- Department of Vascular Surgery, Peking Union Medical College Hospital, Beijing, China
Patients with Takayasu arteritis (TA) and descending aorta involvement often experience insidious onset and slow progression, leading to irreversible vascular lesions despite medication therapy. Surgical management plays a crucial role in resolving hemodynamic disturbances and has shown promise in improving the outcomes of this patient population, owing to significant advancements in surgical expertise. However, studies focusing on this rare disease are lacking. This review summarizes the characteristics of patients with stenosis in descending aorta, emphasizing surgical approaches, perioperative management, and disease outcomes. The operative approach depends on lesion location and extent. Existing studies have confirmed that the choice of surgical modality significantly influences postoperative complications and long-term prognosis in patients, highlighting the effectiveness of bypass surgery as a favorable option in clinical practice with a satisfactory long-term patency rate. To mitigate postoperative complications, it is advisable to conduct regular imaging follow-ups to prevent the deterioration of the condition. Notably, the occurrence of restenosis and pseudoaneurysm formation deserves particular attention due to their impact on patient survival. The use of perioperative medication remains a topic of debate, as previous studies have presented divergent perspectives. The primary objective of this review is to provide a comprehensive perspective on surgical treatment and offer customized surgical approaches for patients in this population.
Introduction
Takayasu arteritis (TA) is a nonspecific inflammatory disease of the aorta and its main branches, causing a range of arterial stenosis/occlusion or dilatation. Previous studies revealed that stenosis (93%) is the most frequent vascular presentation, and the abdominal aorta is the most frequent lesion location in the Asian population (1, 2). Patients with stenosis in the descending aorta (including the thoracic and abdominal aorta) may present life-threatening complications before 40, causing poor prognosis (3, 4). Surgical treatments are required in 18%–70% of all TA patients, with a substantial proportion experiencing stenosis in the descending aorta (5, 6).
Bypass surgery has been associated with a good long-term patency rate but is complex and requires a multidisciplinary approach. Endovascular therapy is less invasive and reproducible, but its patency rate is inferior to the former. Indeed, each method has pros and cons, which necessitates the tailored surgery design. It is imperative to evaluate each patient's condition individually, assess the surgical benefit and risks, and choose the appropriate surgical approach. A comprehensive evaluation of large systemic vessels is necessary to determine the optimal surgical approach. The utilization of advanced technology can assist in developing a precise surgical plan. In our center, for complex cases, we employ hemodynamic simulation to calculate pressures at various anatomical sites, identify optimal anastomosis locations, and re-evaluate the pressure to assess the effectiveness of the planned surgery. This approach allows us to validate the efficacy of the surgical intervention.
This article aims to present a comprehensive overview of current practices in the management of patients with descending aorta involvement. We will summarize the findings of previous studies, explore the impact of different surgical approaches on prognosis, and propose optimized management strategies for this specific patient population. We advocate that perioperative treatment and surgical modalities will continue to advance, offering hope and improved outcomes for these patients.
Methods
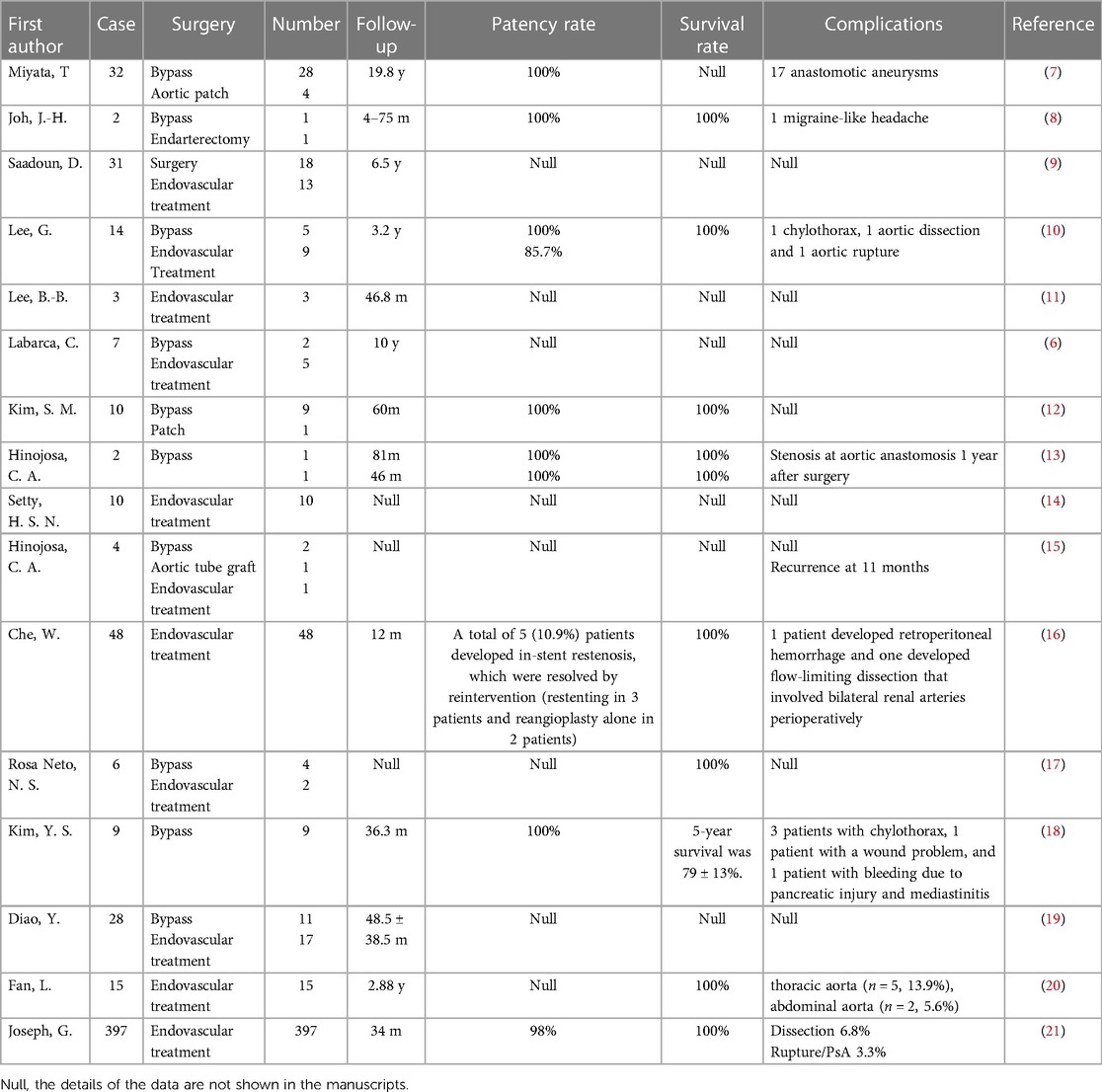
Considering the rarity of Takayasu arteritis, a comprehensive search was conducted to ensure that the objective reflects clinical practice. The study selection specifically focused on a population that underwent surgery targeting the descending aorta, which includes the thoracic and/or abdominal aorta. The research encompassed retrospective case-control analyses, case series, and case reports (Table 1). Studies that analyzed the outcomes of TA patients with multiple lesions, including thoracic and/or abdominal aortic stenosis, but were not exclusively focused on that specific aspect were marked "Null" in Table 1.
Diagnostic criteria
The diagnostic criteria for Takayasu arteritis established by the American College of Rheumatology (ACR) in 1990 are widely accepted (22). However, these criteria were developed using a small sample size, limiting their generalizability and independent validation, impacting their applicability in clinical practice. In 1995, a modification to the diagnostic criteria was proposed, eliminating age restrictions. This modification resulted in an increased diagnostic sensitivity (92.5%) and specificity (95.0%) (23, 24). The most recent classification criteria for Takayasu arteritis developed jointly by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) in 2022 have shown superior performance compared to the previous criteria. The 2022 criteria demonstrated a sensitivity of 93.8% and a specificity of 99.2%. These criteria were developed using a cohort of 316 TA patients and further validated using an independent dataset comprising an additional 146 TA patients from an international cohort (25). Notably, the 2022 criteria emphasized the importance of clinical symptoms, vascular physical examination findings, and vascular imaging in the classification of the disease. These criteria exhibited excellent performance across patients from different regions.
Demographics and angiographic patterns
Ascending aorta and aortic arch involvement is more commonly observed in patients from East Asia, while South Asian patients tend to exhibit a higher prevalence of abdominal aorta and renal artery involvement, and among Mexican patients, Numano V disease is the most frequently encountered subtype (26–28). Besides, gender plays a role in the distribution of vascular involvement in TA (29). In terms of vascular involvement in Takayasu arteritis, females are more commonly affected by thoracic aorta involvement, while males tend to have a higher susceptibility to abdominal aorta involvement (30, 31). Specifically, lesions in the abdominal aorta are diffusely distributed, with approximately 69% occurring in the suprarenal region, 23% in the juxtarenal region, and 8% in the infrarenal aorta (27). The Numano angiographic classification is widely utilized to categorize TA patients; however, it exhibits limitations in differentiating patients based on clinical presentation and formulating appropriate treatment plans (32). In this review, we focus on patients with descending aorta involvement (including Numano IIb, III, IV, V) as they often display similar clinical presentations and require similar surgical and medical approaches.
Signs and symptoms
The clinical presentation of Takayasu arteritis varies depending on the specific lesions involved. In patients with stenosis in the descending aorta, symptoms can arise from hypertension proximal to the aortic stenosis or hypotension distal to it. If the lower abdominal aorta is affected, claudication may be observed. Involvement of the suprarenal or juxtarenal aorta can lead to impaired renal perfusion and subsequent renal hypertension. Stenosis of the thoracic aorta can result in hypertension due to increased workload on the heart. Notably, hypertension is the most common symptom in TA patients with descending aorta involved with a prevalence of 60%–100%, probably associated with renal hypoperfusion or ischemia, stenosis of the descending aorta, severe aortic regurgitation, and reduced aortic compliance (12, 27, 33). If untreated, most patients die before 35 due to the complications of uncontrolled hypertension (3). Lower extremity claudication is the second most common symptom, presenting in 15%–50% of patients (4, 33). Other symptoms, including headache and syncope, can be observed in these patients (33). The formation of aneurysms (24%) is not rare in TA patients, and >50% of TA patients may develop aneurysms in the course of the disease. More importantly, multiple synchronous lesions (stenotic and aneurysmatic) may coexist in the same patients (34).
Hypertension, being the most prevalent symptom in Takayasu arteritis patients with descending aorta involvement, holds significant value as an indicator for assessing disease control and prognosis. However, the involvement of upper limb arteries may result in inaccurate blood pressure measurements, leading to delayed diagnosis and poor prognosis. For patients without bilateral upper limb arteries involved, the higher value from the arms is recorded; for patients with unilateral upper limb artery affected, the reading from the unaffected side is used; for patients with stenosis in bilateral upper limb arteries, the central blood pressure is collected to reflect the core blood pressure (35).
Assessment of disease activity
The definition of active disease in TA is based on the National Institutes of Health (NIH) guidelines (36, 37). Current acute-phase reactants used to assess disease activity include erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Elevated ESR is one of the strongest indicators of disease progression (15). However, it is important to note that vascular damage can progress without systemic inflammation. Current evidence suggests that 30%–40% of patients may appear clinically stable (in quiescence) but can still be confirmed to be in the active phase based on surgical histopathology findings (5, 33, 38).
[18F] Fluorodeoxyglucose combined positron emission and computed tomography (18F-FDG-PET-CT) and magnetic resonance imaging (MRI) can assess arterial inflammation by measuring the degree of vessel wall edema. These imaging examinations can confirm vascular wall inflammation, especially in patients with normal levels of inflammation markers (38, 39).
It should be borne in mind that 18F-FDG-PET-CT cannot accurately distinguish arteritis from metabolically active vascular remodeling due to the lack of inflammatory cell selectivity. However, new means of imaging examination have emerged to address this limitation. One such approach involves targeting macrophage activation, as macrophages play a significant role in inflammatory infiltrates. The somatostatin receptor subtype-2 (SST2), expressed on activated macrophages, has been identified as a biomarker for vasculitis. A recent study demonstrated that SST2 positron emission tomography (PET)/magnetic resonance imaging (MRI) showed potential in defining disease activity in TA patients with a more sensitive and accurate diagnosis (40).
Contrast-enhanced ultrasound (CEUS) is also a promising approach to assessing disease activity. A previous study showed that the severe stenosis depicted by CEUS in the carotid artery wall was correlated with vascular inflammation detected by PET/CT (41–44). Given the convenience of CEUS imaging, we introduced CEUS imaging as a routine in surveillance protocol.
Imaging
Imaging assessment primarily focuses on the aorta and its major branches in diagnosing Takayasu arteritis. While various imaging modalities are available, angiography remains the cornerstone for diagnosing TA. Computed tomographic arteriography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA) are the most frequently preoperative study used to define anatomy, which can facilitate the assessment of the extent and severity of the arterial injury (45). CTA is the imaging modality of choice for diagnosing and monitoring Takayasu arteritis in nearly 60% of patients. The widespread availability, cost-effectiveness, and superior image resolution compared to MRA account for the popularity of CTA. Its accessibility, affordability, and ability to provide detailed and high-quality images make CTA an invaluable tool in the evaluation and management of TA patients during both the diagnostic and follow-up stages.
Doppler ultrasonography (Doppler US) is also used to quantify the severity of luminal narrowing as a less invasive approach. Reports indicate that among TA patients who received imaging assessment, 58.8% underwent CTA, while 29.9% underwent MRA, and Doppler ultrasonography was used in 11.3% of all patients (46).
Current evidence suggests that 18F-FDG-PET-CT facilitates early diagnosis in 7% of patients and may improve prognosis (15, 47). PET-CT has also become a diagnostic test for assessing arterial inflammation and monitoring the response to immunomodulatory therapy (6, 48). Repeat PET-CT should be considered to confirm disease activity during this period.
The EULAR 2018 guidelines suggest that if the patient presents with recurrent or new symptoms, regular imaging assessment is needed during follow-up (49). Among these imaging methods, MRI is the most frequently used for follow-up because it avoids the use of radiation (46).
Cardiovascular manifestations
As the most common symptom, hypertension is one of the most valued indicators to assess disease control and prognosis. However, the involvement of upper limb arteries may result in inaccurate blood pressure measurements, leading to delayed diagnosis and poor prognosis.
Heart involvement is not rare in TA patients, emphasizing the need for conducting electrocardiograms (ECGs). It is now understood that congestive heart failure induced by stenosis lesions in descending aorta is the main cause of death in patients with Takayasu arteritis (50). Another symptom, aortic regurgitation, is present in 13% to 44% of cases due to increased afterload on the heart (51, 52). Thus, ECG is recommended as a routine examination in all TA patients to reflect valvular and atrioventricular abnormalities. The ejection fraction, the aortic regurgitation, the diameter of the ascending aorta, the diameter of the aortic sinus, the aortic valve annular diameter, and the left ventricular end-diastolic diameter have been reported as indicators in previous studies (53, 54). ECG can also be used for follow-up examination since surgery can mitigate TA-related hypertension and relieve left ventricular hypertrophy (18).
Coronary involvement is a commonly observed lesion in patients with Takayasu arteritis. When there is suspicion of coronary stenosis, state-of-the-art CT coronary angiography has emerged as a reliable non-invasive method (55, 56). This imaging technique offers high isotropic spatial resolution ranging from 0.23 mm to 0.35 mm, while maintaining a low radiation dose profile. CT coronary angiography provides detailed visualization of the coronary arteries, aiding in the assessment of coronary stenosis in TA patients with accuracy and precision.
Management
Surgical treatment
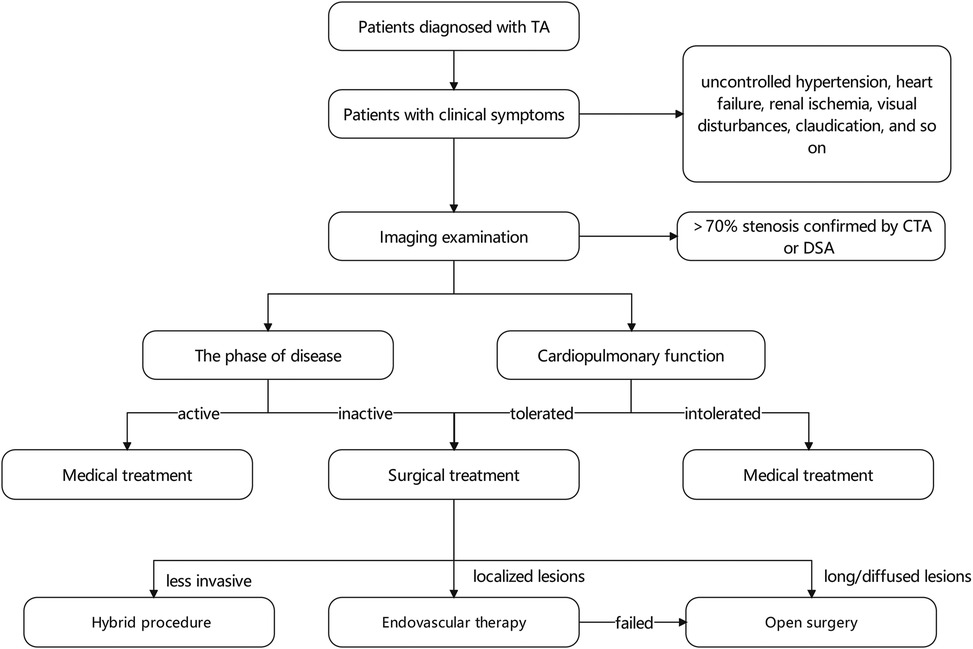
Severe stenosis, defined as a narrowing of 70% or more, can result in hemodynamic disturbances that lead to symptomatic end-organ ischemia. In such cases, surgical interventions are crucial in addressing stenosis in the descending aorta and its visceral artery branches. The indications for TA patients with descending aorta involvement mainly include refractory hypertension, cardiac ischemia, aortic regurgitation, and extremity claudication. In order to optimize patient outcomes, preoperative blood pressure control is recommended, aiming to maintain blood pressure within the normal range. However, if hypertension is primarily caused by a significant narrowing of the descending aorta, surgical intervention is preferred regardless of the patient's hypertension status. While it is generally advisable to avoid surgery during the acute phase of the disease, if patients are in a critical condition, surgery becomes necessary (17).
Aortic stenosis can often involve adjacent visceral arteries, with the splanchnic and renal arteries being common coexisting lesions (27). About 80% of patients with abdominal aortic stenosis also exhibit renal artery stenoses (27, 57). Renal reconstructions are usually performed in patients with decreased renal function or refractory renovascular hypertension. Mesenteric reconstructions are conducted in patients with abdominal pain or other related symptoms. However, although more than 50% of patients present with splanchnic occlusive lesions, only 6% experienced symptomatic bowel ischemia, suggesting prophylactic treatment is required to improve splanchnic stenosis (27).
The location and extent of the lesion should be taken into account during the selection of the surgical procedure. To date, no standard therapy is applicable to all patients. Methods for aortic reconstruction include bypass surgery, interposition graft, patch angioplasty, and endovascular therapy (12). Bypass procedures may be favored in patients having too extensive coarctation segments or complex lesions (15). In other cases with short coarctation, arterial patch, interposition graft, and endovascular therapy may be attractive (58) (Figure 1).
Endovascular technologies (including angioplasty and stent-graft repair) are suitable for localized stenoses distant from the renal, celiac, and superior mesenteric arteries. These minimally invasive procedures are advantageous for young patients since they can be repeated and may obviate the need for open surgery. In particular, endovascular interventions allow luminal dilation in children with a developing aorta without interfering with the vessels. The first percutaneous transluminal angioplasty(PTA) for a patient with abdominal aortic coarctation was performed in 1983 (59). However, due to the relatively high failure rate, balloon angioplasty alone is less effective than stent-graft repair (60, 61). It is widely recognized that endovascular therapy has certain inherent limitations. For instance, the arterial wall may become weakened following balloon angioplasty, resulting in aneurysm formation in 5% to 20% of cases (62, 63). Furthermore, the elastic fibers disruption in the media vessel wall and vascular fibrosis in the adventitia contribute to poor patient response to both endovascular therapies, leading to restenosis rates in 25% to 60% of cases (58, 64–66). Moreover, vessel stiffness can limit endovascular therapy's effectiveness, resulting in under-dilatation and risk of stent graft rupture (67).
Aorta endarterectomy is indicated for young patients with short and isolated segment (4, 8, 68). Butcher and his colleagues first reported a satisfactory result in cases with aortoiliac arterial occlusion treated by endarterectomy (69). However, endarterectomy is not recommended for TA patients. Theoretically, aortic endarterectomy addresses only the intimal fibrosis, while fibrosis in the adventitial or periadventitial layers may persist, posing a challenge to treatment efficacy (27). While it may offer the possibility of a thoracoabdominal bypass in the later stages of life, it is widely considered a standby option.
Considering that the TA patients are relatively young, long-term durability is a vital factor in choosing surgical approaches, leading to bypass surgery as the most frequent choice in TA with descending aorta involvement (26). This approach can resolve extensive arteriopathy by an ingenious surgical design, such as using a sequential bypass to re-establish the blood supply of the ischemic organs. Some studies also revealed that the need for open surgery remains, even when percutaneous procedures presented with an increasing and widening ambit (21). To date, several techniques for extra-anatomic bypass have been proposed.
The left posterolateral thoracotomy enables the procedure to be performed on the ascending aorta, aortic arch, and entire descending aorta. However, some studies have raised concerns about potential complications, including massive bleeding, paraplegia, and chylothorax (70–72). Median sternotomy was first reported by Vijayanagar R. et al. (73) in 1980. The benefits and disadvantages of this surgical modality have been established. Optimal operative exposure for the entire descending aorta can be achieved. It also allows simultaneous cardiac procedures or reoperation to be conducted. However, the requirement of both thoracotomy and laparotomy poses a great challenge to patients' cardiorespiratory functions, making it more appropriate for younger patients. For elderly patients, aorta-femoral bypass is a better option to augment the vascular bed and retrograde renal blood flow with a lower risk of damaging the collateral circulation.
Considering the chronic inflammatory state of patients, open surgery can be overly invasive. However, there is evidence supporting the use of hybrid aortic repair as a more favorable option in a less invasive way for complex lesions. This approach involves bypassing the supra-aortic vessels or debranching the visceral or renal arteries before performing stent grafting on the aortic arch and descending aorta. Joseph G. et al. (21) proposed that about 80% patients treated with surgical procedures underwent also endovascular procedures. Hybrid aortic repair offers advantages such as shorter operation time, reduced surgical complexity, and increased success rates. It is particularly beneficial for patients with a heavily calcified aorta (74–76).
Regarding the choice of the anastomotic site, the utilization of supra celiac bare area for distal anastomosis was first reported by Wukasch D. C. et al. in 1977, which featured reduced bleeding and decreased incidence of complications due to the short course of the graft (77, 78). However, it should be noted that this less invasive approach may have certain drawbacks. For instance, inadequate exposure may be an issue in patients with abdominal obesity or barrel-shaped thorax, and managing bleeding from the distal anastomosis may be challenging. As an alternative, an ascending-to-infrarenal abdominal aortic bypass may be considered. Although longer grafts increase the risk of complications from adjacent organs, the long midline incision facilitates exposure of the whole length of the descending aorta, making anastomosis and hemostasis easier (79). More importantly, the ascending-to-infrarenal abdominal aortic bypass can also provide significant antegrade flow to permit optimal renal perfusion, which relieves renovascular hypertension (4). According to the current literature, the double-suture aortic anastomotic technique is applied to prevent postoperative anastomotic aneurysms (80).
A statistically significant difference in restenosis rates has been reported between different graft materials. Polytetrafluoroethylene (PTFE) grafts demonstrated a superior patency rate to Dacron grafts at a 7-year follow-up (100% vs. 58%, P = 0.005) (58). The graft diameter was consistent with the mean diameter of descending aorta. A 14 mm–16 mm graft was deemed sufficient for most women, while an average man required a 16 mm–18 mm graft for adequate perfusion (27). Notably, oversized grafts compared to the aorta have been recommended in children to accommodate future growth (4, 27, 64).
Perioperative medications
Corticosteroids are generally the first-line treatment to control the disease activity, and cytotoxic drugs are added for those patients with disease progression on steroid therapy (64). However, the optimal timing for initiating immunosuppressive therapy upon confirmation of the diagnosis remains a subject of debate. Hinojosa C. A. et al. (15) believed that administering the immunosuppressive drugs as early as possible could arrest disease progression and reverse early clinical symptoms. Perera and colleagues proposed a similar finding that immunosuppression before the endovascular intervention significantly improved results (P = 0.001) (45). In contrast, Young Su Kim and co-workers formulated that fibrosis and calcification are predominantly disease-specific alterations rather than vascular wall inflammation for those in the chronic inactive phase, which means using immunosuppressive agents such as cytotoxic agents or steroids is unnecessary (18).
Similarly, no consensus has been reached on the efficacy of postoperative medication use. Some authors postulated that the restenosis rate is lower with post-surgical immunosuppressive treatment, while others argued that there were no differences among patients treated with or without corticosteroids (5, 81, 82). A previous study indicated that for patients on medication therapy, 93% in the open surgery group and 86% in the interventional procedure group exhibited good long-term patency (45). However, other studies showed there was no difference between groups (47, 83).
Previous studies reached an agreement regarding antiplatelet agent use since the TA-related hypercoagulable state can lead to arterial ischemic events, and patients can benefit from anticoagulant therapy (39, 84).
In recent years, the pathogenesis of TA has been better understood, which has led to the development of targeted biotherapies aimed at inhibiting signaling pathways. Recent studies have shown promising results for biological disease-modifying agents (bDMARDs), such as TNF-α inhibitors and IL-6 inhibitors, as well as targeted synthetic disease-modifying agents (tsDMARDs), such as JAK inhibitors75–80. Although limited evidence exists for some bDMARDs, such as Rituximab, Abatacep, and Ustekinumab, they also require further investigation (85–87).
Complications and prognosis
The prognosis of TA is heavily influenced by the presence or severity of complications. A study from the late 1980s, conducted at a time when diagnostic imaging and medical treatments were less advanced, found that most patients with descending aorta involvement would not survive past the age of 35 years (3). It has been established that graft-related complications, including restenosis and anastomotic aneurysms, are the most common complications after surgery (88). Other study also proposed that peri-interventional dual antiplatelet therapy, concurrent surgery, and technical failure were predictors for complications (P < 0.05) (20).
Several studies have revealed that the most common complication in both open and endovascular groups is restenosis, and patients who underwent endovascular procedures showed a higher rate of restenosis (P < .001). The patency rate of surgical bypass varied from 64 to 100%, while that of PTA ranged from 29% to 83% (5, 7, 58, 82, 89–91). Consistently, our prior retrospective study, which examined 116 TA patients who received surgery or endovascular interventions (such as PTA and stent-graft repair), revealed that both surgical approaches were effective and safe. However, open surgical repair was found to be more suitable for complex lesions due to its longer durability (19). Moreover, other factors are related to restenoses, such as hypertension (P = 0.01), dyslipidemia (P = 0.01), and high-dose steroids (P = 0.012) (6).
It has been reported that pseudoaneurysms at the anastomotic site occur with an incidence of 12.2%, 21.2%, and 37.3% in the 10-year, 20-year, and 30-year follow-ups, respectively (12). This complication probably results from hypertension and the degradation of graft materials (92). Notably, most patients presented with no symptoms or signs and were detected incidentally, which led to devastating results. Therefore, even though the patients showed no signs of anastomotic aneurysms, regular follow-ups are needed (27).
It is widely thought that postoperative complications are associated with disease activity. However, the association between postoperative complications and disease activity has been controversial. Kim, S. M. et al. (12) thought disease activity could affect outcomes and long-term survival. However, Fields C. E. et al. (5) found that long-term survival was not affected by disease activity, supported by findings reported by Weaver F. A. et al. (93).
Studies reported that the overall survival rate at 20 years was 62.3%–73.5% (7, 12) and death was mainly attributed to cardiovascular events (7, 50). Among these, the incidence of congestive heart failure-induced death ranged from 3% to 40% (7, 10). Further investigation also revealed that the risk factors for heart failure include pulmonary hypertension, aortic valve or coronary artery involvement, onset age >38 years, and serum tumor necrosis factor (TNF)-α concentration >10 pg/ml (54).
Ishikawa K. et al. (94) identified four predictors for mortality risk factors: complications (retinopathy, secondary hypertension, aortic regurgitation, and aneurysmal formation), the pattern of the clinical course, age, and year of diagnosis. Other studies confirmed that postoperative hypertension (P = 0.028), type of disease (P = 0.0142), age at operation (P = 0.0052), and presence of an aneurysmal lesion (P = 0.0106) were significantly associated with postoperative events and survival rate (7, 33).
Discussion
The need for multiple vascular surgeries involving both endovascular and surgical procedures is not uncommon in TA patients due to the prolonged duration of the disease. Table 1 presents several studies highlighting the surgical management of descending aorta stenosis associated with TA. Most patients benefit from the correction of abnormal hemodynamics and the relief of hypertension. Current evidence suggests that 74%–90% of patients experience improvement in hypertension-related symptoms after the surgery (3, 27, 33). Surgery also played a role in relieving left ventricular burden. A study revealed that almost all patients demonstrated improved cardiac function, with some cases showing significant enhancements in interventricular septal diameter (IVSD, P = 0.016) and left ventricular mass index (LVMI, P = 0.017) (18). An updated retrospective study from our research team also demonstrated that surgery could significantly improve the prognosis of patients.
Taketani T. et al. (33) reported that after surgical treatment, the overall survival and event-free survival rate were 62.3% and 58.4% at 20-year follow-up, and postoperative hypertension was a significant predictor of event-free survival (P = 0.028). Kalangos A. et al. (58) demonstrated the safety and effect of the surgery, with hypertension being controlled and cardiac function returning to normal postoperatively. Stanley J. C. et al. (27) assessed the outcomes of different operative treatments in patients with abdominal aortic coarctation (4 were diagnosed with inflammatory aortitis) and found that more than 90% of patients benefit from surgery. The above studies overlap in their assertion that surgery is safe and effective for TA in all arterial areas. Herein, we focused on patients with descending aorta involvement and comprehensively analyzed the surgical methods and clinical outcomes of this patient population. We aimed to review the relevant literature in detail and summarize the unique characteristics of these patients.
With significant inroads achieved in surgical techniques, multidisciplinary decision-making, targeted biotherapies, and comprehensive postoperative monitoring and treatment, surgeries can be performed with low morbidity and improved quality of life (58). In addition, a deeper understanding of the pathophysiology of arterial reconstruction in TA patients helps to reduce surgical complications.
Preoperative evaluation is critical in guiding surgical decisions regarding the method and timing of interventions in TA patients. Despite their relatively young age, TA patients often present with severe cardiac, renal, and pulmonary complications due to the insidious nature of the disease and its atypical clinical manifestations. The involvement of multiple arterial bifurcations further adds to the complexity of the condition. As a result, comprehensive medical evaluations are essential prior to surgery. These evaluations encompass various aspects, such as assessing vascular lesions, determining disease activity, evaluating cardiopulmonary function, and overall disease status. While erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are commonly used as acute-phase indicators in TA, it should be noted that these serum markers may not always accurately reflect vascular wall inflammation. In fact, approximately 30%–40% of patients in the active phase of the disease may exhibit normal ESR and/or CRP levels (26). More importantly, combining clinical presentation, serum markers, and imaging examinations such as PET-CT and MRI is crucial for accurately assessing disease activity in TA patients, especially when acute-phase reactants exhibit poor sensitivity during periods of low disease activity.
The presence of nonspecific symptoms often leads to delayed diagnosis of TA, resulting in disease progression and the occurrence of ischemic events. Early diagnosis and prompt treatment are essential in order to improve the prognosis of patients with TA (95).
Besides, although the indications for surgery have not been definitively established, we advocate the safety and efficacy of surgical intervention. Prior to 1988, the average life expectancy of patients with atypical TA was only 35 years, likely due to less advanced and effective diagnostic techniques that led to delayed treatment (96). Kalangos A. et al. (58) revealed that patients couldundergo reconstructive procedures with satisfactory midterm and long-term outcomes regardless of the extent and severity of vascular lesions. We also demonstrated in another article that surgical revascularization is superior in relieving symptoms and improving the prognosis compared to conservative treatment. Moreover, given that these patients are complicated with coexisting renal and splanchnic artery occlusion, surgery aims to restore the renal and splanchnic artery flow based on the symptoms.
More importantly, the inconsistency of findings may be attributed to different follow-up duration. It has long been thought that at least a 20-year follow-up is mandatory to reflect the impact of surgical therapy since about 10% of patients were treated with secondary surgeries in the late stages of follow-up (5, 27). However, the debate regarding the choice of surgical options remains unsettled due to the limited number of studies with long-term follow-up, varying prognoses among patients, and inconsistent durations of follow-up.
It should be borne in mind that TA is a rare disease, and it is challenging to obtain a large cohort of patients undergoing surgical treatment. Further research is required to confirm the efficacy and safety of these procedures, which will enable us to offer improved treatment options for patients with TA.
Conclusion
The ongoing advancements in surgical techniques establish surgery as a viable treatment option for TA. While more studies are required to establish definitive criteria for surgical indications, existing data indicate that most patients can benefit from surgery.
Author contributions
YF conducted relevant literature and wrote the article, and YC designed the article and revised it. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-191).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Seyahi E. Takayasu arteritis: an update. Curr Opin Rheumatol. (2017) 29(1):51–6. doi: 10.1097/BOR.0000000000000343
2. Vanoli M, Daina E, Salvarani C, Sabbadini MG, Rossi C, Bacchiani G, et al. Takayasu's arteritis: a study of 104 Italian patients. Arthritis Rheum. (2005) 53(1):100–7. doi: 10.1002/art.20922
3. Cohen JR, Birnbaum E. Coarctation of the abdominal aorta. J Vasc Surg. (1988) 8(2):160–4. doi: 10.1016/0741-5214(88)90404-1
4. Hetzer R, Absi D, Miera O, Solowjowa N, Schulz A, del Maria Javier MF, et al. Extraanatomic bypass technique for the treatment of midaortic syndrome in children. Ann Thorac Surg. (2013) 96(1):183–9. doi: 10.1016/j.athoracsur.2013.03.025
5. Fields CE, Bower TC, Cooper LT, Hoskin T, Noel AA, Panneton JM, et al. Takayasu's arteritis: operative results and influence of disease activity. J Vasc Surg. (2006) 43(1):64–71. doi: 10.1016/j.jvs.2005.10.010
6. Labarca C, Makol A, Crowson CS, Kermani TA, Matteson EL, Warrington KJ. Retrospective comparison of open versus endovascular procedures for takayasu arteritis. J Rheumatol. (2016) 43(2):427–32. doi: 10.3899/jrheum.150447
7. Miyata T, Sato O, Koyama H, Shigematsu H, Tada Y. Long-term survival after surgical treatment of patients with Takayasu's Arteritis. Circulation. (2003) 108(12):1474–80. doi: 10.1161/01.CIR.0000089089.42153.5E
8. Joh J-H, Kim D-K, Park K-H, Kim D-I. Surgical management of Takayasu's Arteritis. J Korean Med Sci. (2006) 21(1):20–4. doi: 10.3346/jkms.2006.21.1.20
9. Saadoun D, Lambert M, Mirault T, Resche-Rigon M, Koskas F, Cluzel P, et al. Retrospective analysis of surgery versus endovascular intervention in takayasu arteritis: a multicenter experience. Circulation. (2012) 125(6):813–9. doi: 10.1161/CIRCULATIONAHA.111.058032
10. Lee GY, Jeon P, Do YS, Sung K, Kim DI, Kim YW, et al. Comparison of outcomes between endovascular treatment and bypass surgery in takayasu arteritis. Scand J Rheumatol. (2014) 43(2):153–61. doi: 10.3109/03009742.2013.822096
11. Lee B-B, Laredo J, Neville R, Villavicencio JL. Endovascular management of takayasu arteritis: is it a durable option? Vascular. (2009) 17(3):138–46. doi: 10.2310/6670.2009.00012
12. Kim SM, Jung IM, Han A, Min SI, Lee T, Ha J, et al. Surgical treatment of middle aortic syndrome with takayasu arteritis or midaortic dysplastic syndrome. Eur J Vasc Endovasc Surg. (2015) 50(2):206–12. doi: 10.1016/j.ejvs.2015.04.032
13. Hinojosa CA, Anaya-Ayala JE, Torres-Machorro A, Lizola R, Laparra-Escareno H. Middle aortic syndrome in Takayasu's Arteritis: report of two surgical cases. Ann Vasc Surg. (2016) 34:270.e13–e17. doi: 10.1016/j.avsg.2015.12.015
14. Setty HSN, Rao M, Srinivas KH, Srinivas BC, Usha MK, Jayaranganath M, et al. Clinical, angiographic profile and percutaneous endovascular management of Takayasu's Arteritis—a single centre experience. Int J Cardiol. (2016) 220:924–8. doi: 10.1016/j.ijcard.2016.06.194
15. Hinojosa CA, Anaya-Ayala JE, Gomez-Arcive Z, Laparra-Escareno H, Torres-Machorro A, Lizola R. Factors associated with need for revascularisation in non-coronary arterial occlusive lesions secondary to Takayasu's Arteritis. Eur J Vasc Endovasc Surg. (2017) 54(3):397–404. doi: 10.1016/j.ejvs.2017.05.020
16. Che W, Xiong H, Jiang X, Dong H, Zou Y, Yang Y, et al. Stenting for middle aortic syndrome caused by takayasu arteritis-immediate and long-term outcomes. Catheter Cardiovasc Interv. (2018) 91(S1):623–31. doi: 10.1002/ccd.27492
17. Rosa Neto NS, Shinjo SK, Levy-Neto M, Pereira RMR. Vascular surgery: the main risk factor for mortality in 146 takayasu arteritis patients. Rheumatol Int. (2017) 37(7):1065–73. doi: 10.1007/s00296-017-3656-y
18. Kim YS, Cho YH, Sung K, Kim D-K, Chung S, Park TK, et al. Clinical outcome of extraanatomic bypass for midaortic syndrome caused by takayasu arteritis. Ann Thorac Surg. (2020) 109(5):1419–25. doi: 10.1016/j.athoracsur.2019.08.032
19. Diao Y, Yan S, Premaratne S, Chen Y, Tian X, Chen Z, et al. Surgery and endovascular management in patients with Takayasu's Arteritis: a ten-year retrospective study. Ann Vasc Surg. (2020) 63:34–44. doi: 10.1016/j.avsg.2019.07.009
20. Fan L, Yang L, Wei D, Ma W, Lou Y, Song L, et al. Clinical scenario and long-term outcome of childhood takayasu arteritis undergoing 121 endovascular interventions: a large cohort over a fifteen-year period. Arthritis Care Res (Hoboken). (2021) 73(11):1678–88. doi: 10.1002/acr.24387
21. Joseph G, Thomson VS, Attumalil TV, Mathen PG, Anandaraj AM, George OK, et al. Outcomes of percutaneous intervention in patients with takayasu arteritis. J Am Coll Cardiol. (2023) 81(1):49–64. doi: 10.1016/j.jacc.2022.10.024
22. Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM, et al. The American college of rheumatology 1990 criteria for the classification of takayasu arteritis. Arthritis Rheum. (1990) 33(8):1129–34. doi: 10.1002/art.1780330811
23. Sharma BK, Jain S, Suri S, Numano F. Diagnostic criteria for takayasu arteritis. Int J Cardiol. (1996) 54(Suppl):S141–7. doi: 10.1016/S0167-5273(96)88783-3
24. de Souza AWS, de Carvalho JF. Diagnostic and classification criteria of takayasu arteritis. J Autoimmun. (2014) 48-49:79–83. doi: 10.1016/j.jaut.2014.01.012
25. Grayson PC, Ponte C, Suppiah R, Robson JC, Gribbons KB, Judge A, et al. 2022 American college of rheumatology/EULAR classification criteria for takayasu arteritis. Ann Rheum Dis. (2022) 81(12):1654–60. doi: 10.1136/ard-2022-223482
26. Petrovic-Rackov L, Pejnovic N, Jevtic M, Damjanov N. Longitudinal study of 16 patients with Takayasu's Arteritis: clinical features and therapeutic management. Clin Rheumatol. (2009) 28(2):179–85. doi: 10.1007/s10067-008-1009-7
27. Stanley JC, Criado E, Eliason JL, Upchurch GR, Berguer R, Rectenwald JE. Abdominal aortic coarctation: surgical treatment of 53 patients with a thoracoabdominal bypass, patch aortoplasty, or interposition aortoaortic graft. J Vasc Surg. (2008) 48(5):1073–82. doi: 10.1016/j.jvs.2008.05.078
28. Joseph G, Goel R, Thomson VS, Joseph E, Danda D. Takayasu arteritis: jACC focus seminar 3/4. J Am Coll Cardiol. (2022) 81(2):172–86. doi: 10.1016/j.jacc.2022.09.051
29. Zhang Z, Wang W, Zhou M, Lu PYJ, Li Y, Chen Y. An observational study of sex differences in takayasu arteritis in China: implications for worldwide regional differences. Ann Vasc Surg. (2020) 66:309–17. doi: 10.1016/j.avsg.2019.12.007
30. Watanabe Y, Miyata T, Tanemoto K. Current clinical features of new patients with takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation. (2015) 132(18):1701–9. doi: 10.1161/CIRCULATIONAHA.114.012547
31. Lim AY, Lee GY, Jang SY, Gwag HB, Choi SH, Jeon ES, et al. Gender differences in clinical and angiographic findings of patients with takayasu arteritis. Clin Exp Rheumatol. (2015) 33(2 Suppl 89):132–37.25572418
32. Hata A, Noda M, Moriwaki R, Numano F. Angiographic findings of takayasu arteritis: new classification. Int J Cardiol. (1996) 54(Suppl):S155–63. doi: 10.1016/S0167-5273(96)02813-6
33. Taketani T, Miyata T, Morota T, Takamoto S. Surgical treatment of atypical aortic coarctation complicating Takayasu's Arteritis–experience with 33 cases over 44 years. J Vasc Surg. (2005) 41(4):597–601. doi: 10.1016/j.jvs.2005.01.022
34. Comarmond C, Biard L, Lambert M, Mekinian A, Ferfar Y, Kahn J-E, et al. Long-Term outcomes and prognostic factors of complications in takayasu arteritis: a multicenter study of 318 patients. Circulation. (2017) 136(12):1114–22. doi: 10.1161/CIRCULATIONAHA.116.027094
35. Qi Y, Yang L, Zhang H, Liang E, Song L, Cai J, et al. The presentation and management of hypertension in a large cohort of takayasu arteritis. Clin Rheumatol. (2018) 37(10):2781–8. doi: 10.1007/s10067-017-3947-4
36. Hoffman GS, Ahmed AE. Surrogate markers of disease activity in patients with takayasu arteritis. A preliminary report from the international network for the study of the systemic vasculitides (INSSYS). Int J Cardiol. (1998) 66:Suppl 1. doi: 10.1016/S0167-5273(98)00181-8
37. Hoffman GS. Takayasu arteritis: lessons from the American national institutes of health experience. Int J Cardiol. (1996) 54(Suppl):S99–102. doi: 10.1016/S0167-5273(96)88778-X
38. Jiang L, Li D, Yan F, Dai X, Li Y, Ma L. Evaluation of takayasu arteritis activity by delayed contrast-enhanced magnetic resonance imaging. Int J Cardiol. (2012) 155(2):262–7. doi: 10.1016/j.ijcard.2010.10.002
39. Wang H, Lai B, Wu X, Han T, Chen H. Late diagnosis of Takayasu's Arteritis with repeated attacks of heart failure and uncontrolled hypertension due to abdominal aortic thrombosis: case report and review of the literature. Blood Press. (2015) 24(6):333–9. doi: 10.3109/08037051.2015.1049423
40. Tarkin JM, Wall C, Gopalan D, Aloj L, Manavaki R, Fryer TD, et al. Novel approach to imaging active takayasu arteritis using somatostatin receptor positron emission tomography/magnetic resonance imaging. Circulation. Cardiovascular Imaging. (2020) 13(6):e010389. doi: 10.1161/CIRCIMAGING.119.010389
41. Lottspeich C, Dechant C, Köhler A, Tischler M, Treitl KM, Treitl M, et al. Assessment of disease activity in takayasu arteritis: potential role of contrast-enhanced ultrasound. Ultraschall in Der Medizin (Stuttgart, Germany: 1980). (2019) 40(5):638–45. doi: 10.1055/a-0817-5423
42. Germanò G, Macchioni P, Possemato N, Boiardi L, Nicolini A, Casali M, et al. Contrast-Enhanced ultrasound of the carotid artery in patients with large vessel vasculitis: correlation with positron emission tomography findings. Arthritis Care Res (Hoboken). (2017) 69(1):143–9. doi: 10.1002/acr.22906
43. Wang Y, Wang Y-H, Tian X-P, Wang H-Y, Li J, Ge Z-T, et al. Contrast-enhanced ultrasound for evaluating arteritis activity in takayasu arteritis patients. Clin Rheumatol. (2020) 39(4):1229–35. doi: 10.1007/s10067-019-04698-9
44. Li Z, Zheng Z, Ding J, Li X, Zhao Y, Kang F, et al. Contrast-enhanced ultrasonography for monitoring arterial inflammation in takayasu arteritis. J Rheumatol. (2019) 46(6):616–22. doi: 10.3899/jrheum.180701
45. Perera AH, Youngstein T, Gibbs RGJ, Jackson JE, Wolfe JH, Mason JC. Optimizing the outcome of vascular intervention for takayasu arteritis. Br J Surg. (2014) 101(2):43–50. doi: 10.1002/bjs.9372
46. Keleşoğlu Dinçer AB, Kılıç L, Erden A, Kalyoncu U, Hazirolan T, Kiraz S, et al. Imaging modalities used in diagnosis and follow-up of patients with Takayasu's Arteritis. Turk J Med Sci. (2021) 51(1):224–30. doi: 10.3906/sag-2005-70
47. Ohigashi H, Haraguchi G, Konishi M, Tezuka D, Kamiishi T, Ishihara T, et al. Improved prognosis of takayasu arteritis over the past decade–comprehensive analysis of 106 patients. Circ J. (2012) 76(4):1004–11. doi: 10.1253/circj.CJ-11-1108
48. Wong SPY, Mok CC, Lau CS, Yip ML, Tam LS, Ying KY, et al. Clinical presentation, treatment and outcome of Takayasu's Arteritis in southern Chinese: a multicenter retrospective study. Rheumatol Int. (2018) 38(12):2263–70. doi: 10.1007/s00296-018-4150-x
49. Hellmich B, Agueda A, Monti S, Buttgereit F, de Boysson H, Brouwer E, et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. (2020) 79(1):19–30. doi: 10.1136/annrheumdis-2019-215672
50. Numano F. Differences in clinical presentation and outcome in different countries for Takayasu's Arteritis. Curr Opin Rheumatol. (1997) 9(1):12–5. doi: 10.1097/00002281-199701000-00003
51. Honig HS, Weintraub AM, Gomes MN, Hufnagel CA, Roberts WC. Severe aortic regurgitation secondary to idiopathic aortitis. Am J Med. (1977) 63(4):623–33. doi: 10.1016/0002-9343(77)90208-X
52. Isomura T, Hisatomi K, Yanagi I, Shimada S, Uraguchi K, Aoyagi S, et al. The surgical treatment of aortic regurgitation secondary to aortitis. Ann Thorac Surg. (1988) 45(2):181–5. doi: 10.1016/S0003-4975(10)62433-2
53. Cheng X, Li Z, Dang A, Lv N, Chang Q, Song Y, et al. Different treatment options for takayasu arteritis patients with moderate-to-severe aortic regurgitation: long-term outcomes. Rheumatol (Oxford, England). (2021) 60(7):3134–43. doi: 10.1093/rheumatology/keaa647
54. Wang Y-J, Ma L-L, Liu Y, Yan Y, Sun Y, Wang Y-S, et al. Risk assessment model for heart failure in Chinese patients with Takayasu's Arteritis. Clin Rheumatol. (2021) 40(10):4117–26. doi: 10.1007/s10067-021-05745-0
55. Amano J, Suzuki A. Coronary artery involvement in Takayasu's Arteritis. Collective review and guideline for surgical treatment. J Thorac Cardiovasc Surg. (1991) 102(4):554–60. doi: 10.1016/S0022-5223(20)31426-4
56. Yuan S-M, Lin H-Z. Coronary artery involvements in takayasu arteritis: systematic review of reports. Gen Thorac Cardiovasc Surg. (2020) 68(9):883–904. doi: 10.1007/s11748-020-01378-3
57. Graham LM, Zelenock GB, Erlandson EE, Coran AG, Lindenauer SM, Stanley JC. Abdominal aortic coarctation and segmental hypoplasia. Surgery. (1979) 86(4):519–29. doi: 10.5555/uri:pii:0039606079901946
58. Kalangos A, Christenson JT, Cikirikcioglu M, Vala D, Buerge A, Simonet F, et al. Long-term outcome after surgical intervention and interventional procedures for the management of Takayasu's Arteritis in children. J Thorac Cardiovasc Surg. (2006) 132(3):656–64. doi: 10.1016/j.jtcvs.2006.04.020
59. Nanni GS, Hawkins IF, Alexander JA. Percutaneous transluminal angioplasty of an abdominal aortic coarctation. AJR. Am J Roentgenol. (1983) 140(6):1239–41. doi: 10.2214/ajr.140.6.1239
60. Ballweg J, Liniger R, Rocchini A, Gajarski R. Use of palmaz stents in a newborn with congenital aneurysms and coarctation of the abdominal aorta. Catheter Cardiovasc Interv. (2006) 68(4):648–52. doi: 10.1002/ccd.20749
61. Eliason JL, Passman MA, Guzman RJ, Naslund TC. Durability of percutaneous angioplasty and stent implantation for the treatment of abdominal aortic coarctation: a case report. Vasc Surg. (2001) 35(5):397–401. doi: 10.1177/153857440103500511
62. Ovaert C, Benson LN, Nykanen D, Freedom RM. Transcatheter treatment of coarctation of the aorta: a review. Pediatr Cardiol. (1998) 19(1):27–44. doi: 10.1007/s002469900243
63. O'Laughlin MP, Perry SB, Lock JE, Mullins CE. Use of endovascular stents in congenital heart disease. Circulation. (1991) 83(6):1923–39. doi: 10.1161/01.CIR.83.6.1923
64. Petrovic-Rackov L, Pejnovic N, Jevtic M. Refractory rapidly progressive Takayasu's Arteritis successfully treated with surgery. Clin Rheumatol. (2007) 26(10):1787–9. doi: 10.1007/s10067-006-0522-9
65. Saxena A, Kothari SS, Sharma S, Juneja R, Srivastava S. Percutaneous transluminal angioplasty of the aorta in children with nonspecific aortoarteritis: acute and follow-up results with special emphasis on left ventricular function. Catheter Cardiovasc Interv. (2000) 49(4):419–24. doi: 10.1002/(SICI)1522-726X(200004)49:4%3C419::AID-CCD15%3E3.0.CO;2-I
66. Deyu Z, Lisheng L, Ruping D, Haiying W, Guozhang L. Percutaneous transluminal renal angioplasty in aortoarteritis. Int J Cardiol. (1998) 66(Suppl 1):S205–11. doi: 10.1016/S0167-5273(98)00170-3
67. Hiraya D, Sato A, Watabe H, Hoshi T, Ieda M. Axillofemoral bypass to improve congestive heart failure for atypical aortic coarctation complicating takayasu arteritis. ESC Heart Failure. (2020) 7(5):3184–8. doi: 10.1002/ehf2.12855
68. Kim DI, Huh SH, Lee BB. Aortic endarterectomy in Takayasu's Arteritis and Leriche's Syndrome. J Cardiovasc Surg (Torino). (2002) 43(5):751.12386597
69. Butcher HR, Jaffe BM. Treatment of aortoiliac arterial occlusive disease by endarterectomy. Ann Surg. (1971) 173(6):925–32. doi: 10.1097/00000658-197106010-00010
70. Jacob T, Cobanoglu A, Starr A. Late results of ascending aorta-descending aorta bypass grafts for recurrent coarctation of aorta. J Thorac Cardiovasc Surg. (1988) 95(5):782–7. doi: 10.1016/S0022-5223(19)35688-0
71. Tsukube T, Yoshimura M, Matsuda H, Okada K, Matsukawa R, Hino Y, et al. Rib-cross thoracotomy for replacement of the thoracoabdominal or total descending aorta. J Vasc Surg. (2003) 37(1):219–21. doi: 10.1067/mva.2003.49
72. Okada K, Tanaka A, Munakata H, Matsumori M, Morimoto Y, Tanaka Y, et al. Extended replacement of aortic arch aneurysms through left posterolateral thoracotomy. Eur J Cardiothorac Surg. (2009) 35(2):270–5. doi: 10.1016/j.ejcts.2008.09.048
73. Vijayanagar R, Natarajan P, Eckstein PF, Bognolo DA, Toole JC. Aortic valvular insufficiency and postductal aortic coarctation in the adult. Combined surgical management through median sternotomy: a new surgical approach. J Thorac Cardiovasc Surg. (1980) 79(2):266–8. doi: 10.1016/S0022-5223(19)37983-8
74. Obitsu Y, Koizumi N, Saiki N, Kawaguchi S, Shigematsu H. Long-term result of hybrid procedure for an extensive thoracic aortic aneurysm in takayasu arteritis: a case report. J Cardiothorac Surg. (2010) 5:28. doi: 10.1186/1749-8090-5-28
75. Okonogi S, Ohki S, Obayashi T, Yasuhara K, Nagasawa A, Miki T. Single-stage hybrid procedure for ruptured calcified thoracic aortic aneurysm caused by Takayasu's Arteritis. Gen Thorac Cardiovasc Surg. (2021) 69(3):610–3. doi: 10.1007/s11748-020-01513-0
76. Liu Z, Zhou M, Liu C, Qiao T, Huang D, Zhang M, et al. Hybrid procedures for thoracoabdominal aortic pathologies. Vascular. (2013) 21(4):205–14. doi: 10.1177/1708538113478772
77. Wukasch DC, Cooley DA, Sandiford FM, Nappi G, Reul GJ. Ascending aorta-abdominal aorta bypass: indications, technique, and report of 12 patients. Ann Thorac Surg. (1977) 23(5):442–8. doi: 10.1016/S0003-4975(10)64164-1
78. Kumar MV, Choudhary SK, Talwar S, Gharde P, Sahu M, Kumar S, et al. Extraanatomic bypass to supraceliac abdominal aorta for Complex thoracic aortic obstruction. Ann Thorac Surg. (2016) 101(4):1552–7. doi: 10.1016/j.athoracsur.2015.10.080
79. Wang R, Sun L-Z, Hu X-P, Ma W-G, Chang Q, Zhu J-M, et al. Treatment of complex coarctation and coarctation with cardiac lesions using extra-anatomic aortic bypass. J Vasc Surg. (2010) 51(5):1203–8. doi: 10.1016/j.jvs.2009.12.027
80. Oishi K, Mizuno T, Fujiwara T, Kuroki H, Yashima M, Takeshita M, et al. Surgical strategy for inflammatory thoracic aortic aneurysms in the endovascular surgery era. J Vasc Surg. (2022) 75:1. doi: 10.1016/j.jvs.2021.06.479
81. Park MC, Lee SW, Park YB, Lee SK, Choi D, Shim WH. Post-interventional immunosuppressive treatment and vascular restenosis in Takayasu's Arteritis. Rheumatol (Oxford, England). (2006) 45(5):600–5. doi: 10.1093/rheumatology/kei245
82. Tyagi S, Gupta MD, Singh P, Shrimal D, Girish MP. Percutaneous revascularization of sole arch artery for severe cerebral ischemia resulting from takayasu arteritis. J Vasc and Int Radiol: JVIR. (2008) 19(12):1699–703. doi: 10.1016/j.jvir.2008.09.017
83. Ham SW, Kumar SR, Rowe VL, Weaver FA. Disease progression after initial surgical intervention for takayasu arteritis. J Vasc Surg. (2011) 54(5):1345–51. doi: 10.1016/j.jvs.2011.04.044
84. Akazawa H, Ikeda U, Yamamoto K, Kuroda T, Shimada K. Hypercoagulable state in patients with Takayasu's Arteritis. Thromb Haemostasis. (1996) 75(5):712–6. doi: 10.1055/s-0038-1650353
85. Pazzola G, Muratore F, Pipitone N, Crescentini F, Cacoub P, Boiardi L, et al. Rituximab therapy for takayasu arteritis: a seven patients experience and a review of the literature. Rheumatol (Oxford, England). (2018) 57(7):1151–5. doi: 10.1093/rheumatology/kex249
86. Langford CA, Cuthbertson D, Ytterberg SR, Khalidi N, Monach PA, Carette S, et al. A randomized, double-blind trial of Abatacept (CTLA-4Ig) for the treatment of takayasu arteritis. Arth & Rheumatol (Hoboken, N.J.). (2017) 69(4):846–53. doi: 10.1002/art.40037
87. Terao C, Yoshifuji H, Nakajima T, Yukawa N, Matsuda F, Mimori T. Ustekinumab as a therapeutic option for takayasu arteritis: from genetic findings to clinical application. Scand J Rheumatol. (2016) 45(1):80–2. doi: 10.3109/03009742.2015.1060521
88. Miyata T. The Asia pacific meeting for vasculitis and ANCA workshop 2012: surgical treatment for Takayasu's Arteritis. Clin Exp Nephrol. (2014) 18(2):296–300. doi: 10.1007/s10157-013-0894-5
89. Reddy E, Robbs JV. Surgical management of Takayasu's Arteritis in children and adolescents. Cardiovasc J Afr. (2007) 18(6):393–6. doi: 10.10520/EJC2306
90. Liang P, Hoffman GS. Advances in the medical and surgical treatment of takayasu arteritis. Curr Opin Rheumatol. (2005) 17(1):16–24. doi: 10.1097/01.bor.0000146607.65808.37
91. Jung JH, Lee YH, Song GG, Jeong HS, Kim J-H, Choi SJ. Endovascular versus open surgical intervention in patients with Takayasu's Arteritis: a meta-analysis. Eur J Vasc Endovasc Surg. (2018) 55(6):888–99. doi: 10.1016/j.ejvs.2018.02.030
92. Berdat PA, Göber V, Carrel T. Extra-anatomic aortic bypass for complex (re-) coarctation and hypoplastic aortic arch in adolescents and adults. Interact Cardiovasc Thorac Surg. (2003) 2(2):133–7. doi: 10.1016/S1569-9293(02)00122-6
93. Weaver FA, Kumar SR, Yellin AE, Anderson S, Hood DB, Rowe VL, et al. Renal revascularization in takayasu arteritis-induced renal artery stenosis. J Vasc Surg. (2004) 39(4):749–57. doi: 10.1016/j.jvs.2003.12.022
94. Ishikawa K, Maetani S. Long-term outcome for 120 Japanese patients with Takayasu's Disease. Clinical and statistical analyses of related prognostic factors. Circulation. (1994) 90(4):1855–60. doi: 10.1161/01.CIR.90.4.1855
95. Clarkson PM, Nicholson MR, Barratt-Boyes BG, Neutze JM, Whitlock RM. Results after repair of coarctation of the aorta beyond infancy: a 10 to 28 year follow-up with particular reference to late systemic hypertension. Am J Cardiol. (1983) 51(9):1481–8. doi: 10.1016/0002-9149(83)90661-6
Keywords: surgery, descending aorta, takayasu arteritis, bypass suegery, endovascular treatment
Citation: Fu Y and Chen Y (2023) Operative experience on descending aorta with Takayasu Arteritis: a review. Front. Cardiovasc. Med. 10:1181285. doi: 10.3389/fcvm.2023.1181285
Received: 7 March 2023; Accepted: 12 June 2023;
Published: 21 June 2023.
Edited by:
Xiangjiu Ding, Shandong University, ChinaReviewed by:
Zilun Li, The First Affiliated Hospital of Sun Yat-sen University, ChinaSara Seitun, Radiology Unit, San Martino Polyclinic Hospital IRCCS, Italy
Copyright: © 2023 Fu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuexin Chen, Y3l1ZXhpbjIwMDdAMTYzLmNvbQ==
 Yining Fu
Yining Fu Yuexin Chen
Yuexin Chen