- 1Heart Institute, Medical School, University of Pecs, Pecs, Hungary
- 2Department of Cardiology, University Medical Center Ljubljana, Ljubljana, Slovenia
- 3Cardiac Electrophysiology Division, Department of Internal Medicine, University of Szeged, Szeged, Hungary
Introduction: Catheter ablation for atrial fibrillation (AF) is the most frequently performed cardiac ablation procedure worldwide. The majority of ablations can now be performed safely with minimal radiation exposure or even without the use of fluoroscopy, thanks to advances in 3-dimensional electroanatomical mapping systems and/or intracardiac echocardiography. The aim of this study was to conduct a meta-analysis to compare the effectiveness of zero fluoroscopy (ZF) versus non-zero fluoroscopy (NZF) strategies for AF ablation procedures.
Methods: Electronic databases were searched and systematically reviewed for studies comparing procedural parameters and outcomes of ZF vs. NZF approaches in patients undergoing catheter ablation for AF. We used a random-effects model to derive the mean difference (MD) and risk ratios (RR) with a 95% confidence interval (CI).
Results: Our meta-analysis included seven studies comprising 1,593 patients. The ZF approach was found to be feasible in 95.1% of patients. Compared to the NZF approach, the ZF approach significantly reduced procedure time [mean difference (MD): −9.11 min (95% CI: −12.93 to −5.30 min; p < 0.01)], fluoroscopy time [MD: −5.21 min (95% CI: −5.51 to −4.91 min; p < 0.01)], and fluoroscopy dose [MD: −3.96 mGy (95% CI: −4.27 to −3.64; p < 0.01)]. However, there was no significant difference between the two groups in terms of total ablation time [MD: −104.26 s (95% CI: −183.37 to −25.14; p = 0.12)]. Furthermore, there was no significant difference in the acute [risk ratio (RR): 1.01, 95% CI: 1.00–1.02; p = 0.72] and long-term success rates (RR: 0.96, 95% CI: 0.90–1.03; p = 0.56) between the ZF and NZF methods. The complication rate was 2.76% in the entire study population and did not differ between the groups (RR: 0.94, 95% CI: 0.41–2.15; p = 0.89).
Conclusion: The ZF approach is a feasible method for AF ablation procedures. It significantly reduces procedure time and radiation exposure without compromising the acute and long-term success rates or complication rates.
Introduction
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia, which is linked to an elevated risk of stroke, heart failure, mortality, and reduced quality of life (1). The electrical isolation of the pulmonary veins is the cornerstone of AF ablation procedures for patients with symptomatic paroxysmal or persistent AF that is refractory to antiarrhythmic drug (AAD) therapy (2). Catheter ablation for AF is by far the most commonly performed cardiac ablation procedure worldwide (2–4).
Radiation exposure during electrophysiology (EP) procedures can vary significantly in clinical practice. During AF ablation procedures, the average fluoroscopy exposure is 15 mSv, which is higher compared to other ablations and carries an excess risk of fatal and non-fatal cancer of 1 in 750 men at the age of 50 years (5, 6). Moreover, based on the stochastic effects of the radiation, there is no safe lower threshold. Thus, completely fluoroless procedures can entirely eliminate radiation hazards for both patients and personnel, although radiation risk can be reduced with minimal fluoroscopic approach also.
Due to the technological progress made in the last decade, with the use of 3-dimensional electroanatomical mapping systems (EAMS) and/or intracardiac echocardiography (ICE), the majority of the ablations can be performed safely with minimal radiation exposure or even without the use of fluoroscopy (7–10).
Low or zero (L/Z) fluoroscopy catheter ablation are available also for pulmonary vein isolation (PVI) procedures. A previous meta-analysis published in 2020 included 2,228 patients who underwent AF ablation, L/Z fluoroscopy-guided approaches were compared to conventional, fluoroscopy-guided procedures. The L/Z fluoroscopy approach was associated with shorter procedural time and reduced fluoroscopy exposure, without compromising safety or efficacy compared to traditional AF ablation techniques (11).
Nonetheless, there is limited available scientific data regarding completely fluoroless AF ablation procedures. Thus, we conducted a systematic review and a meta-analysis to analyse the feasibility, safety and efficacy of zero-fluoroscopy approach for AF ablation procedures.
Methods
Search strategy and data acquisition
Electronic databases [PubMed, Excerpta Medica Database (EMBASE), Cochrane Central Register of Controlled Trials (CENTRAL)] were systematically searched for relevant articles published between January of 2000 and December of 2022, using the search string “zero-fluoroscopy or fluoroless or non-fluoroscopic” and “ablation” and “atrial fibrillation”. Additionally, manual searches of reference lists of relevant studies were conducted to identify any additional articles that were not found in the database search. Reviews and duplicate articles were excluded. The analyses were performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.
In this meta-analysis, we included studies that fulfilled the following criteria:
(1) Randomized or non-randomized prospective and retrospective studies enrolling consecutive patients with paroxysmal or persistent AF who underwent catheter ablation for AF; (2) studies having at least 1 zero-fluoroscopic (ZF) and 1 non-zero-fluoroscopic arm (NZF); (3) studies written in English. Case reports, letters, conference abstracts and presentations as well as full text papers not in the English language were excluded. ZF was defined as no radiation used during the procedure. “Low fluoroscopic” and “minimal fluoroscopic” procedures were not considered as ZF approach. All approaches other than ZF were considered NZF. Selection and data abstraction were done independently by two reviewers (DD and PK) and any disagreements were resolved by consensus.
We extracted the following data from the included studies: the first author's name, publication year, study design, number of patients in each group, baseline characteristics of the study population, as well as procedural and clinical outcome data for our meta-analysis.
Endpoints of interest
The primary endpoints of the study were the skin-to-skin procedure time and any procedure related complications, including vascular complications (groin hematoma, pseudoaneurysm, arteriovenous fistula), cardiac effusion/tamponade, stroke/cardioembolic events, phrenic nerve palsy and death. The secondary outcomes were fluoroscopy exposure, total ablation time, and acute and long-term success rates.
Statistical analysis
We performed the analyses in R statistical software package version 4.2.2 (R Development Core Team, 2010) with the help of the “dmetar” package (12). A random-effects model was used to derive risk ratios (RR) with 95% confidence interval (CI) on dichotomous outcomes and mean difference (MD) on continuous data. The significance of the pooled estimates was determined by the Z-test, and p < 0.05 was considered as statistically significant. Heterogeneity was tested with a chi-square heterogeneity statistic for which a p value <0.2 was considered potentially heterogeneous. Consistency was assessed by the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than due to chance. Values of I2 < 25% were considered as low and values of I2 > 75% were considered as high. To assess the stability of acquired effect estimates, a leave-one-out sensitivity analysis was performed. Quality assessment was performed with Cochrane's tool for assessing bias, wherein studies are scored as high, low, or unclear risk of bias in five domains: selection, performance, detection, attrition, and reporting. Funnel plot was drawn to assess publication bias, and asymmetry was assessed by visual estimation and by Egger's linear regression test.
Results
Study characteristics
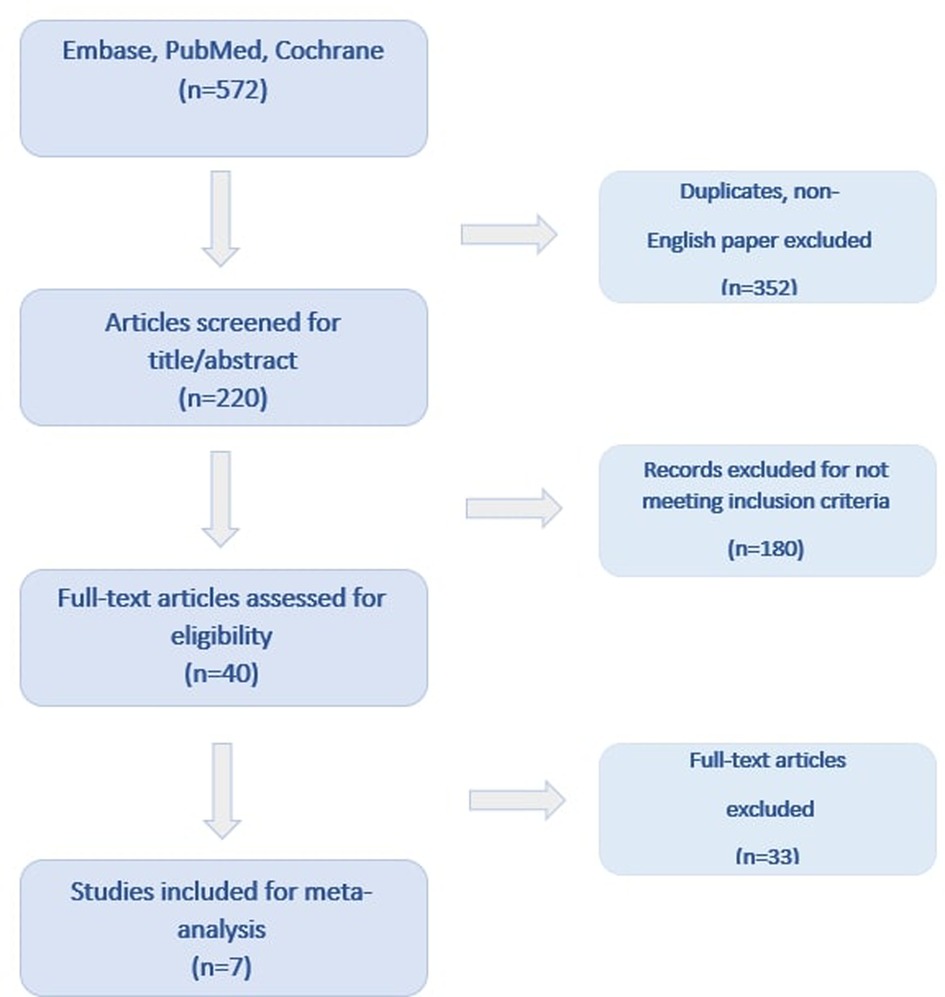
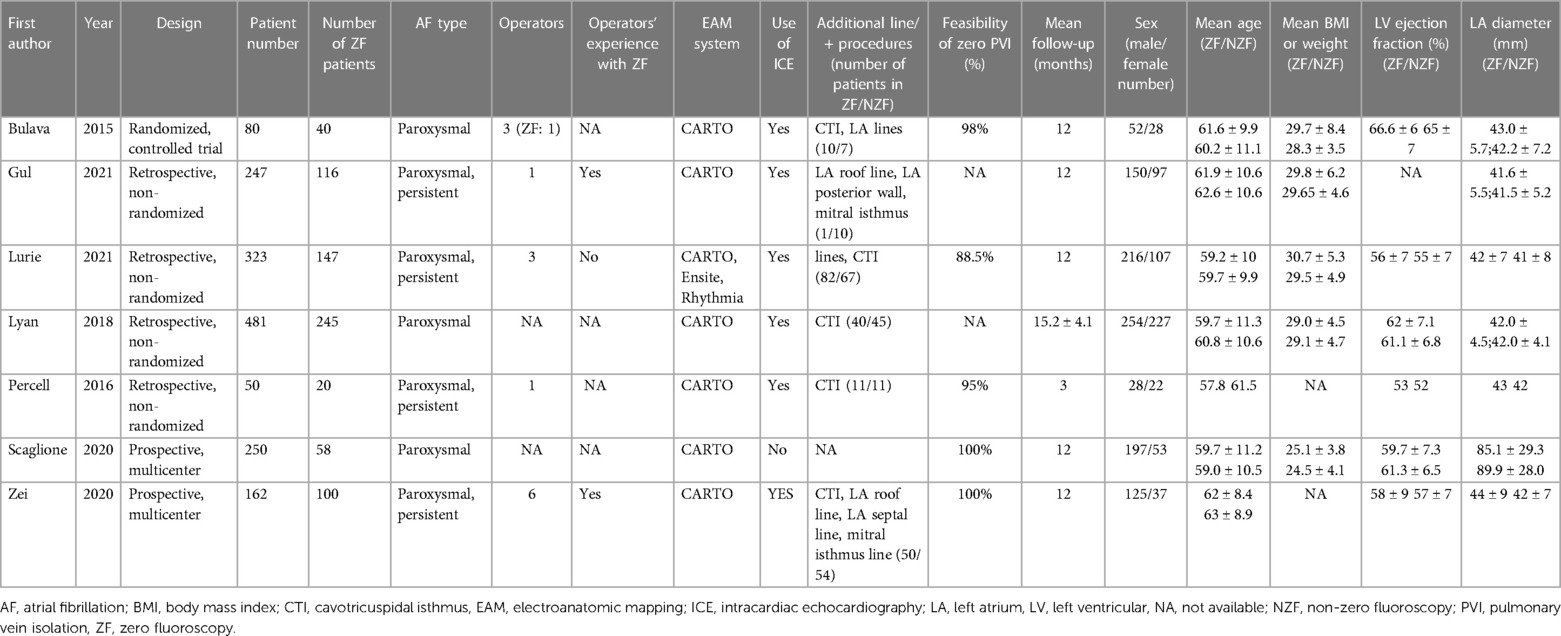
Seven studies involving 1,593 patients (726 patients in the ZF and 867 patients in the NZF group) included in our analysis. Among the included studies, 1 was a randomized controlled trial (RCT) (13) and 6 were observational, non-randomized (14–19). The results of the literature search are presented in Figure 1 and the main characteristics of the trials and study populations are summarized in Table 1. Except for 1 trial that enrolled patients with patent foramen ovale (PFO) (18), ICE was also applied to achieve the ZF strategy in addition to EAMSs. In 1 trial, AF ablation procedures were performed using CARTO, Ensite Precision and Rhythmia EAMSs (15), while in the other studies, CARTO 3 system was exclusively used. The ZF approach was feasible in 95.1% of the patients, and in remaining cases, fluoroscopy was used. The mean length of the follow-up period varied between 3 and 15.2 months.
Procedural and outcome data
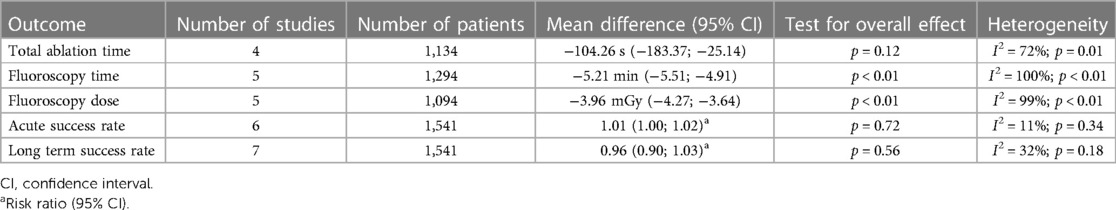
The ZF approach was associated with a significant decrease in procedural procedure time compared to the NZF approach [MD: −9.11 min (95% CI: −12.93 to −5.30 min; p < 0.01; Figure 2)]. Additionally, the ZF group had reduced fluoroscopy time [MD: −10.02 min (95% CI, −18.67 to −1.37 min; p = 0.02)] and fluoroscopy dose [MD: −3.96 mGy (−4.27 to −3.64 mGy; p < 0.01)]; however, the total ablation time was similar [MD: −138.90 s (95% CI: −316.27 to 38.48 s; p = 0.12)]. The acute success rate was 99.35% and did not differ between the groups (RR = 1.00, 95% CI, 0.99–1.01; p = 0.71). No difference in long-term success rate was found between the groups (RR: 0.98, 95% CI, 0.90–1.06; p = 0.56).
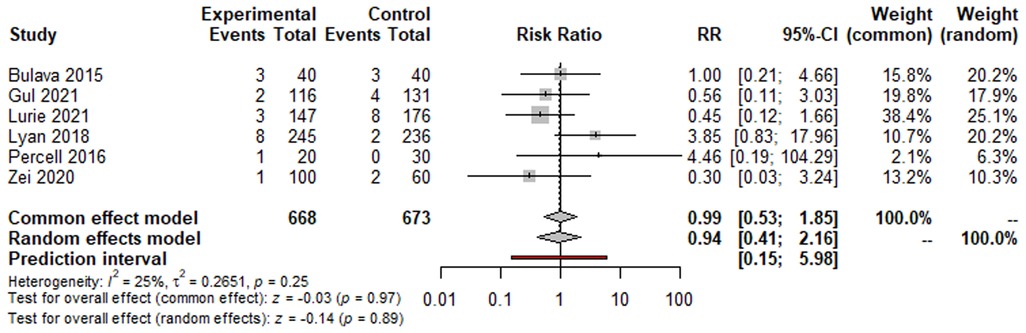
Regarding safety outcomes, there were 18 cases of complications in the ZF arm (2.69%) and 19 cases in the NZF arm (2.82%). The risk of complications was not significantly different between the two study arms (RR: 0.94, 95% CI: 0.41–2.15; p = 0.89; Figure 3). The results for secondary outcomes are summarized in Table 2 and Supplementary Figure S1.
A leave-one-out analysis indicated no difference between the groups for primary outcomes (Supplementary Figure S2). Furthermore, funnel plot analyses revealed no sign of possible publication bias (Supplementary Figure S3).
Discussion
In this meta-analysis of 7 studies involving 1,593 patients who underwent AF ablation procedures, we found that the use of ZF ablation was associated with a significant reduction in procedural and fluoroscopy time compared to the NZF approach, while maintaining similar efficacy and safety outcomes.
Medical radiation exposure is the most significant anthropogenic source of radiation (20). During EP procedures, fluoroscopy is primarily used for catheter placement and accounts for 95% of the total fluoroscopy time (21). The amount of radiation exposure varies among different types of ablation procedures, with AF ablation procedures associated with the highest doses. These procedures expose patients up to an average dose of 15 mSv per procedure, equivalent to 750 chest x-rays (21). Fluoroscopy increases the life-time risk of cataract, dermatitis, and cancer via stochastic and deterministic effects, thus preventing potentially life-threatening effects of ionizing radiation. For example, a typical PVI procedure raises the absolute lifetime of fatal cancer risk by 0.08% (21). Therefore, radiation exposure must be minimized according to the ALARA principle, which aims to reduce exposure “as low as reasonably achievable” (22).
In the last two decades, technology has significantly improved, and nowadays, EAMSs offer reliable alternatives to fluoroscopy for visualizing catheter positions during EP procedures. By using EAMSs, radiation exposure can be substantially reduced, and completely fluoroless ablations have become available (23). Ensite NavX (St. Jude Medical, Inc., St. Paul, MN, USA) system based on impedance measurements between catheter electrodes and patches put on patient's chest and abdomen, CARTO 3 system (Biosense Webster, Inc., Diamond Bar, CA, USA) uses magnetic location. Some studies showed different results comparing EAMSs (24). In our meta-analysis only CARTO system was used 6 of 7 studies as a support system for PVI. In recent years new ablation techniques were successfully developed for PVI such as ablation index, high-power short- duration, or pulse field ablation, which methods lead differences in procedure time and required fluoroscopy time (25–27). In our meta-analysis, ablation index was used in 1 publication (14) and contact force was available in 6 study (13, 15–19), based on findings during EPS additional lesion delivery was performed (Table 1). The ZF approach was initially used for ablations of supraventricular tachycardias (SVTs) (28). A recent meta-analysis compared Z/MF versus conventional, fluoroscopy-guided techniques for SVT ablations and found that the Z/MF approach reduces radiation exposure and ablation time without compromising the acute and long-term success rates or increasing the complication rate (28).
Fluoroscopic guidance is currently considered the standard method for transseptal puncture (TSP), which remains a major obstacle for widespread adoption of zero-fluoroscopy AF ablation, despite the availability of both transesophageal and intracardiac echocardiographic techniques for achieving fluoroless TSP (23, 29, 30). In addition, the use of intracardiac echocardiography (ICE) has been shown to reduce fluoroscopy exposure during both SVT and AF ablation procedures (7, 31). In our analysis, except for 1 study that enrolled patients with PFO, ICE was used in ZF arms. Besides ICE, recently developed steerable sheaths that can be visualized using EAMSs have also been shown to contribute to the reduction of fluoroscopy exposure and the performance of fluoroless procedures (32).
A previous meta-analysis published in 2020, including 2,218 patients from 15 studies, compared L/Z fluoroscopy method to the conventional strategy for PVI. Consistent with our findings, this meta-analysis also showed a significant reduction in procedural and fluoroscopy time, while complication rates and acute- and long-term success rates did not differ between groups (11). However, the definition of a low fluoroscopy strategy is not well-defined and differed across the enrolled studies.
In our opinion, the ZF approach is characterized by the operator's decision at the beginning of the procedure to pursue fluoroless ablation before inserting catheters, even though radiation may be required later and thus the ZF strategy is deemed unsuccessful. In addition to above, that during PVI most fluoroscopy required at the beginning of the procedure: catheter positioning and transeptal puncture. After the beginning phase operator staff can remove lead aprons and prevent orthopaedic problems. In our analysis, the ZF strategy was achievable in more than 95% of the procedures in the ZF arm.
Z/MF approach is now more extensively used compared to ZF for AF ablation, mainly due to the costs and technical challenges related to systematic use of transoesophageal or ICE for transseptal puncture.
In addition to the technological aspects, operators' experience is also of paramount importance when implementing Z/MF procedures. For obvious reasons, total fluoroless or minimal fluoroscopic PVI procedures have a learning curve of 20–40 cases (33, 34).
Atrial fibrillation may be precipitated secondary factors by hypertension, hyperthyroidism (35), lifestyle factors such as endurance sport (36), smoking (37), cardiomyopathies (38) and channelopathies (39, 40). Considering these factors may prevent future AF attacks regardless of the ablation strategy or may re-evaluate its indication. Thus, further investigation needed.
Limitations
There are several limitations that should be acknowledged in our analysis. Firstly, we only included 1 randomized controlled trial (RCT), with the majority of data originating from observational studies. However, the lack of heterogeneity in this aspect suggests that the effects of the ZF approach are consistent across different trial designs and are not affected by potential bias. Secondly, significant differences in patient demographics and different modern mapping system including different specific modern tools for AF ablation could have an impact on the results but were not considered in this analysis. The use of a random-effects model helped to mitigate the potential effect of heterogeneity, and the high level of significance supports the validity of our findings. Finally, data on operators' prior experience with the ZF approach for AF ablations were insufficient, which could have an effect on both procedural and safety outcomes.
Conclusion
In summary, our analysis of 1,593 patients indicates that the ZF approach is a safe and feasible method for patients undergoing catheter ablation for AF. The significant reduction in procedure time and radiation exposure, without compromising the acute and long-term success rates or complication rates, suggest that the ZF approach can be considered as a viable alternative to the NZF approach for AF ablation procedures.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
PK and DD contributed to conception and design of the study. AK and PK performed the statistical analysis. DD, PK, MV, and AK wrote sections of the manuscript. Tables and figures were designed by PK, DD, AK, and KJ. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1178783/full#supplementary-material
References
1. Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the framingham heart study. Circulation. (1998) 98:946–52.9737513
2. Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GA, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
3. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. (2012) 367:1587–95. doi: 10.1056/NEJMoa1113566
4. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. J Am Med Assoc. (2019) 321(13):1261–74. doi: 10.1001/jama.2019.0693
5. Gerber TC, Jeffrey Carr J, Arai AE, Dixon RL, Ferrari VA, Gomes AS, et al. Ionizing radiation in cardiac imaging: a science advisory from the American heart association committee on cardiac imaging of the council on clinical cardiology and committee on cardiovascular imaging and intervention of the council on cardiovascular radi. Circulation. (2009) 119(7):1056–65. doi: 10.1161/CIRCULATIONAHA.108.191650
6. Ginocchio IF. The 2007 recommendations of the international commission on radiological protection. ICRP publication 103. Ann ICRP. (2007) 37:1–332.
7. Kupo P, Saghy L, Bencsik G, Kohari M, Makai A, Vamos M, et al. Randomized trial of intracardiac echocardiography-guided slow pathway ablation. J Interv Card Electrophysiol. (2022) 63(3):709–14. doi: 10.1007/s10840-022-01126-y
8. Fadhle A, Hu M, Wang Y. The safety and efficacy of zero-fluoroscopy ablation versus conventional ablation in patients with supraventricular tachycardia. Kardiol Pol. (2020) 78(6):552–8. doi: 10.33963/KP.15293
9. Bergonti M, Dello Russo A, Sicuso R, Ribatti V, Compagnucci P, Catto V, et al. Long-term outcomes of near-zero radiation ablation of paroxysmal supraventricular tachycardia: a comparison with fluoroscopy-guided approach. JACC Clin Electrophysiol. (2021) 7(9):1108–1117. doi: 10.1016/j.jacep.2021.02.017
10. Di Cori A, Zucchelli G, Segreti L, Barletta V, Viani S, Paperini L, et al. Predictors of zero x-ray procedures in supraventricular arrhythmias ablation. Int J Cardiovasc Imaging. (2020) 36(9):1599–607. doi: 10.1007/s10554-020-01884-8
11. Huang HD, Abid QU, Ravi V, Sharma P, Larsen T, Krishnan K, et al. Meta-analysis of pulmonary vein isolation ablation for atrial fibrillation conventional vs low- and zero-fluoroscopy approaches. J Cardiovasc Electrophysiol. (2020) 31(6):1403–12. doi: 10.1111/jce.14450
12. Viechtbauer W. Conducting meta-analyses in R with the metafor. J Stat Softw. (2010) 36(3):1–48. doi: 10.18637/jss.v036.i03
13. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. (2015) 38(7):797–806. doi: 10.1111/pace.12634
14. Gul EE, Azizi Z, Alipour P, Haseeb S, Malcolm R, Terricabras M, et al. Fluoroless catheter ablation of atrial fibrillation: integration of intracardiac echocardiography and cartosound module. J Atr Fibrillation. (2021) 14(2):1–7. doi: 10.4022/jafib.20200477
15. Lurie A, Amit G, Divakaramenon S, Acosta JG, Healey JS, Wong JA. Outcomes and safety of fluoroless catheter ablation for atrial fibrillation. CJC Open. (2021) 3(3):303–10. doi: 10.1016/j.cjco.2020.11.002
16. Lyan E, Tsyganov A, Abdrahmanov A, Morozov A, Bakytzhanuly A, Tursunbekov A, et al. Nonfluoroscopic catheter ablation of paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. (2018) 41(6):611–9. doi: 10.1111/pace.13321
17. Percell J, Sharpe E, Percell R. SANS FLUORO (SAy No series to FLUOROscopy): a first-year experience. J Innov Card Rhythm Manag. (2016) 7(11):2529–34. doi: 10.19102/icrm.2016.071102
18. Scaglione M, Ebrille E, Caponi D, Battaglia A, Di Donna P, Anselmino M, et al. Zero-fluoroscopy atrial fibrillation ablation in the presence of a patent foramen ovale: a multicentre experience. J Cardiovasc Med. (2020) 21(4):292–8. doi: 10.2459/JCM.0000000000000943
19. Zei PC, Quadros KK, Clopton P, Thosani A, Ferguson J, Brodt C, et al. Safety and efficacy of minimal- versus zerofluoroscopy radiofrequency catheter ablation for atrial fibrillation: a multicenter, prospective study. J Innov Card Rhythm Manag. (2020) 11(11):4281–91. doi: 10.19102/icrm.2020.111105
20. Mettler FA, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, et al. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources - 1950-2007. Radiology. (2009) 253(2):520–31. doi: 10.1148/radiol.2532082010
21. Heidbuchel H, Wittkampf FHM, Vano E, Ernst S, Schilling R, Picano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. (2014) 16(7):946–64. doi: 10.1093/europace/eut409
22. Limacher MC, Douglas PS, Germano G, Laskey WK, Lindsay BD, McKetty MH, et al. Radiation safety in the practice of cardiology. J Am Coll Cardiol. (1998) 31(4):892–913. doi: 10.1016/S0735-1097(98)00047-3
23. Gaita F, Guerra PG, Battaglia A, Anselmino M. The dream of near-zero x-rays ablation comes true. Eur Heart J. (2016) 37(36):2749–55. doi: 10.1093/eurheartj/ehw223
24. Khaykin Y, Oosthuizen R, Zarnett L, Wulffhart ZA, Whaley B, Hill C, et al. CARTO-guided vs. NavX-guided pulmonary vein antrum isolation and pulmonary vein antrum isolation performed without 3-D mapping: effect of the 3-D mapping system on procedure duration and fluoroscopy time. J Interv Card Electrophysiol. (2011) 30(3):233–40. doi: 10.1007/s10840-010-9538-9
25. Natale A, Reddy VY, Monir G, Wilber DJ, Lindsay BD, McElderry HT, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. (2014) 64:647–56. doi: 10.1016/j.jacc.2014.04.072
26. Xu M, Yang Y, Zhang D, Jiang W. Meta-analysis of high power short duration in atrial fibrillation ablation–a superior efficient ablation strategy. Acta Cardiol. (2022) 77(1):14–32. doi: 10.1080/00015385.2021.1939512
27. Sousa PA, Puga L, Adão L, Primo J, Khoueiry Z, Lebreiro A, et al. Two years after pulmonary vein isolation guided by ablation index—a multicenter study. J Arrhythmia. (2022) 38(3):346–52. doi: 10.1002/joa3.12696
28. Debreceni D, Janosi K, Vamos M, Komocsi A, Simor T, Kupo P. Zero and minimal fluoroscopic approaches during ablation of supraventricular tachycardias: a systematic review and meta-analysis. Front Cardiovasc Med. (2022) 9:1–10. doi: 10.3389/fcvm.2022.856145
29. Bayrak F, Chierchia GB, Namdar M, Yazaki Y, Sarkozy A, De Asmundis C, et al. Added value of transoesophageal echocardiography during transseptal puncture performed by inexperienced operators. Europace. (2012) 14(5):661–5. doi: 10.1093/europace/eur366
30. Žižek D, Antolič B, Prolič Kalinšek T, Štublar J, Kajdič N, Jelenc M, et al. Intracardiac echocardiography-guided transseptal puncture for fluoroless catheter ablation of left-sided tachycardias. J Interv Card Electrophysiol. (2021) 61(3):595–602. doi: 10.1007/s10840-020-00858-z
31. Bencsik G, Pap R, Makai A, Klausz G, Chadaide S, Traykov V, et al. Randomized trial of intracardiac echocardiography during cavotricuspid isthmus ablation. J Cardiovasc Electrophysiol. (2012) 23(9):996–1000. doi: 10.1111/j.1540-8167.2012.02331.x
32. Janosi K, Debreceni D, Janosa B, Simor T, Kupo P. Visualizable vs. standard, non-visualizable steerable sheath for pulmonary vein isolation procedures: randomized, single-center trial. Front Cardiovasc Med. (2022) 9:1033755. doi: 10.3389/fcvm.2022.1033755
33. Tahin T, Riba A, Nemeth B, Arvai F, Lupkovics G, Szeplaki G, et al. Implementation of a zero fluoroscopic workflow using a simplified intracardiac echocardiography guided method for catheter ablation of atrial fibrillation, including repeat procedures. BMC Cardiovasc Disord. (2021) 21(1):1–8. doi: 10.1186/s12872-021-02219-8
34. Kochar A, Ahmed T, Donnellan E, Wazni O, Tchou P, Chung R. Operator learning curve and clinical outcomes of zero fluoroscopy catheter ablation of atrial fibrillation, supraventricular tachycardia, and ventricular arrhythmias. J Interv Card Electrophysiol. (2021) 61(1):165–70. doi: 10.1007/s10840-020-00798-8
35. Frost L, Vestergaard P, Mosekilde L. Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med. (2004) 164(15):1675–8. doi: 10.1001/archinte.164.15.1675
36. Mont L, Elosua R, Brugada J. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace. (2009) 11(1):11–7. doi: 10.1093/europace/eun289
37. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, et al. Smoking and incidence of atrial fibrillation: results from the atherosclerosis risk in communities (ARIC) study. Hear Rhythm. (2011) 8(8):1160–6. doi: 10.1016/j.hrthm.2011.03.038
38. Mascia G, Olivotto I, Brugada J, Arbelo E, Di Donna P, Della Bona R, et al. Sport practice in hypertrophic cardiomyopathy: running to stand still? Int J Cardiol. (2021) 345:77–82. doi: 10.1016/j.ijcard.2021.10.013
39. Platonov PG, McNitt S, Polonsky B, Rosero SZ, Zareba W. Atrial fibrillation in long QT syndrome by genotype. Circ Arrhythmia Electrophysiol. (2019) 12(10):1–9. doi: 10.1161/CIRCEP.119.007213
Keywords: zero fluoroscopy, meta-analysis, atrial fibrillation, catheter ablation, pulmonary vein isolation
Citation: Debreceni D, Janosi K, Bocz B, Turcsan M, Lukacs R, Simor T, Antolič B, Vamos M, Komocsi A and Kupo P (2023) Zero fluoroscopy catheter ablation for atrial fibrillation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1178783. doi: 10.3389/fcvm.2023.1178783
Received: 3 March 2023; Accepted: 7 June 2023;
Published: 16 June 2023.
Edited by:
Masateru Takigawa, Tokyo Medical and Dental University, JapanReviewed by:
Nathaniel Thompson, University of Vermont, United StatesGiuseppe Mascia, University of Genoa, Italy
Mario Matta, AOU Città della Salute e della Scienza, Italy
© 2023 Debreceni, Janosi, Bocz, Turcsan, Lukacs, Simor, Antolič, Vamos, Komocsi and Kupo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Kupo cGV0ZXIua3Vwb0BnbWFpbC5jb20=
 Dorottya Debreceni
Dorottya Debreceni Kristof Janosi1
Kristof Janosi1 Botond Bocz
Botond Bocz Marton Turcsan
Marton Turcsan Reka Lukacs
Reka Lukacs Mate Vamos
Mate Vamos Peter Kupo
Peter Kupo