- 13rd Department of Cardiology, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, Katowice, Poland
- 2Department of Cardiology, Scanmed Center of Cardiology, Chorzow, Poland
Introduction: Recently published studies suggest that percutaneous coronary intervention (PCI) has no significant impact on outcomes in patients with heart failure and stable coronary artery disease. The use of percutaneous mechanical circulatory support is growing, but its value is still uncertain. If large areas of viable myocardium are ischemic, the benefit from revascularization should be evident. In such instances, we should strive for complete revascularization. The use of mechanical circulatory support in such cases is vital because it provides hemodynamic stability throughout the complex procedure.
Case report: We present a case of a 53-year-old male heart transplant candidate with type 1 diabetes mellitus, initially considered unsuitable for revascularization and qualified for heart transplantation, transferred to our center due to acute decompensated heart failure. At this time, the patient had temporary contraindications for heart transplantation. As the patient was considered no-option, we have decided to reassess the possibility of revascularization. The heart team opted for a high-risk mechanically supported PCI with the aim of complete revascularization. A complex multivessel PCI was performed with optimal effect. The patient was weaned off dobutamine on the second day post-PCI. Four months post-discharge, he remains stable, is in NYHA II class, and has no chest pain. Control echocardiography showed improved ejection fraction. The patient is not a heart transplant candidate anymore.
Conclusions: This case report shows that we must strive for revascularization in select heart failure cases. The outcome of this patient suggests that heart transplant candidates with potentially viable myocardium should be considered for revascularization, especially as the shortage of donors persists. In the most complex coronary anatomy and severe heart failure, mechanical support in the procedure might be essential.
Introduction
Recently published studies suggest that percutaneous coronary intervention (PCI) has no significant impact on outcomes in patients with heart failure (HF) and stable coronary artery disease (CAD) (1, 2). In patients with severe CAD and HF, the use of percutaneous mechanical circulatory support (MCS) is growing. However, its impact on outcomes is still uncertain, and, in many instances, the potential benefits are diminished by complications (3–5).
Nonetheless, we believe that a significant population of ischemic HF patients benefits from these interventions, especially when revascularization is achieved in large areas of viable myocardium. In such instances, we should strive for complete revascularization, including interventions on chronically occluded arteries (6, 7). MCS is vital because it provides hemodynamic stability throughout the complex procedure.
Case report
We present a case of a 53-year-old male heart transplant candidate with type 1 diabetes mellitus who was initially hospitalized at the intensive care unit in our center in March 2022 due to acute decompensated heart failure (ADHF). He was considered unsuitable for revascularization by the heart team, as his severe left ventricular impairment with a left ventricular ejection fraction (LVEF) of 15% and advanced multivessel coronary artery disease were considered extremely high risk and futile both for PCI and coronary artery bypass grafting. The patient was qualified for heart transplantation (OHT) by the heart transplant team. He was treated medically and discharged in stable condition.
In May 2022, the patient was transferred to our cardiology department due to a second ADHF episode, with severe congestion, hypotension, pleural effusion, severe asymmetric lower limb edema, and signs of infection. On admission, his echo showed a LVEF of 18% and an end-diastolic left ventricle dimension of 64 mm, with moderate mitral and tricuspid insufficiencies and good right ventricular function. The patient was stabilized with inotropes, intravenous diuretics, and pleurocentesis. As Doppler ultrasound and CT-angio confirmed lower limb deep vein thrombosis (DVT) with an infected ulceration, the patient was temporarily taken off the OHT list. Subsequently, after initial stabilization on oral medical treatment, he was transferred back to the referring cardiology department for further observation and rehabilitation. He was readmitted after 25 days in deteriorated clinical condition, on dobutamine support, hypotensive, in NYHA III/IV class. At this time, an urgent heart transplant was considered the only option. Therefore, he underwent right heart catheterization (cardiac output 3.55 L/min; cardiac index 1.92 L/min/m2; pulmonary vascular resistance 2.25 Wood units). Nonetheless, after the heart transplant team reassessment, he was still deemed unsuitable for OHT (due to persistent significant DVT and unclear infectious status with significantly elevated inflammation markers despite treatment).
At this time, we have decided to reassess the possibility of revascularization, as the patient was considered no-option, dobutamine-dependent, unsuitable for urgent OHT. In our healthcare system, only OHT-qualified patients are potential candidates for long-term mechanical circulatory support; therefore, such treatment was not available. The patient's coronary angiography before initial OHT qualification revealed a critical, calcified proximal left anterior descending artery (LAD) lesion, a chronic total occlusion (CTO) of the circumflex artery (LCx), a significant left main stenosis, and diffuse disease of the right coronary artery (RCA) with a critical lesion at the crux cordis. The heart team opted for a high-risk MCS PCI with the aim of complete revascularization. As revascularization was considered the last viable treatment option, myocardial viability or ischemia testing was not performed.
At the beginning of the procedure, two Abbott Perclose ProGlide devices were inserted after an ultrasound-guided femoral puncture. The Abiomed Impella CP catheter was inserted into the left ventricle. With the single access technique (puncture of the Impella hemostatic sheath), we were able to engage the left coronary artery with an EBU 3.5 catheter and perform a control angiography, which revealed similar coronary artery status to that before OHT qualification. A Pilot 50 guidewire easily crossed the LAD lesion; however, we were unable to introduce the HD IVUS Acist Kodama catheter. After meticulous 2.5-mm × 20-mm NC balloon predilatation, two sirolimus-eluting stents were implanted up to the ostium of the LAD (Ultimaster Tansei 2.5 mm × 28 mm and 3.0 mm × 38 mm). As IVUS at this point revealed stent underexpansion and further postdilatation provided insufficient results, 70 impulses of intravascular lithotripsy (Shockwave 3.5 mm × 15 mm) were performed to very good effect.
Subsequently, the circumflex artery was recanalized using a microcatheter-supported Pilot 150 guidewire and followed by the implantation of three everolimus-eluting stents (Xience Pro 2.25 mm × 23 mm, 2.5 mm × 23 mm, 2.5 mm × 18 mm). TIMI 3 flow was restored with optimal angiographic and IVUS results. Finally, the left main PCI was performed using a provisional technique with implantation of a 4.0-mm × 12-mm sirolimus-eluting Ultimaster Tansei stent, postdilated with a 4.5-mm × 6-mm NC balloon. Optimal angiographic and IVUS results were achieved. The patient remained hemodynamically stable through the procedure. The Impella CP was removed in the cathlab, and the Proglide presuture device provided hemostasis.
After 6 days, the patient was taken back to the cathlab. Coronary angiography showed optimal results of the previous procedure. After predilatation, a PCI of the right coronary artery with the use of three sirolimus-eluting stents was performed (Ultimaster Tansei 3.5 × 38, 2.75 × 24, 2.5 × 33).
Initial and follow-up echocardiography is presented in Supplementary Video 1. The angiographic images of the procedures are presented in Supplementary Video 2. The IVUS images are presented in Supplementary Video 3. The summary of initial and final angiography and echocardiography is presented in Figure 1.
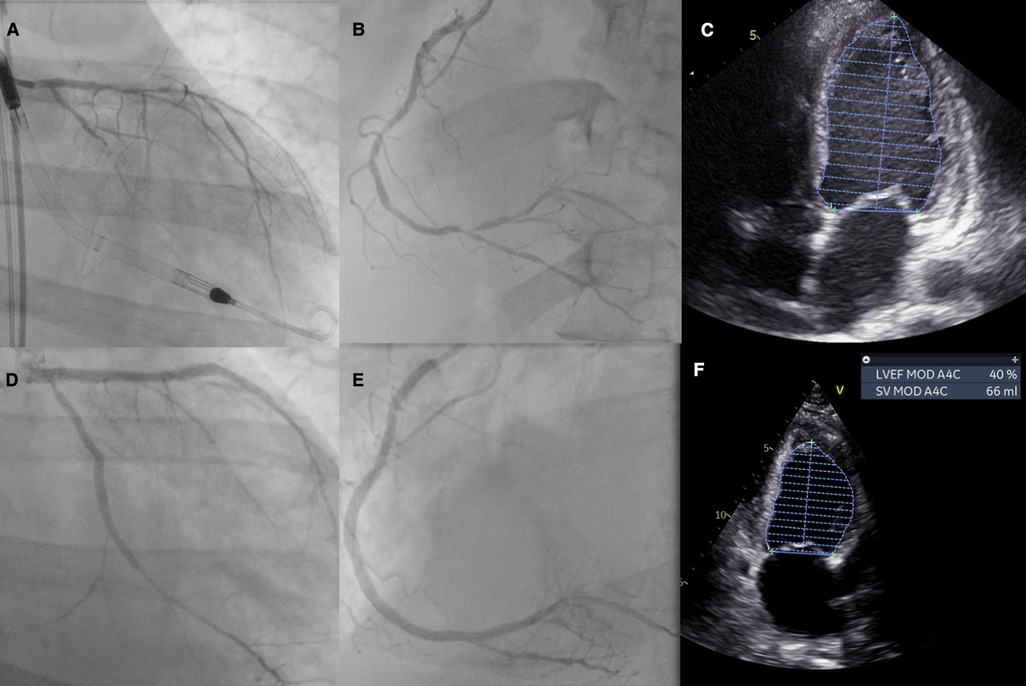
Figure 1. Patient summary: (A) initial left coronary artery anatomy with visible Impella support, (B) initial right coronary artery anatomy, (C) initial echocardiography with an LVEF of 18%, (D) final PCI effect of the left coronary artery, (E) final PCI effect of the right coronary artery, and (F) echocardiography on follow-up with an LVEF of 37%–38%. LVEF, left ventricular ejection fraction.
The patient was weaned off dobutamine on the second day post-PCI. The in-hospital stay was complicated by pneumonia, which was subsequently treated in the referring cardiology department. Four months post-discharge, he was controlled at the outpatient clinic. The patient remains stable, is in NYHA II class, and has no chest pain. Control echocardiography showed an LVEF of 37% and a mild mitral insufficiency. The patient is currently not an OHT candidate. Persistent DVT, despite antithrombotic treatment, remains the most important clinical problem at the time of follow-up.
Discussion
In general, it is the policy at our center to utilize all of the available treatment methods for severe HF patient before OHT qualification. This includes almost routine revascularization. In these patients, long-term results are generally satisfactory, especially when complete revascularization is possible (6). There are, however, select cases when the patient is not revascularized. This concerns mostly patients with extremely complicated coronary anatomy and the most severe left ventricular impairment, especially when the benefit of revascularization is doubtful. In such cases, myocardial viability testing may be useful in the decision-making process. However, severe dilatation of the left ventricle and thinning of the myocardium may be considered surrogates for lack of viability. Moreover, in hemodynamically unstable patients, inotrope-dependent, when OHT is considered urgent (in-hospital), revascularization is rarely performed to avoid potential complications or the need for a dual (or triple) antithrombotic regimen. If the heart team decides to perform PCI in OHT candidates, especially in complex coronary anatomy (unprotected left main lesion, multivessel disease, last patent vessel), a periprocedural hemodynamic compromise might be expected. In such cases, MCS should be considered to provide patient stability throughout the procedure.
This case report presents the treatment and outcomes of a patient in whom, at an early stage of treatment, there was a decision to treat CAD conservatively. Such a decision, a premature one in our opinion, complicated the patient's further clinical course. Performing complete revascularization produced left ventricular improvement exceeding expectations, even with no initial proof of myocardial viability. MCS support enabled the operators to perform the procedure safely and optimally. In such complex HF cases, the ever-changing clinical scenario might compel the physicians to challenge initial treatment decisions in the best interest of the patient.
Conclusions
We have presented a case of a dramatic clinical and LVEF improvement in an OHT candidate with temporary contraindications after complete revascularization. High-risk PCI was the only viable option and provided results exceeding our expectations. This case report shows that in select HF cases, we must strive for revascularization, despite recent trial results. The question remains whether revascularization during initial OHT qualification could have prevented the ADHF episodes. The patient should have never entered the OHT waiting list, especially with the significant comorbidities. The outcome of this patient suggests that OHT candidates with potentially viable myocardium should be considered for revascularization, especially as shortage of donors persists. In the most complex coronary anatomy and severe LVEF impairment, MCS might be essential.
Data availability statement
The datasets presented in this article are not readily available because this is a case report. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LP contributed to the design of the manuscript, analyzed the data, prepared the manuscript, and contributed to the design of the case report study. JP, JS, and MS contributed to the design of the manuscript, design of the case report study, and reviewed the manuscript. MG contributed to the preparation of the manuscript, design of the case report study, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1169165/full#supplementary-material
References
1. Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O'Brien SM, Boden WE, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. (2020) 382(15):1395–407. doi: 10.1056/NEJMoa1915922
2. Perera D, Clayton T, O'Kane PD, Greenwood JP, Weerackody R, Ryan M, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. (2022) 387(15):1351–60. doi: 10.1056/NEJMoa2206606 36027563
3. O'Neill WW, Kleiman NS, Moses J, Henriques JP, Dixon S, Massaro J, et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. (2012) 126(14):1717–27. doi: 10.1161/CIRCULATIONAHA.112.098194
4. Protty M, Sharp ASP, Gallagher S, Farooq V, Spratt JC, Ludman P, et al. Defining percutaneous coronary intervention complexity and risk: an analysis of the United Kingdom BCIS database 2006–2016. JACC Cardiovasc Interv. (2022) 15(1):39–49. doi: 10.1016/j.jcin.2021.09.039
5. Chieffo A, Burzotta F, Pappalardo F, Briguori C, Garbo R, Masiero G, et al. Clinical expert consensus document on the use of percutaneous left ventricular assist support devices during complex high-risk indicated PCI: Italian society of Interventional Cardiology Working Group Endorsed by Spanish and Portuguese Interventional Cardiology Societies. Int J Cardiol. (2019) 293:84–90. doi: 10.1016/j.ijcard.2019.05.065
6. Pyka Ł, Hawranek M, Tajstra M, Gorol J, Lekston A, Gąsior M. Complete percutaneous revascularisation feasibility in ischaemic heart failure is related to improved outcomes: insights from the COMMIT-HF registry. Kardiol Pol. (2017) 75(5):453–61. doi: 10.5603/KP.a2017.0018
7. Tajstra M, Pyka Ł, Gorol J, Pres D, Gierlotka M, Gadula-Gacek E, et al. Impact of chronic total occlusion of the coronary artery on long-term prognosis in patients with ischemic systolic heart failure: insights from the COMMIT-HF registry. JACC Cardiovasc Interv. (2016) 9(17):1790–7. doi: 10.1016/j.jcin.2016.06.007
Keywords: heart transplant, revascularisation, congestive heart failure, mechanical circulatory support, outcomes, myocardial recovery
Citation: Pyka L, Szkodzinski J, Piegza J, Swietlińska M and Gąsior M (2023) Case Report: do heart transplant candidates benefit from mechanically supported revascularization?. Front. Cardiovasc. Med. 10:1169165. doi: 10.3389/fcvm.2023.1169165
Received: 18 February 2023; Accepted: 30 May 2023;
Published: 23 June 2023.
Edited by:
Jamshid Karimov, Cleveland Clinic, United StatesReviewed by:
Nagarajan Muthialu, Great Ormond Street Hospital for Children NHS Foundation Trust, United KingdomFabrizio De Rita, Newcastle upon Tyne Hospitals NHS Foundation Trust, United Kingdom
© 2023 Pyka, Szkodzinski, Piegza, Swietlinska and Gąsior. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukasz Pyka bC50LnB5a2FAZ21haWwuY29t
 Lukasz Pyka
Lukasz Pyka Janusz Szkodzinski1
Janusz Szkodzinski1