Cer(d18:1/18:0)/Cer(d18:1/24:0), and Cer(d18:1/24:1)/Cer(d18:1/24:0) were still significantly related to adverse events [HR: 2.58 (95% CI: 1.74–3.82); HR: 1.65 (95% CI: 1.22–2.22); and HR: 1.99 (95% CI: 1.40–2.82), respectively] (Figure 1).
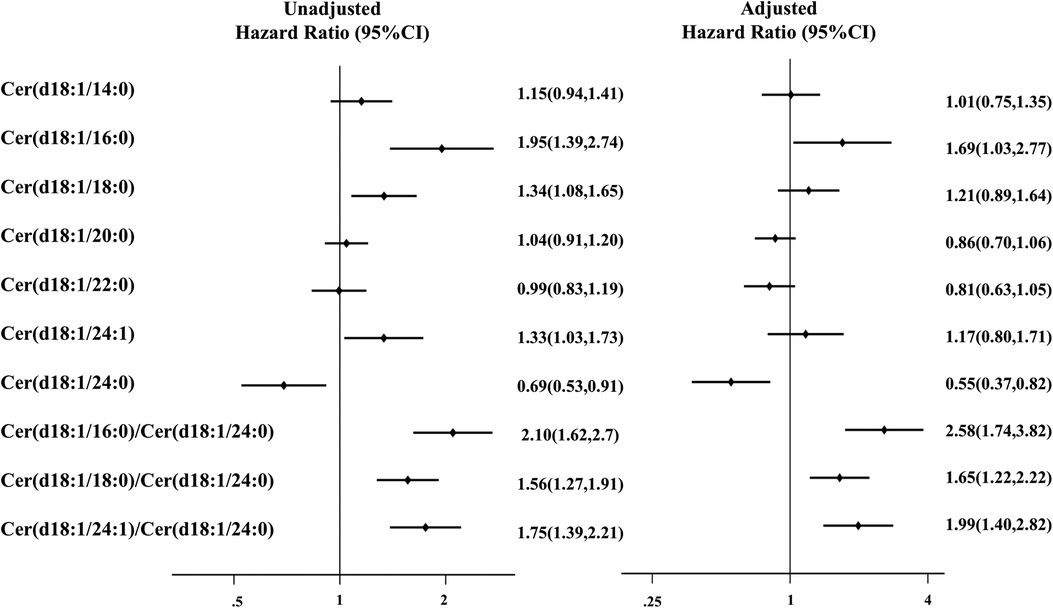
Figure 1. Association of combined endpoint with baseline ceramide lengths and ratios in the discovery cohort. Model was adjusted for sex, age, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, diabetes, hypertension, hyperlipidemia, chronic renal disease, atrial fibrillation, coronary heart disease, and smoking. Effect size (odds ratio and 95% confidence interval) is presented for each ceramide length and ratio.
Univariate and multivariate logistic regression analyses of ceramide lengths and ratios for different organ dysfunctions in the discovery cohort are shown in Table 2. Increases of Cer(d18:1/18:0) and the ceramide ratios Cer(d18:1/18:0)/Cer(d18:1/24:0) were correlated with cardiac injury; very-long-chain ceramides [Cer(d18:1/24:0) and Cer(d18:1/24:1)] and Cer(d18:1/16:0) were correlated with liver dysfunction; Cer(d18:1/16:0) and Cer(d18:1/16:0)/Cer(d18:1/24:0) were correlated with renal dysfunction (all P < 0.005). Before the detailed analysis, we quantified the appropriateness of our statistical models using AIC and BIC (Supplementary Table S7). The final model was selected considering both the statistical significance and Bayesian information criteria and detailed equation was shown in Supplementary Table S10.
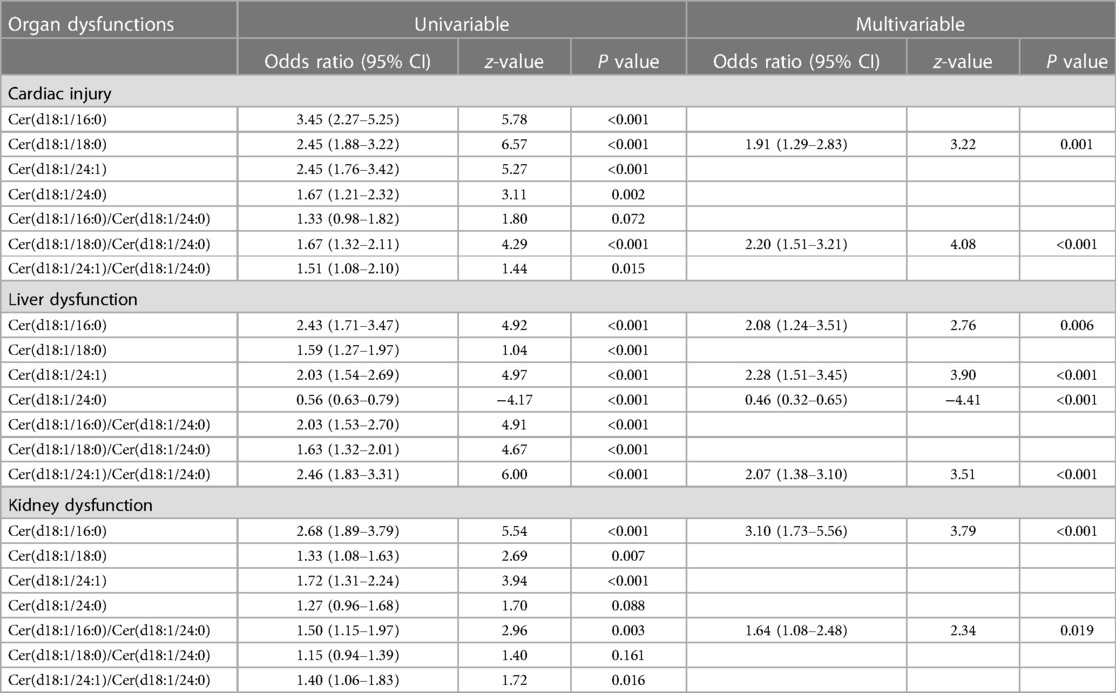
Table 2. Univariate and multivariate logistic regression of ceramide lengths and ratios for different organ dysfunctions in the discovery cohort.
Furthermore, we constructed ceramide heart, liver, and kidney scores based on plasma ceramide concentrations and distinct ratios. The restricted cubic spline of ceramide levels, cardiac injury/liver dysfunction, and kidney dysfunction scores are shown in Figure 2. The Hosmer–Lemeshow goodness-of-fit test for three logistic regression models shows that the models are acceptable (ceramide heart score: P = 0.123; ceramide liver score: P = 0.859; ceramide kidney score: P = 0.136) (Supplementary Table S6). We examined the correlations between these scores and the clinical characteristics in the discovery cohort of patients with WHF (Figure 3, Supplementary Figure S4). We found that the ceramide liver score was correlated with chronic metabolic injuries, including body mass index (BMI), glutamyl-transpeptidase (GGT), diabetes, orthopnea, and ascites; The ceramide kidney score significantly correlated with kidney function impairment and signs of water and sodium retention (edema score and orthopnea). Ceramide heart score associated with LVEF and cardiac tissue damage marker CKMB, suggesting a clinical phenotype related to myocardial damage. Our results demonstrated different patterns of clinical characteristics among the ceramide heart, liver, and kidney scores.
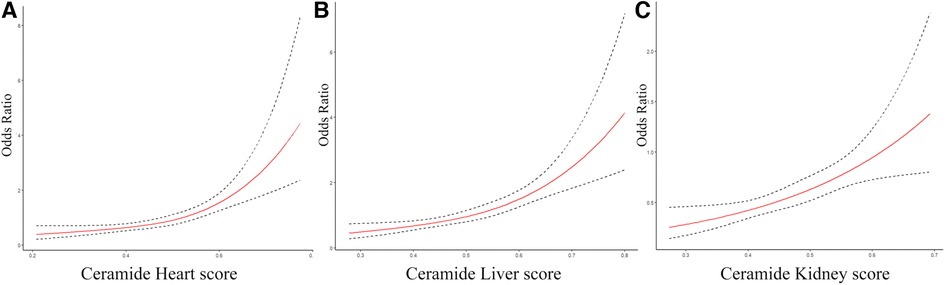
Figure 2. The restricted cubic spline of ceramide heart, liver, and kidney scores for different organ dysfunctions. (A) Restricted cubic spline of the association between the ceramide heart score and the cardiac injury. (B) Restricted cubic spline of the association between the ceramide liver score and liver dysfunction. (C) Restricted cubic spline of the relationship between the ceramide kidney score and kidney dysfunction. The solid lines indicate estimates of the odds ratios of organ damage across continuous levels of different ceramide scores, fitted using logistic regression analysis. The dashed lines indicate the 95% confidence intervals.
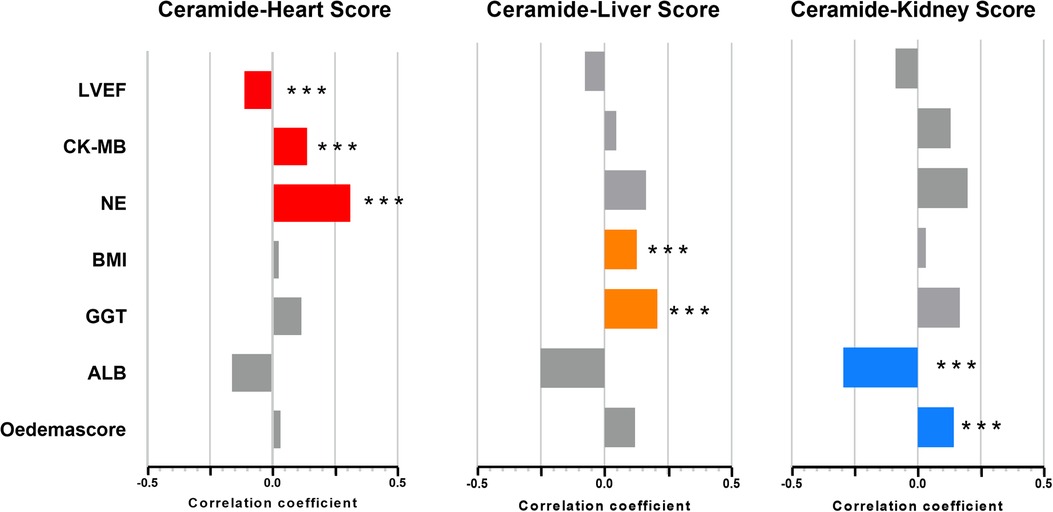
Figure 3. Association of ceramide cardiac injury/liver dysfunction and kidney dysfunction scores and clinical characteristics. DM, diabetes mellitus; LVEF, left ventricular ejection fraction; NE, neutrophilic granulocytes; WBC, white blood cells. *P < 0.05, **P < 0.01, ***P < 0.001.
Finally, we developed a novel CHFS comprising the ceramide heart, liver, and kidney scores to model the clinical use of ceramide in patients with WHF. Test of multicollinearity for all variables resulted in the variance inflation factor (VIF) scores ranging from 1.07 to 2.19, indicating no concerns about multicollinearity (Supplementary Table S7). The CHFS was independently correlated with all-cause mortality in both the discovery set and validation cohort (HR: 2.76; 95% CI: 1.75–4.33; P < 0.001; HR: 3.47; 95% CI: 1.36–8.87; P = 0.009) and composite events (HR: 2.91; 95% CI: 1.23–6.88; P < 0.001; HR: 3.45; 95% CI: 1.10–10.80; P = 0.034) (Supplementary Table S3). We tested assumptions of proportional hazards using Schoenfeld residuals in both discovery and validation set (Supplementary Table S8). The global test showed no deviation from proportionality (discovery set: P = 0.329, validation set: P = 0.264) (Supplementary Table S9). The effect of the addition of CHFS to the ADHERE score (age, sex, urea concentration, heart rate, and systolic blood pressure) in predicting the composite endpoint or all-cause mortality was assessed using the category-free (continuous) net reclassification index (NRI) (Supplementary Table S4). We examined whether the addition of CHFS to the ADHERE logistic regression variables improved the overall WHF patient classification for all-cause mortality in the entire cohort (NRI: 0.31; 95% CI: 0.18–0.44). The ceramide heart failure score provided favorable risk reclassification for combined endpoint and all-cause motility beyond traditional ADHERE and BNP levels [net reclassification improvement, 0.42 (95% CI: 0.13–0.70) and 0.49 (95% CI: 0.20–0.78), respectively]. We observed no interaction between the CHFS and BNP levels in association with the composite event (interaction, P = 0.135, Figure 4). The hazard of the composite event for higher CHFS appeared to be greatest in patients with lower BNP levels and worsening heart failure in the validation set.
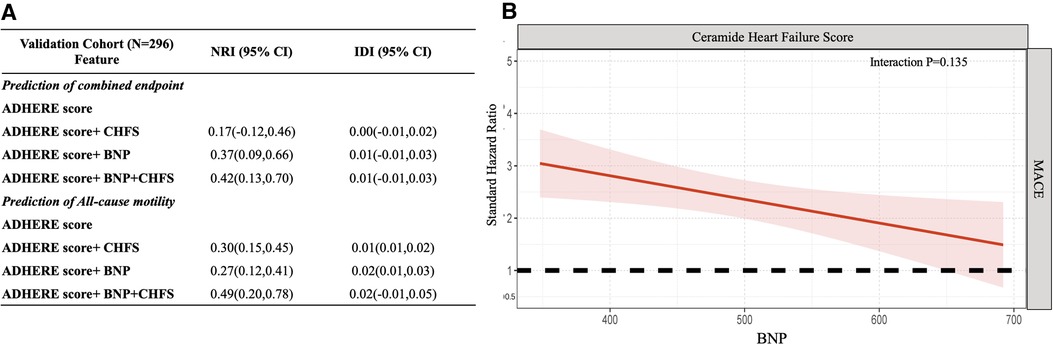
Figure 4. Abnormal ceramides from organ dysfunction indicate the greatest risk in worsening heart failure. (A) Additional value of CHFS for prediction of combined endpoint and all-cause motility in the validation cohort. (B) The standard hazard for ceramide heart failure score across BNP levels for the composite endpoint in worsening heart failure patients. This figure demonstrates that the hazard of CHFS is higher in lower BNP worsening heart failure patients. ADHERE predictors: age, sex, urea, heart rate, and systolic blood pressure. CHFS consists of the combined ceramide heart, liver, and kidney scores. Point estimates and confidence limits for reclassification (NRI) and fit statistics are described in the Methods section. ADHERE, Acute Decompensated Heart Failure National Registry; BNP, brain natriuretic peptide; CHFS, ceramide heart failure score; CI, confidence interval; NRI, net reclassification index; WHF, worsening heart failure.
We stratified worsening heart failure patients by LV ejection fraction and (Supplementary Table S5). After adjusting for clinical variables, CHFS were significantly associated with poor outcomes in patients with HFpEF [hazard ratio, 3.85 95% CI: 1.50–9.87; P = 0.005].
In this study, we found that specific ceramide forms [Cer(d18:1/16:0), Cer(d18:1/18:0), Cer(d18:1/24:1), and Cer(d18:1/24:0)] were associated with HF severity. We then discovered a relationship between abnormal ceramide lengths and ratios and the risk of adverse events that subsequently progressed to refractory HF or death in patients with WHF in the discovery set. After adjusting for clinical risk factors, the ceramide ratios remained significantly associated with adverse events. Furthermore, we determined the ceramide heart, liver, and kidney scores with C18:0 and C18:0/Cer(d18:1/24:0), Cer(d18:1/16:0) and Cer(d18:1/24:1)/Cer(d18:1/24:0), and Cer(d18:1/16:0) and Cer(d18:1/16:0)/Cer(d18:1/24:0), respectively. Finally, we established that the CHFS (the summary of the three ceramide organ scores mentioned above) can be used as a risk stratification tool to identify high-risk patients with WHF. Abnormal plasma ceramide levels and ratios provide additional information about the injury/dysfunction of the heart and peripheral organs and the risk of HF deterioration, which may indicate specific pathophysiological characteristics of patients with WHF (33).
Studies have shown that ceramide destroys mitochondrial respiration and leads to the decompensation of mitochondrial function, thus affecting cardiac function (34). Inflammation is an important factor in the development of diastolic dysfunction in patients with WHF. Our data suggest that Cer(d18:1/18:0) and the ceramide ratio Cer(d18:1/16:0)/Cer(d18:1/24:0) are strongly correlated with myocardial ischemia and inflammation status. Studies have shown that CerS4 is expressed in the heart, has a high affinity for C18 and C20 acyl-CoA, and contributes to heart failure (35). In cardiomyocytes, CerS2 overexpression causes oxidative stress and mitochondrial dysfunction via lipid overload, eventually leading to apoptosis (36).
In patients with worsening heart failure, myocardial ischemia and hypoxia cause a serious decline in cardiac output, leading to a decrease in circulating blood volume and poor perfusion of major peripheral organs. The imbalance of metabolic pathways, such as the inflammatory response, insulin resistance, weakening of anabolism, and accumulation of oxygen free radicals caused by abnormal hemodynamic changes, simultaneously affects cardiac function and aggravates disease progression (34). Studies revealed that under acute kidney damage state, production of reactive oxygen species and proximal tubular cell death induced activation of CerS6, causing the elevation of C16:0) both in kidney tissue and plasma (37–39). Our data suggest that Cer(d18:1/16:0) and Cer(d18:1/18:0) levels are associated with kidney damage.
Previous studies have pointed out that the difference between long-chain (C16:0-C18:0) and ultra-long-chain (C20:0-C24:0) ceramide is produced by different ceramide synthase isomers (CerS1/5/6 and CerS2/4, respectively). Different concentrations of plasma ceramides may also reflect changes in the composition and function of cellular membranes within tissues. Our study showed that Cer(d18:1/16:0) and ceramide ratio Cer(d18:1/24:1)/Cer(d18:1/24:0) indicated chronic metabolic vulnerability and liver injury. By reducing ER stress and PEPCK expression, plasma very-long-chain ceramide (C24, C24:1) secreted by CerS2 reduced cellular stress and glucose homeostasis (40–42).
CerS isoforms may partially explain the altered ceramide composition, providing a biological explanation for the ceramide ratios. Studies have confirmed that de novo ceramide levels must be within a narrow range to maintain normal cardiac homeostasis. Thus, several hypothesized biological mechanisms link different ceramide and their ratios to cardiac dysfunction and increased mortality. (1) Ceramide metabolism and apoptosis in cardiac dysfunction: De Paola et al. showed that Cer(d18:1/16:0) generates reactive oxygen species and contributes to cardiomyocyte apoptosis. Interestingly, Cer(d18:1/24:0) counteracts Cer(d18:1/16:0)-mediated cytochrome c release in a dose-dependent manner, possibly by interfering with mitochondrial outer membrane channel formation and reducing membrane permeability (36, 43). (2) Ceramide promotes inflammatory activation during cardiac dysfunction: studies have revealed that ceramides contribute to inflammatory processes both as regulators of cytokine production and downstream effectors that mediate cytokine-induced stress responses (44–46). Ceramides can also induce vascular dysfunction by deactivating endothelial nitric oxide synthase (47). (3) Ceramides as potential inducers of fibrosis: ceramides stimulate the proteolytic processing of cAMP-responsive element-binding protein 3-like protein 1 (CREB3L1), a transcription factor that induces collagen production (48, 49). Although these actions have not been explored in the heart, these and other mechanisms may explain how ceramides contribute to myocardial energy metabolism, inflammation, and fibrotic responses that drive HF progression.
In line with these previous findings, our clinical results supported that the plasma ceramide length and ratio reflected the impairment of heart/liver/kidney organ function in WHF patients and indicated myocardial injury, inflammation, and water sodium retention. Abnormal plasma ceramides are associated with an increased risk of disease progression. Our study proved that CHFS is a practical tool for refining the risk stratification of a worsening adverse prognosis in patients with WHF. Further research is needed to discern the potential of specific ceramides to serve as therapeutic targets for HF-protective drugs.
This study has several limitations. Because the study was retrospective and involved a single-center cohort, it may have been subjected to bias owing to uncontrolled confounding factors. We cannot prove a causal relationship between the duration of ceramide use and progression to end-stage HF and death. Moreover, ceramide is a sphingolipid derived from diet and other factors. Despite these limitations, our results provide new insights into the value of ceramides in evaluating the prognosis of patients with WHF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The local Ethics Committee approved this study. The study protocol met the requirements of the Helsinki declaration. All participants provided written informed consent. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
LR performed the data analysis and wrote the manuscript. FL performed the validation. XT performed the methodology. YF performed data curation. BK and YZ prepared resources together. HJ and LJ performed the investigation. YW performed the writing review. JD performed the project administration. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key R & D Program of China (2021YFA0805100) and the National Natural Science Foundation of China (91939303 and 81790622).
We thank Angela Morben, DVM, and ELS from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English language of the draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1185595/full#supplementary-material
1. Luczak ED, Wu Y, Granger JM, Joiner MA, Wilson NR, Gupta A, et al. Mitochondrial CaMKII causes adverse metabolic reprogramming and dilated cardiomyopathy. Nat Commun. (2020) 11(1):4416. doi: 10.1038/s41467-020-18165-6
2. Frantz S, Falcao-Pires I, Balligand JL, Bauersachs J, Brutsaert D, Ciccarelli M, et al. The innate immune system in chronic cardiomyopathy: a European Society of Cardiology (ESC) scientific statement from the working group on myocardial function of the ESC. Eur J Heart Fail. (2018) 20(3):445–59. doi: 10.1002/ejhf.1138
3. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J. (2019) 40(26):2155–63. doi: 10.1093/eurheartj/ehz158
4. Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, et al. A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J. (2014) 35(7):431–41. doi: 10.1093/eurheartj/eht459
5. Zymlinski R, Sokolski M, Biegus J, Siwolowski P, Nawrocka-Millward S, Sokolska JM, et al. Multi-organ dysfunction/injury on admission identifies acute heart failure patients at high risk of poor outcome. Eur J Heart Fail. (2019) 21(6):744–50. doi: 10.1002/ejhf.1378
6. Harjola VP, Mullens W, Banaszewski M, Bauersachs J, Brunner-La Rocca HP, Chioncel O, et al. Organ dysfunction, injury and failure in acute heart failure: from pathophysiology to diagnosis and management. A review on behalf of the Acute Heart Failure Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail. (2017) 19(7):821–36. doi: 10.1002/ejhf.872
7. Diab KJ, Adamowicz JJ, Kamocki K, Rush NI, Garrison J, Gu Y, et al. Stimulation of sphingosine 1-phosphate signaling as an alveolar cell survival strategy in emphysema. Am J Respir Crit Care Med. (2010) 181(4):344–52. doi: 10.1164/rccm.200906-0826OC
8. Bertero E, Maack C. Metabolic remodelling in heart failure. Nat Rev Cardiol. (2018) 15(8):457–70. doi: 10.1038/s41569-018-0044-6
9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
10. Royston P, Altman DG. Risk stratification for in-hospital mortality in acutely decompensated heart failure. JAMA. (2005) 293(20):2467–8; author reply 2468. doi: 10.1001/jama.293.20.2467-c
11. Smith JG, Newton-Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. (2010) 56(21):1712–9. doi: 10.1016/j.jacc.2010.05.049
12. Januzzi JL Jr, Sakhuja R, O'Donoghue M, Baggish AL, Anwaruddin S, Chae CU, et al. Utility of amino-terminal pro-brain natriuretic peptide testing for prediction of 1-year mortality in patients with dyspnea treated in the emergency department. Arch Intern Med. (2006) 166(3):315–20. doi: 10.1001/archinte.166.3.315
13. Tang WHW, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. (2019) 16(3):137–54. doi: 10.1038/s41569-018-0108-7
14. Wittenbecher C, Eichelmann F, Toledo E, Guasch-Ferre M, Ruiz-Canela M, Li J, et al. Lipid profiles and heart failure risk: results from two prospective studies. Circ Res. (2021) 128(3):309–20. doi: 10.1161/CIRCRESAHA.120.317883
15. Zhou J, Bai L, Zhang XJ, Li H, Cai J. Nonalcoholic fatty liver disease and cardiac remodeling risk: pathophysiological mechanisms and clinical implications. Hepatology. (2021) 74(5):2839–47. doi: 10.1002/hep.32072
16. Baek J, He C, Afshinnia F, Michailidis G, Pennathur S. Lipidomic approaches to dissect dysregulated lipid metabolism in kidney disease. Nat Rev Nephrol. (2022) 18(1):38–55. doi: 10.1038/s41581-021-00488-2
17. Summers SA, Chaurasia B, Holland WL. Metabolic messengers: ceramides. Nat Metab. (2019) 1(11):1051–8. doi: 10.1038/s42255-019-0134-8
18. Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharmacol Rev. (2008) 60(2):181–95. doi: 10.1124/pr.107.07113
19. Vykoukal J, Fahrmann J, Gregg J, Tang Z, Basourakos S, Irajizad E, et al. Caveolin-1-mediated sphingolipid oncometabolism underlies a metabolic vulnerability of prostate cancer. Nat Commun. (2020) 11(1):4279. doi: 10.1038/s41467-020-17645-z
20. Yun H, Sun L, Wu Q, Zong G, Qi Q, Li H, et al. Associations among circulating sphingolipids, Β-cell function, and risk of developing type 2 diabetes: a population-based cohort study in China. PLoS Med. (2020) 17(12):e1003451. doi: 10.1371/journal.pmed.1003451
21. Ahmad A, Mitrofanova A, Bielawski J, Yang Y, Marples B, Fornoni A, et al. Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes. FASEB J. (2017) 31(2):771–80. doi: 10.1096/fj.201600618R
22. Ke B, Tan X, Ren L, Fan Y, Zhang Y, Li F, et al. Aldosterone dysregulation predicts the risk of mortality and rehospitalization in heart failure with a preserved ejection fraction. Sci China Life Sci. (2022) 65(3):631–42. doi: 10.1007/s11427-021-1945-6
23. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37(27):2129–200. doi: 10.1093/eurheartj/ehw128
24. Shiba N, Nochioka K, Miura M, Kohno H, Shimokawa H, Investigators C. Trend of westernization of etiology and clinical characteristics of heart failure patients in Japan—first report from the chart-2 study. Circ J. (2011) 75(4):823–33. doi: 10.1253/circj.cj-11-0135
25. Chirinos JA, Zhao L, Jia Y, Frej C, Adamo L, Mann D, et al. Reduced apolipoprotein M and adverse outcomes across the spectrum of human heart failure. Circulation. (2020) 141(18):1463–76. doi: 10.1161/CIRCULATIONAHA.119.045323
26. Yin W, Li F, Tan X, Wang H, Jiang W, Wang X, et al. Plasma ceramides and cardiovascular events in hypertensive patients at high cardiovascular risk. Am J Hypertens. (2021) 34(11):1209–16. doi: 10.1093/ajh/hpab105
27. Felker G, Hasselblad V, Tang W, Hernandez A, Armstrong P, Fonarow G, et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail. (2012) 14(11):1257–64. doi: 10.1093/eurjhf/hfs110
28. Besseling P, Pieters T, Nguyen I, de Bree P, Willekes N, Dijk A, et al. A plasma creatinine- and urea-based equation to estimate glomerular filtration rate in rats. Am J Physiol Renal Physiol. (2021) 320(3):F518–24. doi: 10.1152/ajprenal.00656.2020
29. Damman K, Valente M, Voors A, O'Connor C, van Veldhuisen D, Hillege H. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. (2014) 35(7):455–69. doi: 10.1093/eurheartj/eht386
30. Ambrosy A, Vaduganathan M, Huffman M, Khan S, Kwasny M, Fought A, et al. Clinical course and predictive value of liver function tests in patients hospitalized for worsening heart failure with reduced ejection fraction: an analysis of the EVEREST trial. Eur J Heart Fail. (2012) 14(3):302–11. doi: 10.1093/eurjhf/hfs007
31. Zhu X, Wang J, Du J, Chen S, Chen S, Li J, et al. Changes in serum liver function for patients with COVID-19: a 1-year follow-up study. Infect Drug Resist. (2022) 15:1857–70. doi: 10.2147/idr.S356181
32. Glance LG, Osler TM, Mukamel DB, Meredith W, Dick AW. Impact of statistical approaches for handling missing data on trauma center quality. Ann Surg. (2009) 249(1):143–8. doi: 10.1097/SLA.0b013e31818e544b
33. Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. (2002) 347(3):161–7. doi: 10.1056/NEJMoa020233
34. Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2021) 18(6):400–23. doi: 10.1038/s41569-020-00480-6
35. Kovilakath A, Jamil M, Cowart LA. Sphingolipids in the heart: from cradle to grave. Front Endocrinol (Lausanne). (2020) 11:652. doi: 10.3389/fendo.2020.00652
36. Law BA, Liao X, Moore KS, Southard A, Roddy P, Ji R, et al. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. (2018) 32(3):1403–16. doi: 10.1096/fj.201700300R
37. Mitsnefes M, Scherer PE, Friedman LA, Gordillo R, Furth S, Warady BA, et al. Ceramides and cardiac function in children with chronic kidney disease. Pediatr Nephrol. (2014) 29(3):415–22. doi: 10.1007/s00467-013-2642-1
38. Kalhorn T, Zager RA. Renal cortical ceramide patterns during ischemic and toxic injury: assessments by HPLC-mass spectrometry. Am J Physiol Renal Physiol. (1999) 277(5):F723–33. doi: 10.1152/ajprenal.1999.277.5.F723
39. Ebel P, Vom Dorp K, Petrasch-Parwez E, Zlomuzica A, Kinugawa K, Mariani J, et al. Inactivation of ceramide synthase 6 in mice results in an altered sphingolipid metabolism and behavioral abnormalities. J Biol Chem. (2013) 288(29):21433–47. doi: 10.1074/jbc.M113.479907
40. Yamane M, Miyazawa K, Moriya S, Abe A, Yamane S. D,L-Threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (DL-PDMP) increases endoplasmic reticulum stress, autophagy and apoptosis accompanying ceramide accumulation via ceramide synthase 5 protein expression in A549 cells. Biochimie. (2011) 93(9):1446–59. doi: 10.1016/j.biochi.2011.04.016
41. Contreras C, González-García I, Martínez-Sánchez N, Seoane-Collazo P, Jacas J, Morgan D, et al. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Rep. (2014) 9(1):366–77. doi: 10.1016/j.celrep.2014.08.057
42. Boslem E, MacIntosh G, Preston A, Bartley C, Busch A, Fuller M, et al. A lipidomic screen of palmitate-treated Min6 Β-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. (2011) 435(1):267–76. doi: 10.1042/bj20101867
43. Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. (2008) 77(2):387–97. doi: 10.1093/cvr/cvm029
44. Stiban J, Perera M. Very long chain ceramides interfere with C16-ceramide-induced channel formation: a plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. (2015) 1848(2):561–7. doi: 10.1016/j.bbamem.2014.11.018
45. Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. (2000) 39(22):6660–8. doi: 10.1021/bi9924415
46. Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab. (2014) 99(1):E45–52. doi: 10.1210/jc.2013-2559
47. Zhang Q, Holland W, Wilson L, Tanner J, Kearns D, Cahoon J, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2a-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. (2012) 61(7):1848–59. doi: 10.2337/db11-1399
48. Chen Q, Denard B, Lee C, Han S, Ye J, Ye J. Inverting the topology of a transmembrane protein by regulating the translocation of the first transmembrane helix. Mol Cell. (2016) 63(4):567–78. doi: 10.1016/j.molcel.2016.06.032
Keywords: worsening heart failure, ADHERE score, biomarker, organ dysfunction, prognosis
Citation: Ren L, Li F, Tan X, Fan Y, Ke B, Zhang Y, Jiang H, Jia L, Wang Y and Du J (2023) Abnormal plasma ceramides refine high-risk patients with worsening heart failure. Front. Cardiovasc. Med. 10:1185595. doi: 10.3389/fcvm.2023.1185595
Received: 14 March 2023; Accepted: 13 June 2023;
Published: 29 June 2023.
Edited by:
Pietro Enea Lazzerini, University of Siena, ItalyReviewed by:
Fuyang Zhang, Fourth Military Medical University, China© 2023 Ren, Li, Tan, Fan, Ke, Zhang, Jiang, Jia, Wang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Wang d2FuZ3l1YW45ODA1MTBAMTYzLmNvbQ== Jie Du amllZHVAY2NtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.