
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 02 August 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1179968
 Martin Nicol1*
Martin Nicol1* Giuseppe Vergaro2
Giuseppe Vergaro2 Thibaud Damy3
Thibaud Damy3 Mounira Kharoubi3
Mounira Kharoubi3 Mathilde Baudet1
Mathilde Baudet1 Elena Sofia Canuti4
Elena Sofia Canuti4 Alberto Aimo2
Alberto Aimo2 Vincenzo Castiglione2
Vincenzo Castiglione2 Michele Emdin2
Michele Emdin2 Bruno Royer5
Bruno Royer5 Stephanie Harel5
Stephanie Harel5 Alain Cohen-Solal1
Alain Cohen-Solal1 Bertrand Arnulf5
Bertrand Arnulf5 Damien Logeart1
Damien Logeart1
Background: Both light-chain (AL) amyloidosis and transthyretin (ATTR) amyloidosis are types of cardiac amyloidosis (CA) that require accurate prognostic stratification to plan therapeutic strategies and follow-ups. Cardiac biomarkers, e.g., N-terminal pro-B-type natriuretic peptide (NT-proBNP) and high-sensitivity cardiac troponin T (Hs-cTnT), remain the cornerstone of the prognostic assessment. An increased level of soluble suppression of tumorigenesis-2 (sST2) is predictive of adverse events [all-cause death and heart failure (HF) hospitalizations] in patients with HF. This study aimed to evaluate the prognostic value of circulating sST2 levels in AL-CA and ATTR-CA.
Methods: We carried out a multicenter study including 133 patients with AL-CA and 152 patients with ATTR-CA. During an elective outpatient visit for the diagnosis of CA, Mayo Clinic staging [NT-proBNP, Hs-cTnT, differential of free light chains (DFLCs)] and sST2 were assessed for all AL patients. Gillmore staging [including estimated glomerular filtration rate (eGFR), NT-proBNP] and Grogan staging (including NT-proBNP and Hs-cTnT) were assessed for TTR-CA patients.
Results: The median age was 73 years [interquartile range (IQR) 61–81], and 53% were men. The endpoint was the composite of all-cause death or first HF-related hospitalization. The median follow-up was 20 months (IQR 3–34) in AL amyloidosis and 33 months (6–45) in TTR amyloidosis. The primary outcome occurred in 70 (53%) and 99 (65%) of AL and TTR patients, respectively. sST2 levels were higher in patients with AL-CA than in patients with ATTR-CA: 39 ng/L (26–80) vs. 32 ng/L (21–46), p < 0.001. In AL-CA, sST2 levels predicted the outcome regardless of the Mayo Clinic score (HR: 2.16, 95% CI: 1.17–3.99, p < 0.001). In TTR-CA, sST2 was not predictive of the outcome in multivariate models, including Gillmore staging and Grogan staging (HR: 1.17, CI: 95% 0.77–1.89, p = 0.55).
Conclusion: sST2 level is a relevant predictor of death and HF hospitalization in AL cardiac amyloidosis and adds prognostic stratification on top of NT-proBNP, Hs cTnT, and DFLC.
Cardiac involvement is associated with a worse prognosis in patients with amyloidosis. Treatment methods for light-chain (AL) amyloidosis and transthyretin (ATTR) amyloidosis have improved over the last decade, and prognostic stratification should be more and more useful for helping physicians expand therapeutic choices. Cardiac biomarkers are the cornerstone of prognostic assessment (1, 2). In AL amyloidosis, the stratification of patients is based on Mayo Clinic staging (3) that includes cardiac troponins, natriuretic peptides [N-terminal pro-B-type natriuretic peptide (NT-proBNP)], and the differential of free light chains in the revisited Mayo Clinic staging (4). In ATTR amyloidosis, the prognostic score is the Gillmore staging, which includes estimated glomerular filtration rate (eGFR) and NT-proBNP (5), and the Grogan staging, which includes troponin T and NT-proBNP (6). Like most prognostic scores, they are imperfect and could be improved by adding new and relevant variables.
Soluble suppression of tumorigenicity-2 (sST2) is the circulating form of the interleukin-33 membrane receptor released in response to inflammation, fibrosis in various organs, and myocardium stress (7, 8). The prognostic value of sST2 blood levels has been shown in various diseases. In heart failure (HF), sST2 levels add prognostic information on top of natriuretic peptides, and sST2 testing has been included in recent guidelines (9, 10). Inflammation and profibrotic pathways are one of the explanations for cardiac damage in systemic amyloidosis (11), but the prognostic usefulness of the sST2 level measurement has been poorly studied in cardiac amyloidosis (CA), except for one large study that highlighted the prognostic role of sST2 in AL amyloidosis (12).
We aimed to assess the prognostic value of sST2 in CA, compare it with previously validated biomarkers, and assess its added value on top of other biomarkers and Mayo Clinic staging (4), Gillmore staging (5), and Grogan staging (6) for AL and ATTR amyloidosis.
This study involved three independent cohorts that included consecutive patients receiving a final diagnosis of CA at Lariboisiere Saint-Louis Hospital (Paris, France, n = 105), Henri Mondor Hospital (Creteil, France, n = 92), and Fondazione Monasterio (Pisa, Italy, n = 103). CA was diagnosed according to the current diagnostic algorithm (13). As part of an elective outpatient visit, all patients underwent clinical examination, blood measurements of high-sensitivity cardiac troponin T (Hs-cTnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and free light chains, 12-lead electrocardiography, and comprehensive echocardiographic examination in accordance with the American Society of Echocardiography recommendations (14). Hs-cTnT and NT-proBNP were obtained by commercialized assays (Roche Diagnostics). Serum free light chains were determined using the Freelite assay (Binding Site, Birmingham, Meylan, France). The 2012 Mayo Clinic score (4) was calculated for each patient with NT-proBNP (cutoff 1,800 ng/L), Hs-cTnT (cutoff 40 ng/L) (15), and the differential of plasma free light chains (cutoff 180 mg/L) in AL amyloidosis patients. The 2004 Mayo Clinic staging (3) and stage 3B patients according to Wechalekar et al. (16) were also reported. All AL and TTR amyloidosis patients were included within the first 2 months after the diagnosis. For each TTR patient, Gillmore staging and Grogan staging were calculated. In TTR amyloidosis, prognostic thresholds were 3,000 ng/L for NT-proBNP, 65 ng/L for Hs-cTnT, and 45 mL/min for eGFR according to Gillmore and Gillmore staging (5) and after applying changes in the cTnT cutoff due to the use of new-generation Hs-cTnT (15).
Administration of specific therapies (TTR tetramer stabilizers) at the time of inclusion was checked. Exclusion criteria were age <18 years, pregnant and breastfeeding women, suffering from Randall's disease, and the presence of forms of amyloidosis other than AL-CA or ATTR-CA. The study was carried out according to the principles outlined in the Declaration of Helsinki. Informed and written consent was obtained from all patients.
Blood levels of sST2 were determined with the Presage ST2 ELISA (Critical Diagnostics, San Diego, CA, USA) during the diagnosis of cardiac amyloidosis. The assay was performed on an Aspect Reader device with Aspect-Plus ST2 Rapid Test assay cartridges marketed by Critical Diagnostics. The assay principle was based on lateral flow immunofluorescence, and the reproducibility coefficients of variation were between 9% and 20% depending on the concentration level between 75 and 30 ng/L. According to a previous study on amyloidosis, the prognostic threshold was 30 ng/L for sST2 (12).
Patients were followed up by an exhaustive review of medical files and medical consultations every 3 months and by phone call to referring doctors and patients. When the conclusion of the medical report was unclear, we looked for the use of intravenous diuretics in electronic health records, and hospitalization was considered due to HF only when IV diuretics were prescribed. Follow-up information was retrieved by physicians blinded to sST2 results. The follow-up started at the time of sampling. The endpoint was the composite of all-cause death or first HF-related hospitalization.
Continuous data are expressed as medians and interquartile ranges (IQRs), whereas categorical data are expressed as numbers and percentages. The unpaired t-test was used to assess differences in key continuous variables between patients with AL- and ATTR-CA and between patients with or without events. The χ2 test assessed differences in categorical data between these subgroups. Survival was evaluated with Cox proportional hazards regression analysis, providing estimated hazard ratios (HRs) and Kaplan–Meier curves. The predictive value of most baseline characteristics was explored using Cox regression analysis. To analyze the predictive value of sST2 regardless of Mayo Clinic staging for AL amyloidosis and Gillmore or Grogan staging for TTR amyloidosis, multivariate models included NT-proBNP, cardiac troponin, sST2, and eGFR for TTR amyloidosis and DFLC for AL and the prognostic scores (Mayo Clinic score in AL amyloidosis and Gillmore and Grogan staging in ATTR amyloidosis).
The median age was 73 years (IQR 61–81), and 53% were men. Among the 141 patients with AL cardiac amyloidosis, eight were lost to follow-up. Among the 133 AL patients studied, 78 and 28 also had renal and neurological involvement, respectively. Patients with AL amyloidosis received a treatment based on bortezomib, dexamethasone, and cyclophosphamide in 85% of cases and daratumumab in 22% as first-line therapy.
In total, 10% of patients (because of relapse) received IMID-based treatment, and 5% of patients received other treatments (1% of autologous stem cells, 1% of bendamustine because of IgM gammopathy at the diagnosis, 3% of other drugs). Most (around 90%) of AL amyloidosis patients were included at the time of first diagnosis of AL amyloidosis. Of the 133 AL amyloidosis and 152 TTR, 77 were in stage 3 and 35 were in stage 3b, according to the 2004 Mayo Clinic staging (3) and Wechalekar et al. (16).
Among the 159 patients with ATTR-CA, 7 were lost to follow-up and 152 were then studied. Among them, 20 had variant ATTR amyloidosis (15 Val122Ile, 2 Val30Met, 3 other mutations) and 31 received tafamidis. None received other specific therapy.
The median follow-up was 18 months [interquartile range (IQR) 3–34] in AL amyloidosis and 33 (6–45) in TTR amyloidosis. In AL amyloidosis, the primary outcome occurred in 69 (52%) patients, including 30 deaths (17 from cardiovascular causes) and 39 HF hospitalizations. In TTR amyloidosis, the primary outcome occurred in 99 (65%) patients, including 47 deaths (30 from cardiovascular causes) and 53 HF hospitalizations.
The main clinical characteristics of patients according to the type of amyloidosis are presented in Table 1. Patients with AL amyloidosis were younger, had lower systolic blood pressure and lower LV wall thickness, higher LVEF and global longitudinal strain than patients with TTR amyloidosis, and they also had global longitudinal strain, unlike those with TTR amyloidosis. sST2 levels were higher in AL patients than in ATTR patients, while NT-proBNP, cardiac troponin T, and eGFR levels were similar in the two groups.
The main clinical characteristics of AL and TTR patients according to cardiac events are separately presented in Tables 2 and 3.
sST2 levels were higher in patients with AL amyloidosis than in patients with ATTR amyloidosis (Table 1). sST2 levels were significantly higher in patients experiencing adverse events than in patients experiencing TTR-CA and AL-CA (Tables 2, 3). Supplementary Tables S1 and S2 list the predictive values of the most studied predictive variables and of sST2 for the risk of HF hospitalization and/or death in univariate analysis in TTR and AL amyloidosis, respectively.
Table 4 presents the various multivariate Cox models that tested respective predictive values of the variables used in Mayo Clinic or Gillmore or Grogan models. In TTR models (Table 4, Model 1), the sST2 level was not predictive of HF and/or death, and only NT-proBNP had strong and independent predictive value. In AL amyloidosis (Table 4, Model 2), the sST2 level strongly predicted the outcome regardless of the Mayo Clinic score. Even in the most severe patients [defined by NT-proBNP >8,500 ng/L and/or systolic blood pressure <100 mmHg according to Wechelekar et al. (16)], sST2 was still predictive of cardiac events (HR: 2.12, CI: 95% 1.25–3.60, p = 0.005). Figure 1 shows the survival curves according to sST2 levels > or <30 ng/L for HF hospitalization and all-cause mortality in TTR and AL amyloidosis.
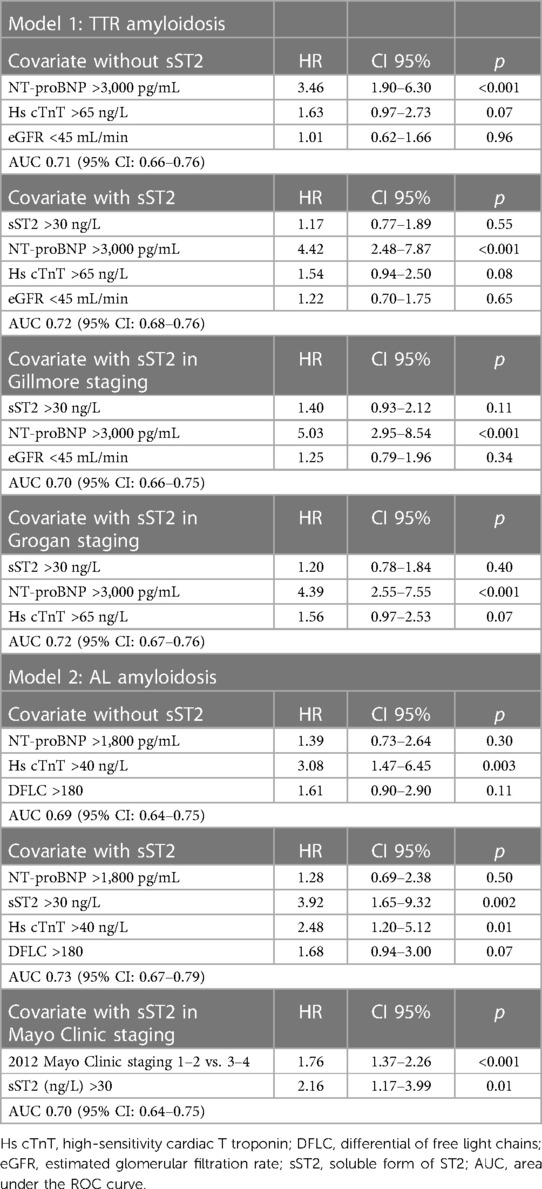
Table 4. Multivariate Cox models for the composite death or heart failure hospitalization in AL and TTR amyloidosis.
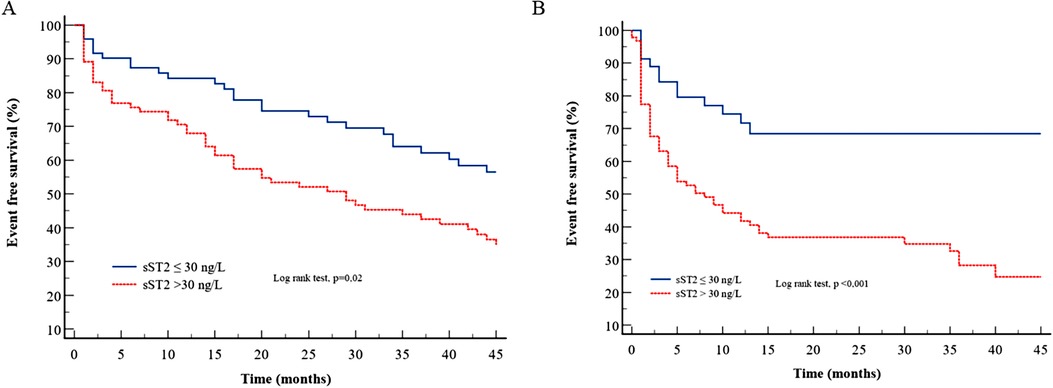
Figure 1. Survival curves for sST2 according to the cutoff of 30 ng/L for the composite endpoint in (A) TTR amyloidosis and (B) AL amyloidosis.
Interestingly, sST2 was also predictive of cardiac events beyond 1 year of follow-up in AL-CA patients (Supplementary Table S3).
Our study highlights the prognostic value of sST2 in cardiac amyloidosis. Increased sST2 levels are strongly associated with both death and HF hospitalization and remain predictive regardless of other validated predictors, including biomarkers in AL cardiac amyloidosis but not in TTR cardiac amyloidosis.
Prognostic stratification is a major issue regarding the choice of chemotherapy, the monitoring of the patients, and therapeutic intensification in the event of immunochemical and/or organ nonresponse. In ATTR amyloidosis, prognostic stratification could be more and more required because treatment options are rapidly expanding beyond tafamidis (17, 18): TTR silencing by mRNA knockdown or silencing, new TTR stabilizers, and amyloid resorption or extraction. Therefore, finding reliable prognostic markers is essential to identify the patients who will benefit from these new therapies. In TTR and AL cardiac amyloidosis, heart failure is a major issue. HF is predictive of subsequent death in these two populations and could explain almost half of the causes of death. Thus, it makes sense to combine HF and death in the outcome analysis of cardiac amyloidosis and to look for relevant biomarkers that predict both endpoints. In addition, we need biomarkers that could help to define the cardiac response to treatment and that could help to refine therapeutic strategies.
sST2 is the circulating form of the interleukin-33 membrane receptor released in response to vascular congestion and inflammatory or profibrotic stimuli. Increased cardiac expression of ST2 has been observed after cardiac insult (19, 20). By binding IL33, sST2 acts as a decoy receptor and removes the protective effects of IL33 against cardiac hypertrophy, reduction of contractility, and fibrosis (21, 8). Previous studies showed that sST2 predicts outcomes in acute and chronic HF regardless of NT-proBNP, cardiac troponin, and LVEF (22–25). Consequently, sST2 testing has been suggested in the guidelines to refine the assessment of the prognosis of HF patients. In amyloidosis, data on the usefulness of sST2 testing are scarce. Some studies have already assessed the prognostic value of sST2 in AL amyloidosis but not in ATTR amyloidosis. Zhang et al. (26) showed, in 56 AL amyloid patients, that sST2 was a powerful and independent prognostic biomarker for all-cause mortality with a cutoff of 12.3 mg/mL. Kim et al. (27) also showed the prognostic role of sST2 in 73 AL amyloid patients with a median follow-up of 18 months, and the authors suggested that the cutoff value could be 32.6 ng/mL. In a large cohort of 502 AL amyloidosis patients, Dispenzieri et al. (12) showed that sST2 levels >30 ng/mL were independently associated with mortality.
To our knowledge, we present the first data on both AL-CA and ATTR-CA. We report that sST2 levels are higher in AL amyloidosis than in ATTR amyloidosis, even though patients with ATTR amyloidosis exhibited the most severe cardiac remodeling and dysfunction, i.e., greater left ventricular hypertrophy, more altered longitudinal strain, lowered cardiac output, and higher cardiac biomarker levels. sST2 levels were strongly predictive of the outcome irrespective of other cardiac biomarkers and predictors. The cytotoxic effect (28, 29) and the inflammatory changes in cardiomyocytes (30) due to the light chains could explain these differences. Indeed, in AL amyloidosis, light chains polymerize into amyloid deposits that probably induce more severe systemic inflammation than in ATTR amyloidosis, in which there is no underlying neoplasia and where organ damage is more targeted: heart and peripheral nervous systems. Kotecha et al. (31) showed that cardiac AL amyloidosis resulted in greater myocardial edema than ATTR amyloidosis, possibly due to the specific toxicity of the light chains on the myocardium. In addition, edema defined by a native T2 >55 ms was an independent predictor of poor prognosis in AL amyloidosis. sST2 blood levels seem to be a marker of systemic inflammation in the case of neoplasia or certain autoimmune diseases independently of cardiac involvement (32).
Compared with natriuretic peptides and troponin, sST2 blood levels are less influenced by obesity, advanced age, and chronic kidney disease (33). These potential advantages for clinicians were also observed in our study. Indeed, sST2 levels were poorly associated with other biomarkers and renal and LV function. In contrast, NT-proBNP and troponin levels were correlated to each other and renal and LV function.
NT-proBNP levels are strongly correlated with Hs-cTnT levels (data not shown). Of these two biomarkers, cTnT gave the main prognostic information of AL amyloidosis, but it was the opposite in TTR amyloidosis. The increase in cTnT levels is due, at least in part, to the direct myocardial toxicity of light chains in AL amyloidosis, which could be a plausible explanation for the strong predictive value of cTnT levels. In addition, sST2 levels were measured at an early stage in most AL patients before the decrease in light chain levels. On the other hand, the increase in NT-proBNP levels is mainly due to the increase in LV wall stress (mainly related to LV hypertrophy and concentric LV remodeling). LV remodeling was lower in AL vs TTR cardiac amyloidosis. In TTR amyloidosis, the severity of such LV remodeling and its subsequent decreased LV function can lead to most cardiac events.
Our study has some limitations. It is a retrospective study, but we collected data from a heterogeneous population and different centers. All patients with AL amyloidosis had a complete evaluation within 2 months of diagnosis, and some patients received chemotherapy before sST2 measurements, which could impact sST2 levels. sST2 levels could be measured in some patients during HF decompensation, and the predictive value of sST2 can differ according to the delay between decompensation and sST2 measurement (25).
In conclusion, the increase in sST2 blood levels (>30 ng/L) is strongly associated with the risk of death or HF hospitalization in AL cardiac amyloidosis but not in TTR amyloidosis. Further studies are needed to estimate the change of sST2 after treatment in both amyloidosis types.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the local etic committee CEERB (Comité d'évaluation et d'éthique pour la recherche biomédicale) of Bichat Hospital. Written informed consent for participation was not required for this study in accordance with national legislation and institutional requirements.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FP declared a past co-authorship with two of the authors AA and VC to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1179968/full#supplementary-material
1. Castiglione V, Franzini M, Aimo A, Carecci A, Lombardi CM, Passino C, et al. Use of biomarkers to diagnose and manage cardiac amyloidosis. Eur J Heart Fail. (2021) 23(2):217–30. doi: 10.1002/ejhf.2113
2. Damy T, Jaccard A, Guellich A, Lavergne D, Galat A, Deux JF, et al. Identification of prognostic markers in transthyretin and AL cardiac amyloidosis. Amyloid Int J Exp Clin Investig Off J Int Soc Amyloidosis. (2016) 23(3):194–202. doi: 10.1080/13506129.2016.1221815
3. Dispenzieri A, Gertz MA, Kyle RA, Lacy MQ, Burritt MF, Therneau TM, et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol Off J Am Soc Clin Oncol. (2004) 22(18):3751–7. doi: 10.1200/JCO.2004.03.029
4. Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol Off J Am Soc Clin Oncol. (2012) 30(9):989–95. doi: 10.1200/JCO.2011.38.5724
5. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, et al. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. (2018) 39(30):2799–806. doi: 10.1093/eurheartj/ehx589
6. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. (2016) 68(10):1014–20. doi: 10.1016/j.jacc.2016.06.033
7. Dieplinger B, Mueller T. Soluble ST2 in heart failure. Clin Chim Acta Int J Clin Chem. (2015) 443:57–70. doi: 10.1016/j.cca.2014.09.021
8. Bayés-Genís A, Núñez J, Lupón J. Soluble ST2 for prognosis and monitoring in heart failure: the new gold standard? J Am Coll Cardiol. (2017) 70(19):2389–92. doi: 10.1016/j.jacc.2017.09.031
9. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. (2017) 136(6):e137–61. doi: 10.1161/CIR.0000000000000509
10. Moura B, Aimo A, Al-Mohammad A, Flammer A, Barberis V, Bayes-Genis A, et al. Integration of imaging and circulating biomarkers in heart failure: a consensus document by the Biomarkers and Imaging Study Groups of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2021) 23(10):1577–96. doi: 10.1002/ejhf.2339
11. Pucci A, Aimo A, Musetti V, Barison A, Vergaro G, Genovesi D, et al. Amyloid deposits and fibrosis on left ventricular endomyocardial biopsy correlate with extracellular volume in cardiac amyloidosis. J Am Heart Assoc. (2021) 10(20):e020358. doi: 10.1161/JAHA.120.020358
12. Dispenzieri A, Gertz MA, Saenger A, Kumar SK, Lacy MQ, Buadi FK, et al. Soluble suppression of tumorigenicity 2 (sST2), but not galactin-3, adds to prognostication in patients with systemic AL amyloidosis independent of NT-proBNP and troponin T. Am J Hematol. (2015) 90(6):524–8. doi: 10.1002/ajh.24001
13. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. (2021) 42(16):1554–68. doi: 10.1093/eurheartj/ehab072
14. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
15. Muchtar E, Kumar SK, Gertz MA, Grogan M, AbouEzzeddine OF, Jaffe AS, et al. Staging systems use for risk stratification of systemic amyloidosis in the era of high-sensitivity troponin T assay. Blood. (2019) 133(7):763–6. doi: 10.1182/blood-2018-10-875252
16. Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. (2013) 121(17):3420–7. doi: 10.1182/blood-2012-12-473066
17. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. (2018) 379(11):1007–16. doi: 10.1056/NEJMoa1805689
18. Damy T, Garcia-Pavia P, Hanna M, Judge DP, Merlini G, Gundapaneni B, et al. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR-ACT) and long-term extension study. Eur J Heart Fail. (2021) 23(2):277–85. doi: 10.1002/ejhf.2027
19. Pascual-Figal DA, Januzzi JL. The biology of ST2: the International ST2 Consensus Panel. Am J Cardiol. (2015) 115(7 Suppl):3B–7B. doi: 10.1016/j.amjcard.2015.01.034
20. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. (2002) 106(23):2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
21. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. (2007) 117(6):1538–49. doi: 10.1172/JCI30634
22. Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupón J, Latini R, et al. sST2 predicts outcome in chronic heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol. (2018) 72(19):2309–20. doi: 10.1016/j.jacc.2018.08.2165
23. Gaggin HK, Szymonifka J, Bhardwaj A, Belcher A, De Berardinis B, Motiwala S, et al. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. (2014) 2(1):65–72. doi: 10.1016/j.jchf.2013.10.005
24. Pascual-Figal DA, Manzano-Fernández S, Boronat M, Casas T, Garrido IP, Bonaque JC, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail. (2011) 13(7):718–25. doi: 10.1093/eurjhf/hfr047
25. Aimo A, Vergaro G, Ripoli A, Bayes-Genis A, Pascual Figal DA, de Boer RA, et al. Meta-analysis of soluble suppression of tumorigenicity-2 and prognosis in acute heart failure. JACC Heart Fail. (2017) 5(4):287–96. doi: 10.1016/j.jchf.2016.12.016
26. Zhang Y, Xiao Y, Liu Y, Fang Q, Tian Z, Li J, et al. Prognostic value of circulating sST2 for the prediction of mortality in patients with cardiac light-chain amyloidosis. Front Cardiovasc Med. (2020) 7:597472. doi: 10.3389/fcvm.2020.597472
27. Kim D, Lee GY, Choi JO, Kim K, Kim SJ, Ju ES, et al. Prognostic values of novel biomarkers in patients with AL amyloidosis. Sci Rep. (2019) 9(1):12200. doi: 10.1038/s41598-019-48513-6
28. Brenner DA, Jain M, Pimentel DR, Wang B, Connors LH, Skinner M, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. (2004) 94(8):1008–10. doi: 10.1161/01.RES.0000126569.75419.74
29. Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. (2010) 107(9):4188–93. doi: 10.1073/pnas.0912263107
30. Jordan TL, Maar K, Redhage KR, Misra P, Blancas-Mejia LM, Dick CJ, et al. Light chain amyloidosis induced inflammatory changes in cardiomyocytes and adipose-derived mesenchymal stromal cells. Leukemia. (2020) 34(5):1383–93. doi: 10.1038/s41375-019-0640-4
31. Kotecha T, Martinez-Naharro A, Treibel TA, Francis R, Nordin S, Abdel-Gadir A, et al. Myocardial edema and prognosis in amyloidosis. J Am Coll Cardiol. (2018) 71(25):2919–31. doi: 10.1016/j.jacc.2018.03.536
32. Lu DP, Zhou XY, Yao LT, Liu CG, Ma W, Jin F, et al. Serum soluble ST2 is associated with ER-positive breast cancer. BMC Cancer. (2014) 14:198. doi: 10.1186/1471-2407-14-198
33. Aimo A, Januzzi JL, Vergaro G, Richards AM, Lam CSP, Latini R, et al. Circulating levels and prognostic value of soluble ST2 in heart failure are less influenced by age than N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin T. Eur J Heart Fail. (2020) 22(11):2078–88. doi: 10.1002/ejhf.1701
Keywords: cardiac amyloidosis, AL, TTR, sST2, biomarker, prognosis
Citation: Nicol M, Vergaro G, Damy T, Kharoubi M, Baudet M, Canuti ES, Aimo A, Castiglione V, Emdin M, Royer B, Harel S, Cohen-Solal A, Arnulf B and Logeart D (2023) Prognostic value of soluble ST2 in AL and TTR cardiac amyloidosis: a multicenter study. Front. Cardiovasc. Med. 10:1179968. doi: 10.3389/fcvm.2023.1179968
Received: 5 March 2023; Accepted: 12 July 2023;
Published: 2 August 2023.
Edited by:
Wouter Kok, Amsterdam University Medical Center, NetherlandsReviewed by:
Federico Perfetto, University of Florence, Italy© 2023 Nicol, Vergaro, Damy, Kharoubi, Baudet, Canuti, Aimo, Castiglione, Emdin, Royer, Harel, Cohen-Solal, Arnulf and Logeart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Nicol bWFydGluLm5pY29sQGFwaHAuZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.