
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 15 September 2023
Sec. Cardiovascular Biologics and Regenerative Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1182209
This article is part of the Research TopicApplication of Stem Cells in the Treatment of Myocardial InfarctionView all 6 articles
 Takuji Kawamura1
Takuji Kawamura1 Yoshito Ito1
Yoshito Ito1 Emiko Ito1
Emiko Ito1 Maki Takeda1
Maki Takeda1 Tsubasa Mikami1
Tsubasa Mikami1 Takura Taguchi1
Takura Taguchi1 Noriko Mochizuki-Oda1
Noriko Mochizuki-Oda1 Masao Sasai1
Masao Sasai1 Tomomi Shimamoto1
Tomomi Shimamoto1 Yukako Nitta1
Yukako Nitta1 Daisuke Yoshioka1
Daisuke Yoshioka1 Masashi Kawamura1
Masashi Kawamura1 Ai Kawamura1
Ai Kawamura1 Yusuke Misumi1
Yusuke Misumi1 Yasushi Sakata2
Yasushi Sakata2 Yoshiki Sawa3
Yoshiki Sawa3 Shigeru Miyagawa1*
Shigeru Miyagawa1*
Introduction: With the expected increase in patients with heart failure and ischemic 15 cardiomyopathy, the development of myocardial regenerative medicine using cell transplantation as a novel treatment method is progressing. This first-in-human clinical trial aimed to confirm the safety of cardiomyocyte patch transplantation derived from allogeneic induced pluripotent stem (iPS) cells based on the results of several preclinical studies.
Study design: The inclusion criteria were left ventricular ejection fraction of 35% or less; heart failure symptoms of New York Heart Association class III or higher despite existing therapies such as revascularization; and a 1-year observation period that included a 3-month immunosuppressive drug administration period after transplantation of iPS cell-derived cardiomyocyte patches to evaluate adverse events, cardiac function, myocardial blood flow, heart failure symptoms, and immune response.
Results: In the first three cases of this trial, no transplanted cell-related adverse events were observed during the 1-year observation period, and improvement in heart failure symptoms was observed. In addition, improvements in left ventricular contractility and myocardial blood flow were observed in two of the three patients. Regarding immune response, an increase in transplant cell-specific antibody titer was observed in all three patients after immunosuppressive drug administration. In one patient with poor improvement in cardiac function and myocardial blood flow, an increase in antibody titer against HLA-DQ was observed even before cell transplantation.
Conclusions: Our case findings demonstrate that the transplantation of iPS cell-derived cardiomyocyte patches for ischemic cardiomyopathy can be safely performed; however, further investigation of the therapeutic effect and its relationship with an immune response is needed by accumulating the number of patients through continued clinical trials.
The number of patients with ischemic cardiomyopathy is increasing worldwide with the aging population (1). Ischemic cardiomyopathy generally worsens from the onset of heart failure symptoms to repeated remission and exacerbation. Left ventricular assist devices and heart transplants are indicated for patients who continue to deteriorate despite receiving appropriate medical therapy, coronary revascularization, and structural interventions for valvular disease (2). However, these replacement therapies have complications and lack donors; therefore, the indications for such treatments have to be limited. In this context, we previously conducted basic research and preclinical studies on induced pluripotent stem cell (iPSC)-derived cardiomyocyte (iPSC-CM) transplantation as an angiogenesis therapy by cell patch transplantation and demonstrated that it is promising in terms of safety and therapeutic efficacy (3–5). Therefore, as previously reported in a study (6), we started an investigator-initiated clinical trial of iPSC-CM patch transplantation as a new treatment method for ischemic cardiomyopathy. The primary objective of this clinical trial was to confirm the safety of iPSC-CM transplantation in a clinical setting. Potential adverse events include undifferentiated cell-derived tumor formation that may persist in transplanted iPSC-CMs and immune reactions to allografted cells. Based on previous preclinical studies, HLA matching is not required for immunosuppressive therapy for allogeneic iPSC-CM transplantation. Therapeutic effects can be obtained by performing immunosuppressive treatment similar to normal heart transplantation (7). However, the immune response that occurs in actual clinical situations remains unknown. Therefore, we review and report about three cases, including those already reported, who underwent iPSC-CM patch transplantation, and present data on general clinical outcomes, safety assessment, and immune reactions.
This clinical trial was approved by the ethical review committee of Osaka University (#199006-A), the Ministry of Health, Labor, and Welfare, Japan (#2019-143), and registered in the Japan Registry of Clinical Trials (https://jrct.niph.go.jp/en-latest-detail/jRCT2053190081). Written informed consent was obtained from all individuals and was saved according to the clinical trial plan.
Patients who met the inclusion criteria mentioned below and confirmed that they did not meet any exclusion criteria were included in the study after obtaining written informed consent. After iPSC-CM patch transplantation, immunosuppressants, including tacrolimus, mycophenolate mofetil, and prednisolone, were administered for 3 months, including the tapering period. Data on adverse events 1 year after transplantation were collected to determine the causal relationship with the iPSC-CM patch. Serial cardiac function was evaluated using echocardiography and cardiac computed tomography (CT), and myocardial blood flow was measured using NH3-positron emission tomography (PET). In addition, the immune response to transplanted cells was evaluated by quantifying the antibodies against the HLA of the cells in the serum.
As this investigator-initiated clinical trial will be the first-in-human trial using iPSC-CMs, this study mainly evaluated the safety of the treatment. To avoid potential bias, the decision to continue this trial was left to an external evaluation committee after the first three cases.
The inclusion criteria were a diagnosis of ischemic cardiomyopathy with a left ventricular ejection fraction (LVEF) of 35% or less and heart failure symptoms of New York Heart Association class III and IV after standard treatment, including coronary revascularization and medication therapy.
The exclusion criteria were as follows: (1) autoimmune disease, (2) allergy or hypersensitivity to the immunosuppressant, (3) severe infection, (4) persistent shock due to worsening heart failure, (5) irreversible organ failure other than heart failure, (6) malignancy, (7) pregnancy, (8) alcoholic or drug addiction in recent 6 months, (9) allergies or hypersensitivity to animals used to obtain raw materials, and (10) severe pulmonary hypertension.
As previously reported (6), three iPSC-CM patches, with 3.3 × 107 cells per patch, the form of which was circular about 3.5 cm in diameter, were transplanted on the left ventricular anterior and the lateral wall under general anesthesia (Supplementary Figure S1). The pericardium was incised through the left fourth or fifth intercostal thoracotomy to observe the surface of the myocardium. Then the patches were fixed to the lateral side of the left anterior descending artery to cover the anterior to the lateral wall of the left ventricle with 7-0 prolene thread, if necessary, with fibrin glue spread on the patches to prevent shifting. The procedure is then terminated with sparse closure of the pericardium. As for the location of the cell patch, we observe the condition of the cardiac surface and choose a location where myocardial viability could be expected. If needed, intra-aortic balloon pumping (IABP) was also performed during surgery.
Postoperative serum samples from each patient were examined over time for the presence or absence of anti-HLA antibodies that are specific or cross-reactive to the transplanted cells to evaluate the immune response against transplanted iPSC-CMs. LABScreen™ Single Antigen (One Lambda, Inc., Los Angeles, California, United States) was used in outsourced inspections to HLA laboratories (https://hla.or.jp) to identify or quantify anti-HLA antibodies, and the immune response was quantified using fluorescence intensity of conjugated antibodies against anti-HLA antibodies.
As previously reported (6), a 51-year-old man who temporarily became dependent on mechanical circulatory support (MCS) after two episodes of acute myocardial infarction was weaned off MCS. Cardioprotective drugs, including beta-blockers and ACE inhibitors, were administered, and despite the maximum possible medical treatment, symptoms of heart failure of NYHA class III persisted. Echocardiography revealed a low LVEF of 30%, meeting the inclusion criteria for this study and no applicable exclusion criteria.
A 76-year-old man developed an inferior wall infarction 18 years ago and had stents placed in the right coronary artery (RCA) #1 and #3 and left circumflex artery (LCx) #13. He developed an anterior wall infarction 13 years ago, and a stent was placed in the left anterior descending artery (LAD) #7; however, unstable angina pectoris developed 1 year later, and a drug-eluting stent (DES) was placed in LAD #6. Finally, DES was placed in the LCx 7 years ago, and since then, follow-up at another hospital has been continued. At the time of referral to our hospital, there was significant stenosis of the RCA and LCx, and percutaneous coronary intervention (PCI) was performed. Besides technically possible PCI, beta-blockers and mineralocorticoid receptor antagonists were administered as cardioprotective drugs, although the heart failure symptoms of NYHA class III persisted. Echocardiography at our hospital revealed an LVEF of 21%, which met the inclusion criteria for this trial. The preoperative screening analysis did not reveal any findings that met the exclusion criteria.
A 65-year-old man underwent CABG and MVP for acute myocardial infarction and papillary muscle rupture 5 years ago. Oral beta-blockers and angiotensin receptor blockers were administered as cardioprotective drugs. The patient had heart failure symptoms of NYHA class III and a lower LVEF of 35%, despite angiographic patent bypass grafts, which met the inclusion criteria for this study and did not meet the relevant exclusion criteria (Table 1).
In cases 1 and 2, IABP was used to support cardiac function. Because case 3 was a prior CABG case, iPSC-CM transplantation was performed after detaching the adhesions in the pericardium. The operation times were 116, 97, and 125 min in cases 1, 2, and 3, respectively. All three patients returned to the ICU while intubated, IABP was removed, and patients were extubated while monitoring hemodynamics.
Four severe adverse events were observed in this clinical trial: chest discomfort, urinary tract infection, acute heart failure, and amiodarone pneumonia. Chest discomfort was observed on the 37th day after the operation in case 1. After the patient was hospitalized and closely examined for acute coronary syndrome, the event was judged to be a symptom of anxiety. Urinary tract infection was reported in case 2 at 13 weeks postoperatively. The diagnosis was made based on bacteriological examination findings. Case 2 had acute heart failure 40 days after the operation. Because the temperature increased quickly in that season, it was speculated to be caused by the afterload mismatch on the left ventricle owing to the hypertension surge. Amiodarone pneumonia was diagnosed using CT at another hospital 13 weeks after surgery in case 3. Image indicating pneumonia and symptoms improved after discontinuing amiodarone. Causal relationships of all four severe adverse events with iPSC-CMs were excluded (Table 2).
Whole-body FDG-PET was performed 6 months after transplantation to identify tumors that could develop from the transplanted iPSCs. Likewise, case 1, which has already been reported (6), cases 2 and 3 did not show any significant uptake of FDG in FDG-PET scan, eliminating the possibility of tumors (Supplementary Figure S2). In addition, We measured blood levels of four biomarkers of alpha-fetoprotein (AFP), carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), and human chorionic gonadotropin (hCG) before surgery, 3 months, 6 months, and 1 year after transplantation to detect tumors arising from iPS cells based on a previous report (8). The results showed that hCG was below the sensitivity of the measurement, and the other parameters were also below the reference values at all time points (Supplementary Figure S3).
Regarding changes in postoperative cardiac function, in cases 1 and 3, the left ventricle, which showed enlargement preoperatively, was reduced 6 months and 1 year after surgery. In addition, the LVEF improved 6 months and 1 year after surgery in cases 1 and 3. In contrast, in case 2, preoperative LVDd/LVDs were enlarged before surgery and did not show noticeable change. Similarly, in case 2, LVEF showed no clear improvement (Figure 1). A similar trend was confirmed using electrocardiogram-gated cardiac CT (Figure 2). In addition, as reported in case 1, the anterolateral wall (segments #1, 7, 13, and 6) of the left ventricle implanted with iPSC-CM sheets showed greater improvement in contraction in case 3; however, there were no apparent changes in ventricular contractility in case 2 (Figure 2).
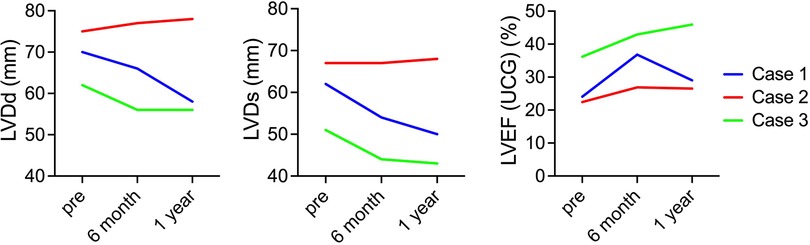
Figure 1. Serial changes in cardiac function. The images show serial changes in LVDd, LVDs, and LVEF on echocardiography. The red, blue, and green charts indicate changes in the first, second, and third cases. LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction.
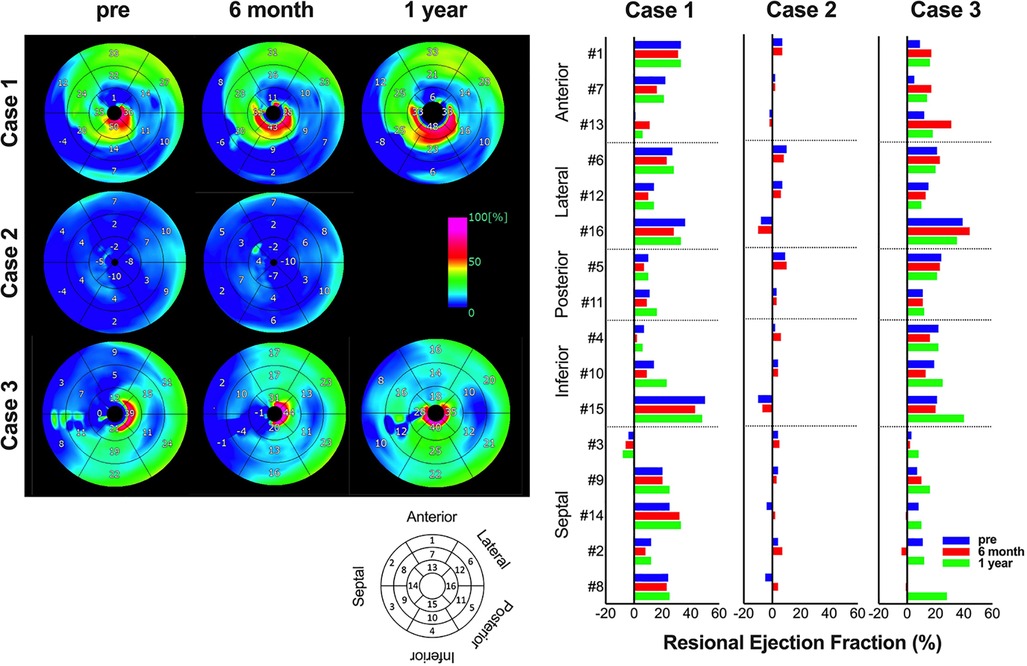
Figure 2. Serial changes in regional cardiac function. The images show serial changes in regional ejection fraction on cardiac computed tomography. Using a model that divides the left ventricle into 17 segments, #1, 7, and 13 are anterior wall, #6, 12, and 16 are lateral wall, #5 and 11 are posterior wall, #4, 10, and 15 are inferior wall, and #3, 9, 14, 2, and 8 are septal wall. The image data are shown on the left, and the graph of quantified values on the right.
Serial changes in myocardial blood flow were measured at rest and under stress using NH3-PET, and this ratio was defined as the coronary flow reserve (CFR). Supplementary Figure S4 shows the regional values of 17 segmentations. In case 1, as previously reported, the preoperative value was not obtained. However, a marked increase in blood flow during stress was observed from 1.67 ml/min/g 6 months after surgery to 3.12 ml/min/g 1 year after surgery. Accordingly, the CFR in case 1 increased. In case 2, resting myocardial blood flow was 0.51 ml/min/g preoperatively, 0.57 ml/min/g 6 months after surgery, and 0.47 ml/min/g 1 year after surgery, showing no significant changes. CFR decreased from 2.53 before surgery to 1.11 6 months after surgery and 1.38 1 year after. In case 3, the resting myocardial blood flow decreased from 1.01 ml/min/g preoperatively to 0.69 ml/min/g 6 months after surgery and 0.67 ml/min/g 1 year after surgery. However, stress myocardial blood flow increased from 2.33 ml/min/g preoperatively to 2.54 ml/min/g 6 months after surgery and decreased to 1.63 ml/min/g 1 year after surgery. CFR increased from 2.36 before surgery to 3.76 6 months after surgery and decreased to 2.42 1 year after surgery, which was higher than the values before surgery.
When the transition of heart failure symptoms was evaluated according to the NYHA classification, all patients had NYHA class 3 heart failure symptoms preoperatively; however, cases 1 and 3 improved to NYHA class I 6 months after surgery, and case 2 improved to NYHA class II 6 months after surgery. In contrast, regarding changes in BNP, cases 1 and 3 showed no significant difference at approximately 100 pg/dl after surgery. In case 2, the preoperative level was 293 pg/dl, which gradually increased after surgery, reaching 482 pg/dl 1 year after the operation. In addition, peak VO2, evaluated by cardiopulmonary exercise testing, increased from 15.4 ml/min/kg before surgery to 18.7 ml/min/kg 6 months after surgery and 20 ml/min/kg 1 year after surgery in case 1 and decreased from 16.7 ml/min/kg before surgery to 14.7 ml/min/kg 6 months after surgery but increased to 16.9 ml/min/kg 1 year after surgery in case 3. In contrast, in case 2, the value was as low as 10 ml/min/kg before surgery, and it did not increase at 6 months after surgery, and the surgery could not be performed after 1 year (Figure 3).
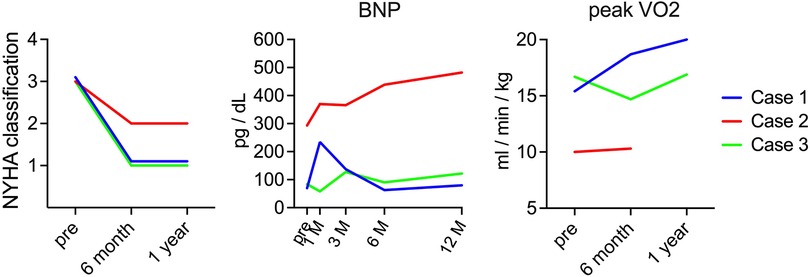
Figure 3. Serial changes in heart failure symptom. The images show serial changes in NYHA classification, BNP, and peak VO2. The red, blue, and green charts indicate changes in cases 1, 2, and 3, respectively. NYHA, New York Heart Association; BNP; brain natriuretic peptide.
The HLA types of iPSC donors and each patient are shown in Table 3. The iPSC donor was homozygous for the six alleles of HLA-A, -B, -C, -DRB1, -DQB1, and -DPB1. Comparing the HLA types of the iPSC donor and each patient clarified the matching of genotype A*24:02 in cases 1 and 3, the matching of HLA-DRB1 serotype DR15 in case 2, and the matching of HLA-DQB1 serotype DQ6 in cases 1 and 2 (Table 3).
Figure 4 describes the changes in fluorescence intensities for representative HLA class 1 allele (HLA-A, B, and C) that are specific or cross-reactive with the HLA type of transplanted cells. Similarly, Figure 5 shows the changes in fluorescence intensities for all evaluated HLA class 2 alleles (HLA-DRB1, DQB1, and DPB1).
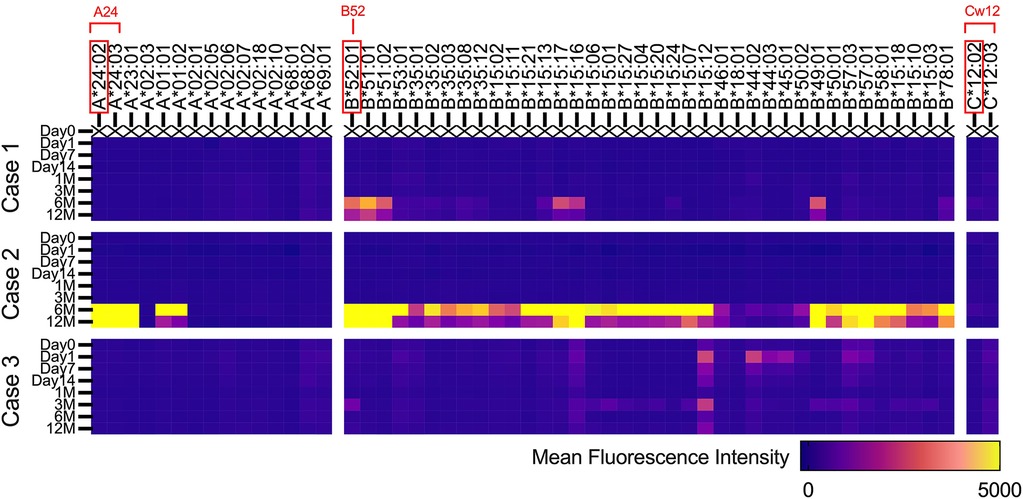
Figure 4. Serial changes in anti-HLA class 1 antibodies. Heatmap showing changes in fluorescence intensities for representative HLA class 1 (HLA-A, B, and C) alleles that are specific or cross-reactive with the HLA type of transplanted iPS cells.
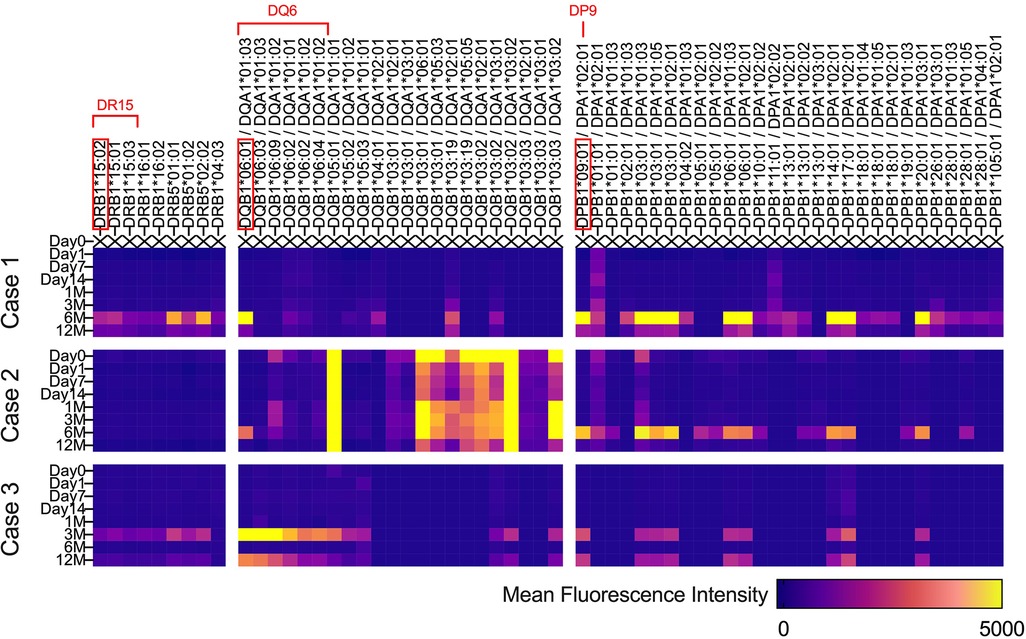
Figure 5. Serial changes in anti-HLA class 2 antibodies. Heatmap showing changes in fluorescence intensities for all evaluated HLA class 2 (HLA-DRB1, DQB1, and DPB1) alleles.
Regarding both HLA class 1 and class 2, all three cases showed increases in fluorescence intensities for multiple anti-HLA antibodies 6 months after transplantation, which was 3 months after the completion of immunosuppressive therapy (Figures 4, 5). In contrast, in case 2, an increase in fluorescence intensity for HLA-DQ was already observed before transplantation (Figure 5).
As described in the Methods section, external evaluation committee members determined the indication for inclusion in the clinical trial based on the evaluation of the preoperative condition of each case. Adverse events and treatment efficacy were evaluated after a 1-year observation period after cell transplantation to determine whether the trial could be continued.
As a result, adverse events during the 1-year postoperative observation period were ruled out as related to the investigational product; therefore, all three patients were judged eligible for inclusion in the trial based on evaluating their preoperative conditions, allowing the trial to continue.
In this first-in-human clinical trial of iPSC-CM transplantation, the first three cases of transplantation and a 1-year follow-up period after transplantation were completed. All adverse events observed during the observation period negatively correlated with iPSC-CM transplantation. In addition, whole-body FDG-PET performed in the latter half of the surgery did not show any tumors that could have been generated from iPSCs (Supplementary Figure S2), thus proving the treatment safe. Improvement in heart failure symptoms was observed in all three cases, especially in two cases (cases 1 and 3). Reduction in left ventricular diameter (Figures 1, 2) and improvement in exercise tolerance (Figure 3) were observed after cell transplantation, suggesting the possibility of a response to cell transplantation treatment.
In this clinical trial protocol, no immunosuppressive agents were administered 3 months after transplantation of allogeneic iPSC-CMs without matching HLA typing, and the transplanted cells were expected to have been lost because of rejection after 3 months. Therefore, the primary mechanism of improvement in cardiac function is the improvement in myocardial blood flow by angiogenesis and functional improvement of residual myocytes, as previously observed in preclinical studies (3, 4). Myocardial blood flow and CFR evaluated using NH3-PET in this study were similar or improved after 6 months in cases 1 and 3 compared with those before transplantation, whereas they were decreased in case 2. In addition, myocardial blood flow, CFR, left ventricular diameter, and contractility improved in cases 1 and 3 at 6 months after transplantation, while there was no clear improvement in left ventricular function in case 2. Therefore, it is suggested that in cases 1 and 3, angiogenesis from the transplanted cells occurred during the immunosuppression period of 3 months after transplantation, and the functional recovery of the remaining myocardium obtained from the improved blood flow was maintained even 6 months after the loss of transplanted cells. In contrast, in case 2, the transplanted cells did not have a sufficient angiogenic effect, which could have resulted in the lack of improvement in cardiac function. Therefore, immune response to the transplanted cells during the immunosuppression period of 3 months after cell transplantation, as conducted in this clinical trial protocol, may be essential to obtain the therapeutic effect.
Because the histological evaluation of the transplanted tissue was practically impossible, we searched for the presence of anti-HLA antibodies that are specific or cross-reactive with the transplanted iPSCs, as generally performed in allogeneic heart transplantation, to evaluate immune response (9). As shown in Figures 4, 5, 3 months after surgery, during which each patient continued to take immunosuppressants, new emergence of anti-HLA antibodies that could react with transplanted iPSCs was not evident in all cases, except for anti-HLA antibodies against HLA-DQ in case 2. Therefore, we speculate that immune rejection against the transplanted tissue was suppressed in cases 1 and 3, whose cardiac function improved. In contrast, in case 2, whose cardiac function did not improve, anti-HLA antibodies against HLA-DQ presented before surgery may have caused the immune rejection. However, at present, it is not possible to demonstrate a causal relationship between the suppression of immune rejection by transplanted iPSCs and the improvement in cardiac function derived from transplanted iPSCs. Therefore, it is crucial to verify the effect of iPSC-CM on cardiac function from the viewpoint of immune response in the future.
Furthermore, residual myocardium, generally referred to as hibernating myocardium (10), is functionally impaired owing to ischemia but can recover through improvement in blood flow and can improve myocardial blood flow by iPSC-CM transplantation. We previously showed that in autologous skeletal myoblast sheet transplantation, the primary mechanism of cardiac function improvement by similar angiogenic therapy is considered to be the improvement in cardiac function, and the lack of progression of left ventricular enlargement is a factor that predicts responders who can obtain therapeutic effects (11). These results suggest that there is not much residual myocardium in cases with progressive left ventricular enlargement (12), which may also be used to predict responders who will benefit from treatment in this clinical trial. In case 2 of this trial, the preoperative LVESV was 305 ml, which was markedly increased compared to that in case 1 (182 ml) and case 3 (122 ml), and even autologous skeletal myoblast sheet transplantation could not have been a responder (11). Therefore, in addition to the immune response to the transplanted cells, it is also possible that the small amount of residual myocardium in case 2 did not respond to angiogenic therapy. It will be important to avoid immune response and predict treatment efficacy by quantifying the residual myocardium to generalize this treatment method in the future.
This study had a limitation. This is the first clinical trial using iPSC-CMs with the main objective of confirming safety. Therefore, the number of cases is overwhelmingly limited to conclude therapeutic efficacy and therapeutic mechanism. After ensuring safety in this clinical trial, increasing the number of cases and conducting a study to assess the therapeutic effect is necessary.
In conclusion, allogeneic iPSC-CM transplantation can be performed safely, supporting further clinical trials. The effect of preoperative HLA antibodies on the therapeutic effect should be investigated in more cases in the future.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethical Review Board of Osaka University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TK: conducting clinical studies and manuscript writing. DY, MK, AK, YM, and YSak: conducting clinical studies. YI, TM and TT: clinical data collection and analysis. EI and MT: preparation and quality control of clinical trial products. MS: coordinating the clinical trial. NM-O: editing of manuscript drafts. TS and YN: coordinating clinical trials. YSaw and SM: design and supervision of the entire project. All authors contributed to the article and approved the submitted version.
This study was funded by the Japan Agency for Medical Research and Development (AMED) under grant number JP20bk0104110.
We thank the staff of the Department of Cardiovascular Surgery and the Department of Frontier Regenerative Medicine for manufacturing the clinical trial products. We also thank the clinical staff at our institution for providing patient care and data collection.
YS and SM are inventors of the patents on the method of manufacturing iPS-CM patches, potentially earning patent income.
The remaining authors declare that the research was conducted in the absence of any commercial or Financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1182209/full#supplementary-material
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145:e895–e1032. doi: 10.1161/CIR.0000000000001063
3. Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. (2012) 126:S29–37. doi: 10.1161/CIRCULATIONAHA.111.084343
4. Kashiyama N, Miyagawa S, Fukushima S, Kawamura T, Kawamura A, Yoshida S, et al. MHC-mismatched allotransplantation of induced pluripotent stem cell-derived cardiomyocyte sheets to improve cardiac function in a primate ischemic cardiomyopathy model. Transplantation. (2019) 103:1582–90. doi: 10.1097/TP.0000000000002765
5. Miki K, Uenaka H, Saito A, Miyagawa S, Sakaguchi T, Higuchi T, et al. Bioengineered myocardium derived from induced pluripotent stem cells improves cardiac function and attenuates cardiac remodeling following chronic myocardial infarction in rats. Stem Cells Transl Med. (2012) 1:430–7. doi: 10.5966/sctm.2011-0038
6. Miyagawa S, Kainuma S, Kawamura T, Suzuki K, Ito Y, Iseoka H, et al. Case report: transplantation of human indruced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front Cardiovasc Med. (2022) 9:950829. doi: 10.3389/fcvm.2022.950829
7. Kawamura T, Miyagawa S, Fukushima S, Maeda A, Kashiyama N, Kawamura A, et al. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Rep. (2016) 6:312–20. doi: 10.1016/j.stemcr.2016.01.012
8. Riegler J, Ebert A, Qin X, Shen Q, Wang M, Ameen M, et al. Comparison of magnetic resonance imaging and serum biomarkers for detection of human pluripotent stem cell-derived teratomas. Stem Cell Rep. (2016) 6:176–87. doi: 10.1016/j.stemcr.2015.12.008
9. Itescu S, Tung TC, Burke EM, Weinberg AD, Mancini D, Michler RE, et al. An immunological algorithm to predict risk of high-grade rejection in cardiac transplant recipients. Lancet. (1998) 352:263–70. doi: 10.1016/S0140-6736(98)09475-6
10. Heusch G. Myocardial stunning and hibernation revisited. Nat Rev Cardiol. (2021) 18:522–36. doi: 10.1038/s41569-021-00506-7
11. Kainuma S, Miyagawa S, Toda K, Yoshikawa Y, Hata H, Yoshioka D, et al. Long-term outcomes of autologous skeletal myoblast cell-sheet transplantation for end-stage ischemic cardiomyopathy. Mol Ther. (2021) 29:1425–38. doi: 10.1016/j.ymthe.2021.01.004
Keywords: cardiomyocyte patch transplantation, iPS cells, myocardial regenerative medicine, heart failure, immune response, HLA antibodies
Citation: Kawamura T, Ito Y, Ito E, Takeda M, Mikami T, Taguchi T, Mochizuki-Oda N, Sasai M, Shimamoto T, Nitta Y, Yoshioka D, Kawamura M, Kawamura A, Misumi Y, Sakata Y, Sawa Y and Miyagawa S (2023) Safety confirmation of induced pluripotent stem cell-derived cardiomyocyte patch transplantation for ischemic cardiomyopathy: first three case reports. Front. Cardiovasc. Med. 10:1182209. doi: 10.3389/fcvm.2023.1182209
Received: 8 March 2023; Accepted: 29 August 2023;
Published: 15 September 2023.
Edited by:
Steve Leu, Kaohsiung Chang Gung Memorial Hospital, TaiwanReviewed by:
Jyotsna Joshi, The Ohio State University, United States© 2023 Kawamura, Ito, Ito, Takeda, Mikami, Taguchi, Mochizuki-Oda, Sasai, Shimamoto, Nitta, Yoshioka, Kawamura, Kawamura, Misumi, Sakata, Sawa and Miyagawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigeru Miyagawa bWl5YS1wQHN1cmcxLm1lZC5vc2FrYS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.