
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 August 2023
Sec. Heart Valve Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1169590
 Jie Li1,†,‡
Jie Li1,†,‡ Yinghao Sun1,‡
Yinghao Sun1,‡ Songyuan Luo1
Songyuan Luo1 Shengneng Zheng1
Shengneng Zheng1 Jiaohua Chen1
Jiaohua Chen1 Ming Fu1
Ming Fu1 Zhenfei Fang2
Zhenfei Fang2 Yan Wang3
Yan Wang3 Guang Li1
Guang Li1 Ruixin Fan1
Ruixin Fan1 Jianfang Luo1*
Jianfang Luo1*
Background: The outcomes of transcatheter aortic valve replacement (TAVR) employing the second-generation retrievable VenusA-Pro and VenusA-Plus delivery systems with the self-expanding VenusA-Valve have not been described yet. This study aims to report the outcomes of these two second-generation delivery systems.
Methods: From January 2022 to April 2023, we prospectively enrolled patients with severe aortic stenosis undergoing TAVR with VenusA-Pro from three centers across China in this first-in-man study and retrospectively identified those undergoing TAVR with VenusA-Plus. All outcomes were reported according to the Valve Academic Research Consortium 3 definition. The primary outcome was 30-day all-cause mortality.
Results: A total of 156 patients were included, of which 46 underwent TAVR with VenusA-Pro and 110 underwent TAVR with VenusA-Plus. The Society of Thoracic Surgeons median score was 2.1%, bicuspid anatomy prevalence rate was 55.1%, and the mean aortic root calcification volume was 693 mm3. The technical success rate was 91.7%, comparable between the VenusA-Pro and VenusA-Plus groups (87.0% vs. 93.6%, P = 0.169). The 30-day all-cause mortality was 2.6%, similar between the VenusA-Pro and VenusA-Plus groups (2.2% vs. 2.7%, P = 0.842). No myocardial infarction occurred. The incidences of stroke (0.6%), major bleeding (3.8%), major vascular complications (5.1%), acute kidney injury (9.0%), permanent pacemaker implantation (5.1%), new-onset atrial fibrillation (5.8%), and moderate-to-severe paravalvular aortic regurgitation (6.0%) were favorable and comparable between the two groups. The clinical outcomes were similar between the patients with bicuspid and tricuspid aortic valve, except that the incidence of permanent pacemaker implantation was lower in patients with bicuspid anatomy (1.2% vs. 10.6%, P = 0.010).
Conclusions: The 30-day outcomes of TAVR with VenusA-Pro and VenusA-Plus were favorable and comparable.
Transcatheter aortic valve replacement (TAVR) has emerged as an alternative to surgical aortic valve replacement in elderly patients with symptomatic severe aortic stenosis (AS) (1). The VenusA-Valve (Venus Medtech Inc., Hangzhou, China), a self-expanding valve with first-generation non-retrievable delivery system, has been widely used in TAVR (2, 3). In China, challenging TAVR cases characterized by bicuspid anatomy and severe aortic valve calcification were more prevalent than in Western countries (4–6). The VenusA-Valve has seen a relatively higher rate of valve malposition and paravalvular aortic regurgitation in such a challenging TAVR population (7), and new-generation retrievable devices may help reduce peri-procedural complications (8). The VenusA-Valve second-generation retrievable and repositionable delivery systems are the VenusA-Pro and VenusA-Plus (Venus Medtech Inc., Hangzhou, China). The first-in-man use of the VenusA-Plus system has been reported previously in a single patient, but not in a larger population (9). Here, we aimed to report the outcomes of both VenusA-Pro and VenusA-Plus second-generation delivery systems. In addition, this was the first-in-man study of the latest VenusA-Pro system.
From January 2022 to April 2023, we prospectively enrolled patients from three centers across China in this first-in-man study of the VenusA-Pro system. In the same time frame, we also retrospectively identified the control group comprising TAVR patients using the VenusA-Plus system in Guangdong Provincial People's Hospital. All TAVR procedures were performed in patients with symptomatic severe aortic stenosis diagnosed according to the guideline (1). The baseline characteristics, procedural characteristics, clinical outcomes, and hemodynamic outcomes within 30 days were collected. The protocols were approved by site-specific institutional review boards. All patients in the prospective cohort had provided written informed consent, but those in the retrospective cohort did not.
The VenusA-Valve features supra-annular design similar to the Medtronic CoreValve (Medtronic Inc., Minneapolis, MN, USA) but with stronger radial force at the inflow end, which may be advantageous in bicuspid anatomy and severe calcification (2). Both VenusA-Pro and VenusA-Plus are second-generation retrievable and repositionable delivery systems that use the same prothesis VenusA-Valve as in the first-generation non-retrievable delivery system. The prosthesis is available in four different sizes (23, 26, 29, and 32 mm). The VenusA-Pro has the same profile as the VenusA-Plus, with an outer diameter ranging from 18 to 19 Fr. Compared to the VenusA-Plus, the VenusA-Pro offers more major advantages. First, the VenusA-Pro features a safety lock to prevent accidental valve deployment. Second, there is an additional marker for delivery system orientation for a better commissural alignment and coronary protection, which should point to the greater curve of the aortic arch. Third, the front end of the sheath has better flexibility when confronted with the horizontal aorta. Lastly, two limiting markers were used as reference during release or retrieval (Figure 1, 2).
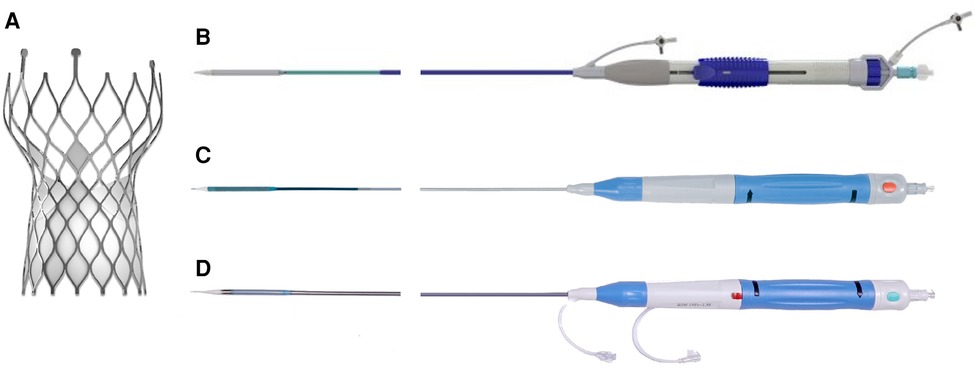
Figure 1. VenusA-Valve prosthesis and iteration of its delivery systems. (A) VenusA-Valve prosthesis, (B) first-generation non-retrievable delivery system, (C) second-generation retrievable VenusA-Plus delivery system, (D) second-generation retrievable VenusA-Pro delivery system. The red button in the middle of the handle is the safety lock.
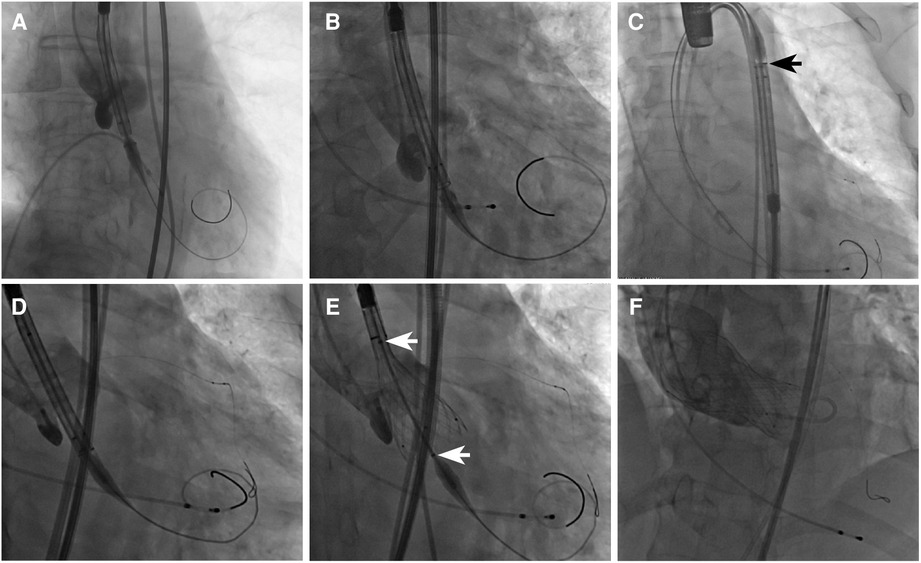
Figure 2. Fluoroscopic view of different delivery systems and their common VenusA-Valve prosthesis. (A) First-generation non-retrievable delivery system, (B) VenusA-Plus, and (C) VenusA-Pro. The black arrow shows the extra marker for orientation, which should point to the greater curve of the aortic arch. (D) VenusA-Pro locating the prosthesis, with left coronary artery under protection. (E) VenusA-Pro releasing the prosthesis after three times of retrieval and adjustment in a challenging case with a horizontal aorta with an aortic root angle of 73°. The white arrows show two limiting markers for reference during release or retrieval. (F) Final position of the VenusA-Valve prosthesis released by the VenusA-Pro delivery system, aortography showing trivial paravalvular aortic regurgitation.
All outcomes were reported according to the Valve Academic Research Consortium 3 definition (10). The primary outcome was 30-day all-cause mortality. Secondary outcomes included technical success, stroke, myocardial infarction, major bleeding (type 2–4), major vascular complications, acute kidney injury (stage 2–4), permanent pacemaker implantation, new-onset atrial fibrillation, and moderate-to-severe paravalvular aortic regurgitation at a 30-day follow-up. Post-procedural hemodynamic outcomes were measured before discharge.
Continuous variables were presented as mean with standard deviation and median with interquartile range and were compared using the Student’s t-test and the Mann–Whitney U-test, respectively. Categorical variables were presented as percentages and compared using the chi-squared test and Fisher’s exact test. All tests were two-tailed and P < 0.05 was considered significant. All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA).
A total of 156 patients were included, of which 46 underwent TAVR with VenusA-Pro and 110 underwent TAVR with VenusA-Plus. The mean age was 71.6 years, and 64.7% were male (Table 1). The Society of Thoracic Surgeons (STS) median score was 2.1%, which was slightly higher in the VenusA-Pro group (2.6% vs. 2.0%, P = 0.026). The percentage of patients with New York Heart Association (NYHA) class III or IV was lower (34.8% vs. 59.1%, P = 0.006), and the incidence of peripheral artery disease (defined according to the criteria in the STS score) was higher (15.2% vs. 2.7%, P = 0.004) in the VenusA-Pro group, whereas the incidences of other comorbidities were comparable between the two groups. The pre-procedural mean aortic valve area (0.61 vs. 0.74 cm2, P = 0.006) and annular perimeter (75.4 vs. 78.5 mm, P = 0.031) were smaller in the VenusA-Pro group, while other echocardiography and computed tomography characteristics were similar between the two groups. It was worth mentioning that over half of the patients (55.1%) had bicuspid anatomy, with a mean aortic root calcification volume of 693 mm3.
The majority of patients underwent the procedure using general anesthesia (93.6%) and transfemoral access (94.2%) (Table 2). The rates of combined percutaneous coronary intervention, pre-dilation, and post-dilation in all patients were 13.5%, 98.7%, and 48.7%, respectively, which were comparable between the two groups. The prostheses sizes were generally smaller in the VenusA-Pro group (P = 0.019). The mean perimeter oversizing was 5.7%, which was comparable between the two groups. The second valve implantation rate was 1.9% in all patients without a significant difference between the two groups. Two patients (1.3%) had tamponade. Two patients (1.3%) were transferred to open surgery, one with the VenusA-Pro was due to annular rupture, while the other one with the VenusA-Plus was due to valve embolization to the ascending aorta. No coronary obstruction occurred. The technical success rate was 91.7%, comparable between the VenusA-Pro and VenusA-Plus groups (87.0% vs. 93.6%, P = 0.169).
The 30-day all-cause mortality was 2.6%, similar between the VenusA-Pro and VenusA-Plus groups (2.2% vs. 2.7%, P = 0.842) (Table 3). Three patients died due to cardiogenic shock, and one died due to septic shock and subsequent multiple organ dysfunction syndrome. No myocardial infarction occurred. The incidence of stroke (0.6%), major bleeding (3.8%), major vascular complications (5.1%), acute kidney injury (9.0%), permanent pacemaker implantation (5.1%), and new-onset atrial fibrillation (5.8%) were favorable and comparable between the VenusA-Pro and VenusA-Plus groups.
The rates of moderate-to-severe paravalvular aortic regurgitation was 6.0% in all patients, without significant difference between the VenusA-Pro and VenusA-Plus groups (2.2% vs. 7.6%, P = 0.202). The post-procedural mean aortic valve area (1.72 cm2), mean transaortic gradient (12.0 mmHg), peak aortic velocity (2.14 m/s), and left ventricular ejection fraction (56.6%) were also similar between the two groups (Table 4).
In this study, based on the pre-procedural computed tomography analysis, 86 (55.1%) patients had bicuspid aortic valve (BAV), 66 had tricuspid aortic valve, three had bioprosthetic aortic valve failure, and one lacked pre-procedural computed tomography due to emergency (Table 5). The patients with bicuspid aortic valve were younger (70.5 vs. 73.3 years, P = 0.005) and had lower incidence of coronary artery disease (18.6% vs. 36.4%, P = 0.014). The pre-procedural mean transaortic gradient (62.6 vs. 53.4 mmHg, P = 0.002) and peak aortic velocity (5.03 vs. 4.65 m/s, P = 0.001) were higher, whereas the incidence of moderate-to-severe aortic regurgitation was lower (33.7% vs. 53.0%, P = 0.017) in patients with bicuspid aortic valve. The aortic root calcification volume was higher in patients with BAV (836 vs. 507 mm3, P < 0.001) than those with tricuspid aortic valve. During TAVR procedure, post-dilation was performed more frequently in patients with bicuspid aortic valve (55.8% vs. 39.4%, P = 0.045). The prosthesis sizes were generally smaller in patients with BAV than those with tricuspid aortic valve (P = 0.044). The mean perimeter oversizing was smaller in patients with BAV than those with tricuspid aortic valve (2.8% vs. 8.6%, P <0.001). The technical success rates were similar between the two groups (91.9% vs. 90.9%, P = 0.835). The incidence of permanent pacemaker implantation was lower in patients with BAV than those with tricuspid aortic valve (1.2% vs. 10.6%, P = 0.010). There was no significant difference in 30-day all-cause mortality (1.2% vs. 4.5%, P = 0.197) and other clinical outcomes between patients with bicuspid and tricuspid aortic valve. The post-procedural mean transaortic gradient (12.7 vs. 10.5 mmHg, P = 0.031) and peak aortic velocity (2.21 vs. 2.02 m/s, P = 0.049) was higher in patients with bicuspid aortic valve. The incidence of moderate-to-severe paravalvular aortic regurgitation were similar between patients with bicuspid and tricuspid anatomy (6.0% vs. 6.5%, P = 0.901).
This was a first-in-man multicenter study of the VenusA-Pro system and the largest report of the VenusA-Plus system so far. The main findings included the following: (1) the 30-day outcomes of TAVR with VenusA-Pro and VenusA-Plus were favorable and comparable, (2) the 30-day clinical outcomes of TAVR with both second-generation delivery systems were similar between bicuspid and tricuspid aortic valve, except that the incidence of permanent pacemaker implantation was lower in those with bicuspid anatomy (Figure 3).
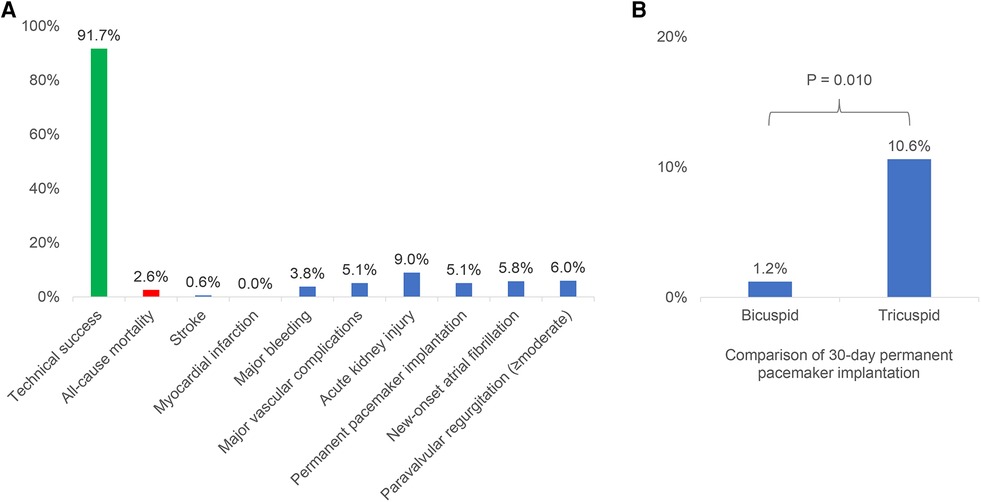
Figure 3. The 30-day outcomes of transcatheter aortic valve replacement with the VenusA-Pro and VenusA-Plus. (A) Incidences of 30-day outcomes of the VenusA-Pro and VenusA-Plus groups. The outcomes were favorable and comparable between the two groups. (B) Comparison of 30-day permanent pacemaker implantation between the patients with bicuspid and tricuspid aortic valve, the incidence was lower in patients with bicuspid anatomy.
In this study of two second-generation retrievable delivery systems using the self-expanding VenusA-Valve prosthesis, the overall 30-day mortality was 2.6%, with no significant difference between the VenusA-Pro and VenusA-Plus groups. The 30-day mortality of the most widely used retrievable self-expanding Evolut R (Medtronic, Minneapolis, MN, USA) was around 3.4% (8), similar to our results. The 30-day mortality of our study was also acceptable compared to that (1.9%) of the latest-iteration self-expanding Evolut PRO/PRO+ (Medtronic, Minneapolis, MN, USA) in the OPERA-TAVI registry (11).
With regard to procedural characteristics, the prosthesis sizes were generally smaller in the VenusA-Pro group as compared to the VenusA-Plus group, which could be explained by a smaller baseline aortic valve area and annular perimeter. The overall technical success rate in our study (91.7%) was comparable to that (93.1%) of the latest-iteration self-expanding Evolut PRO/PRO+ (11).
With respect to peri-procedural complications, the risk of stroke or myocardial infarction in this study was low, similar to that of the latest-iteration self-expanding valves (11). The rates of bleeding and vascular complications in this study were reasonable compared to previous reports (8, 12). The overall risk of acute kidney injury was 9.0% in this study, numerically lower than the 30-day incidence (17%) reported in a BRAVO-3 trial substudy (13), and similar to that (6.0%) reported in a previous review of Evolut R (8). The incidence of permanent pacemaker implantation was 5.1% in our study, generally lower than those reported in the previous studies (11, 14, 15), which may be explained by a downsizing strategy and a higher release position of the valve and subsequently less compression on the conduction system in patients with bicuspid anatomy with severe calcification. The overall incidence of new-onset atrial fibrillation in this study (5.8%) was numerically lower than that (9.9%) reported in a previous review (16).
As for hemodynamic performance, the post-procedural mean transaortic gradient in this study (12.0 mmHg) was slightly higher than that of the Evolut PRO/PRO+ (7.0 mmHg), and this might be explained by a lower perimeter oversizing rate (5.7%) as compared to that (18.9%) in a previous study (11). As was shown in the baseline characteristics, over half of the patients had bicuspid anatomy, and the mean aortic root calcification volume was near 700 mm3, indicating a population of challenging anatomy. Considering the strong radial force of the VenusA-Valve (though released by second-generation retrievable systems) in this study, the downsizing strategy (17) was frequently used to avoid severe valve migration toward the ventricle and subsequent unacceptable paravalvular aortic regurgitation. The rate of moderate-to-severe paravalvular aortic regurgitation was 6.0% in this study, slightly higher than that (3.2%) of the Evolut PRO/PRO+ (11), which might be explained by the absence of a VenusA-Valve external wrap, downsizing strategy, and high calcium burden (possibly bulky calcification) at the aortic root in our study. Actually, three generations of the Medtronic CoreValve System have seen lower incidence of moderate-to-severe paravalvular aortic regurgitation in a matched population, with 8.3% in CoreValve, 5.4% in Evolut R, and 3.4% in Evolut PRO (P = 0.032) (18). As paravalvular aortic regurgitation has been proved to be a risk factor on short-term and long-term mortality (19), further efforts should be made to minimize its incidence and severity.
The proportion of bicuspid aortic valve in this study was over 50%, similar to that (48.5%) reported in a previous study from China (6), and much higher than in other countries (4). The mean aortic root calcification volume was higher in patients with BAV than those with tricuspid aortic valve (836 vs. 507 mm3), both of which were higher than that (382 mm3) reported in Western patients with bicuspid aortic valve stenosis undergoing TAVR (20). The higher calcium burden could account for generally smaller prostheses, a higher post-dilation rate (55.8% vs. 39.4%), and a higher post-procedural mean transaortic gradient (12.7 vs. 10.5 mmHg) and peak aortic velocity (2.21 vs. 2.02 m/s) in patients with bicuspid anatomy. Previous studies have shown comparable survival rates after TAVR in patients with bicuspid and tricuspid anatomy (21, 22), similar to our findings. Nevertheless, excessive calcification has been related to poorer outcomes in patients with bicuspid anatomy (20). It was worth mentioning that the risk of permanent pacemaker implantation was significantly lower in patients with bicuspid anatomy (1.2% vs. 10.6%), which might be explained by a downsizing strategy as reflected by a lower perimeter oversizing rate (2.8% vs. 8.6%) and also by a higher release strategy, both of which were frequently used in severely calcified bicuspid anatomy.
There were several limitations in this study. First, there can be selection bias in such an observational study. Second, the statistic power maybe insufficient considering the small sample size, unmeasured confounding factors, and unbalanced baseline characteristics. Lastly, long-term outcomes were not available. Future studies with larger sample size and longer follow-up are warranted to further testify our findings.
In this study of the VenusA-Pro and VenusA-Plus delivery systems using the self-expanding VenusA-Valve, we found that the 30-day outcomes of TAVR with VenusA-Pro and VenusA-Plus were favorable and comparable (2). The clinical outcomes of both second-generation delivery systems were similar between patients with bicuspid and tricuspid aortic valve, except that the incidence of permanent pacemaker implantation was lower in patients with bicuspid anatomy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the institutional review boards of Guangdong Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
JLI, YS, and JL contributed to conception and design of the study. JLI and YS organized the database, performed the statistical analysis, and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by Venus Medtech (Hangzhou, China) and funded by Startup Project of National Natural Science Foundation of China (8200020121), Guangzhou Clinical Major Technical Project (2023FTJCZ0017), High-level Hospital Construction Project (DFJH201807), and Guangzhou Science and Technology Project (2023B03J1256).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2021) 77(4):e25–197. doi: 10.1016/j.jacc.2020.11.018
2. Liao YB, Zhao ZG, Wei X, Xu YN, Zuo ZL, Li YJ, et al. Transcatheter aortic valve implantation with the self-expandable VenusA-valve and CoreValve devices: preliminary experiences in China. Catheter Cardiovasc Interv. (2017) 89(S1):528–33. doi: 10.1002/ccd.26912
3. Leone PP, Scotti A, Ho EC, Assafin M, Doolittle J, Chau M, et al. Prosthesis tailoring for patients undergoing transcatheter aortic valve implantation. J Clin Med. (2023) 12(1):338–60. doi: 10.3390/jcm12010338
4. Xiong TY, Ali WB, Feng Y, Hayashida K, Jilaihawi H, Latib A, et al. Transcatheter aortic valve implantation in patients with bicuspid valve morphology: a roadmap towards standardization. Nat Rev Cardiol. (2022) 20:52–67. doi: 10.1038/s41569-022-00734-5
5. Jilaihawi H, Wu Y, Yang Y, Xu L, Chen M, Wang J, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv. (2015) 85(Suppl 1):752–61. doi: 10.1002/ccd.25863
6. Li YM, Xiong TY, Xu K, Fang ZF, Jiang L, Jin J, et al. Characteristics and outcomes following transcatheter aortic valve replacement in China: a report from China aortic valve transcatheter replacement registry (CARRY). Chin Med J (Engl). (2021) 134(22):2678–84. doi: 10.1097/CM9.0000000000001882
7. Li J, Sun Y, Zheng S, Li G, Dong H, Fu M, et al. Anatomical predictors of valve malposition during self-expandable transcatheter aortic valve replacement. Front Cardiovasc Med. (2021) 8:600356. doi: 10.3389/fcvm.2021.600356
8. Sun Y, Li J, Fan R, Li G, Fu M, Luo S, et al. Outcomes of Evolut R versus CoreValve after transcatheter aortic valve implantation: a meta-analysis. Heart Lung Circ. (2020) 29(2):288–94. doi: 10.1016/j.hlc.2018.12.013
9. Liu XB, He YX, Liu CH, Wang LH, Gao F, Yu L, et al. First-in-man implantation of the retrievable and repositionable VenusA-Plus valve. World J Emerg Med. (2018) 9(1):64–6. doi: 10.5847/wjem.j.1920-8642.2018.01.010
10. VARC-3 Writing Committee, Genereux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve academic research consortium 3: updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol. (2021) 77(21):2717–46. doi: 10.1016/j.jacc.2021.02.038
11. Costa G, Saia F, Pilgrim T, Abdel-Wahab M, Garot P, Valvo R, et al. Transcatheter aortic valve replacement with the latest-iteration self-expanding or balloon-expandable valves: the multicenter OPERA-TAVI registry. JACC Cardiovasc Interv. (2022) 15(23):2398–407. doi: 10.1016/j.jcin.2022.08.057
12. Noble S, Stortecky S, Heg D, Tueller D, Jeger R, Toggweiler S, et al. Comparison of procedural and clinical outcomes with Evolut R versus Medtronic CoreValve: a Swiss TAVI registry analysis. EuroIntervention. (2017) 12(18):e2170–6. doi: 10.4244/EIJ-D-16-00677
13. Chandrasekhar J, Sartori S, Mehran R, Aquino M, Vogel B, Asgar AW, et al. Incidence, predictors, and outcomes associated with acute kidney injury in patients undergoing transcatheter aortic valve replacement: from the BRAVO-3 randomized trial. Clin Res Cardiol. (2021) 110(5):649–57. doi: 10.1007/s00392-020-01787-7
14. Sammour Y, Krishnaswamy A, Kumar A, Puri R, Tarakji KG, Bazarbashi N, et al. Incidence, predictors, and implications of permanent pacemaker requirement after transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2021) 14(2):115–34. doi: 10.1016/j.jcin.2020.09.063
15. Genereux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. (2012) 59(25):2317–26. doi: 10.1016/j.jacc.2012.02.022
16. Ryan T, Grindal A, Jinah R, Um KJ, Vadakken ME, Pandey A, et al. New-onset atrial fibrillation after transcatheter aortic valve replacement: a systematic review and meta-analysis. JACC Cardiovasc Interv. (2022) 15(6):603–13. doi: 10.1016/j.jcin.2022.01.018
17. Liu X, He Y, Zhu Q, Gao F, He W, Yu L, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv. (2018) 91(5):986–94. doi: 10.1002/ccd.27467
18. Forrest JK, Kaple RK, Tang GHL, Yakubov SJ, Nazif TM, Williams MR, et al. Three generations of self-expanding transcatheter aortic valves: a report from the STS/ACC TVT registry. JACC Cardiovasc Interv. (2020) 13(2):170–9. doi: 10.1016/j.jcin.2019.08.035
19. Athappan G, Patvardhan E, Tuzcu EM, Svensson LG, Lemos PA, Fraccaro C, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. (2013) 61(15):1585–95. doi: 10.1016/j.jacc.2013.01.047
20. Yoon SH, Kim WK, Dhoble A, Milhorini Pio S, Babaliaros V, Jilaihawi H, et al. Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J Am Coll Cardiol. (2020) 76(9):1018–30. doi: 10.1016/j.jacc.2020.07.005
21. Makkar RR, Yoon SH, Leon MB, Chakravarty T, Rinaldi M, Shah PB, et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. (2019) 321(22):2193–202. doi: 10.1001/jama.2019.7108
Keywords: aortic stenosis (AS), bicuspid aortic valve (BAV), transcatheter aortic valve replacement (TAVR), VenusA-Pro, VenusA-Plus
Citation: Li J, Sun Y, Luo S, Zheng S, Chen J, Fu M, Fang Z, Wang Y, Li G, Fan R and Luo J (2023) Transcatheter aortic valve replacement with the VenusA-Pro and VenusA-Plus systems: preliminary experience in China. Front. Cardiovasc. Med. 10:1169590. doi: 10.3389/fcvm.2023.1169590
Received: 19 February 2023; Accepted: 7 August 2023;
Published: 24 August 2023.
Edited by:
Verena Veulemans, University Hospital of Düsseldorf, GermanyReviewed by:
David Meier, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland© 2023 Li, Sun, Luo, Zheng, Chen, Fu, Fang, Wang, Li, Fan and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfang Luo amlhbmZhbmdsdW9Ac2luYS5jb20=
†Present address Jie Li Department of Cardiology, Nyingchi People's Hospital, Tibet, China
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.