
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 27 June 2023
Sec. Atherosclerosis and Vascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1167132
This article is part of the Research Topic Oxidative Stress, Inflammation and Atherosclerosis-Related Diseases: From basic to clinical research View all 11 articles
Objective: To investigate the association of methyltransferase-like protein 14 (METTL14) expression with coronary heart disease (CHD).
Methods: Three hundred and sixteen patients who attended Henan Provincial People's Hospital between June 2019 and February 2021 with principal symptoms of pain or tightness in the chest and who underwent coronary angiography for definitive diagnosis were enrolled. The uric acid, TG, TC, LDL-C, HDL-C, apolipoprotein A1, free fatty acid, lipoprotein a, homocysteine, CRP, and SAA levels were examined. The levels of METTL14, TNF-α, MCP-1, VCAM-1, ICAM-1, and IL-6 were evaluated by ELISA.
Results: Patients with CHD had significantly higher m6A methyltransferase activity. In addition, the incidence of diabetes and hypertension, as well as the concentrations of TC, CRP, and SAA were higher in CHD patients. Patients with coronary lesion branches also had significantly increased TG, LDL-C, CRP, and SAA levels. TNF-α, MCP-1, VCAM-1, ICAM-1, and IL-6 expression was also markedly increased in the CHD group (P < 0.001) as was the expression of METTL14 (P < 0.001). The METTL14 expression levels also differed significantly in relation to the number of branches with lesions (P < 0.01) and were correlated with SAA, VCAM-1, ICAM-1, IL-6, and the Gensini score. ROC curve analyses of METTL14 in CHD indicated an AUC of 0.881 (0.679, 0.894) with a cut-off value of 342.37, a sensitivity of 77%, and a specificity of 84%. MCP-1, VCAM-1, IL-6, SAA, and METTL14 were found to independently predict CHD risk.
Conclusions: METTL14 levels were found to be positively associated with inflammatory markers and to be an independent predictor of CHD risk.
Coronary heart disease (CHD) is increasing in incidence. The current tests used to assist in the diagnosis of CHD have limited specificity and sensitivity and are invasive; thus, the identification of specific biomarkers is important for the early detection of CHD. CHD is both chronic and progressive and evidence suggests a close association between CHD and inflammation, which can be assessed by a variety of inflammatory indicators, suggesting that the levels of these indicators may be useful for preventing, diagnosing, and treating CHD (1–4). Thus, a comprehensive analysis of potential indicators and biomarkers for CHD development and progression would be highly useful for the assessment of CHD risk.
The importance of epigenetic modification is increasingly recognized. Nucleic acid methylation is a major form of epigenetic modification, with N6-methyladenosine (m6A) methylation representing approximately 80% of RNA modifications in eukaryotes (5). m6A methylation is controlled by a series of enzymes, specifically, the “writers” or m6A methyltransferases, such as METTL3/14, VIRMA, RBM15/15B, WTAP, and ZC3H13, responsible for the methylation, the “erasers” or demethylases, such as ALKBH5, FTO, and ALKBH3, that remove the modification, and “readers”, such as hnRNP, YTHDF1/2/3, eIF3, and IGF2BP1/2/3 (6, 7). Evidence suggests the close involvement of m6A methylation in regulating RNA function and these modifications have been associated with pathological processes in in physiological and pathological processes of cardiovascular diseases (8). Methyltransferase-like 14 (METTL14), a well-known m6A writer protein, widely participated in the progression of major diseases, such as cardiovascular pathogenesis (9). In addition, METTL14 plays an important role in maintaining cardiac homeostasis (10). Knocking down METTL14 could inhibit the development of atherosclerosis in high-fat diet-treated APOE-/- mice (11). Here, the clinical significance of alterations in the METTL14 levels of CHD patients was investigated to clarify the association between METTL14 and the severity of CHD. It is hoped that these findings will suggest new directions for CHD prevention and treatment.
A total of 316 patients who attended Henan Provincial People's Hospital between June 2019 and February 2021 with principal symptoms of pain or tightness in the chest and who received coronary angiography for definitive diagnosis were recruited. The patients were allocated to a CHD group and a control group based on the angiographic findings. Patients in the control group had no stenosis of the coronary arteries or only myocardial bridge alterations (i.e., congenital abnormal coronary artery development where a part of the coronary artery crosses the myocardium). The inclusion criterion for the CHD group was the presence of a >50% stenosis in at least one coronary artery (left main, left circumflex, left anterior descending, or right coronary artery). Patients who had undergone earlier coronary angiography and coronary artery bypass grafting were excluded, as were those with hematological disorders, tumors, severe liver and renal insufficiencies, acute or chronic infectious diseases, active bleeding from all causes, peripheral vascular disease, diabetes mellitus, cardiac arrhythmia, or chronic obstructive pulmonary disease. The study was approved by the Ethics Committee of the hospital, and all participants provided written informed consent.
Five-milliliter venous blood samples were collected after an overnight fast. After centrifugation (3,000 rpm, 15 min), the uric acid, TG, TC, LDL-C, free fatty acid, lipoprotein a, homocysteine, HDL-C, Apolipoprotein A1 (ApoA1), CRP, and plasma amyloid A (SAA) levels were determined.
ELISA kits (Abcam, Cambridge, UK) were used to measure the plasma levels of METTL14, TNF-α, MCP-1, VCAM-, ICAM-1, and IL-6.
The m6A methylase activity was determined using an Epigenase m6A Methylase Activity Assay kit (Epigentek, NY, USA).
Total RNA was isolated from plasma using a TRIzol kit (Invitrogen, Carlsbad, CA, USA). The RNA concentration was measured with a spectrophotometer (NanoDrop® 2000; Thermo Fisher Scientific, Waltham, MA, USA). All experimental procedures were performed according to the manufacturer's instructions. Ploidy differences in expression levels were determined using the 2-ΔΔCt method.
METTL14 Forward: 5′-GTT GGA ACA TGG ATA GCC GC-3′; Reverse: 5′-CAA TGC TGT CGG CAC TTT CA-3′.
GAPDH Forward:5′-GGTGGTCTCCTCTGACTTCAA-3′; Reverse: 5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
FTO forward: 5′-CTTCACCAAGGAGACTGCTATTTC; Reverse: 5′-CAAGGTTCCTGTTGAGCACTCTG-3′.
The Gensini score equals the sum of all segment scores (each segment score equals a segment weighting factor multiplied by a severity score). The segment weighting factors range from 0.5 to 5.0. The severity scores reflecting the specific percentage of luminal diameter reduction in the coronary artery segment are 32 for 100%, 16 for 99%, 8 for 90%, 4 for 75%, 2 for 50%, and 1 for 25% reduction. Thus, segments supplying a larger area of the myocardium are more heavily weighted and the highest scores are associated with multiple severe proximal lesions. Scoring was performed according to internationally recognized methods and the score of the individual patients represented the summed scores for each branch (12).
Data were analyzed using SPSS 23.0. Data distributions were assessed by Kolmogorov–Smirnov tests and normally distributed data were expressed as means ± standard deviations and compared using independent-sample t-tests for two groups and one-way ANOVAs for multiple groups. Data that did not conform to a normal distribution were expressed as medians (interquartile spacing) and compared with the Mann–Whitney U rank-sum test for two groups and the Kruskal–Wallis rank-sum test for multiple groups. Associations between variables were assessed by Pearson or Spearman correlation analysis, and the sensitivity and specificity of METTL14 were evaluated by ROC curves and logistic regression analysis of risk factors for CHD. The discrimination ability of the logistic regression model was assessed by estimating the area under the receiver operating characteristic (ROC) curve. Model calibration was assessed using the Hosmer–Lemeshow test for good-ness of fit. Differences were statistically significant at P < 0.05.
Firstly, we used ELISA to determine the activity of methyltransferase in the plasma of patients with CHD, finding the activity of the methyltransferase significantly raised in CHD patients relative to the controls (Figure 1). In addition, the mRNA levels of METTL14 were markedly increased while the FTO levels were significantly reduced in the CHD group (Figure 2).
No significant differences were observed between the groups in relation to age, sex, smoking, drinking, UA, TC, TG, LDL-C, FFA, LPa, HCY, HDL-C, or ApoA1 (P > 0.05). However, as shown in Table 1, the incidence of diabetes and hypertension, as well as the concentrations of TC, CRP, and SAA were higher in CHD patients.
Patients with CHD were then classified by the number of coronary branches containing lesions. Seventy-two patients had a single lesion in one branch, 80 had lesions in two branches, and 64 had lesions in three branches. Analysis of the clinical parameters of the patients in these different subgroups showed no inter-group differences in terms of age, sex, hypertension, diabetes, smoking, alcohol consumption, LDL-C, FFA, LPa, HCY, HDL-C, and ApoA1 while significant differences were observed for TG, LDL-C, CRP, and SAA (Table 2).
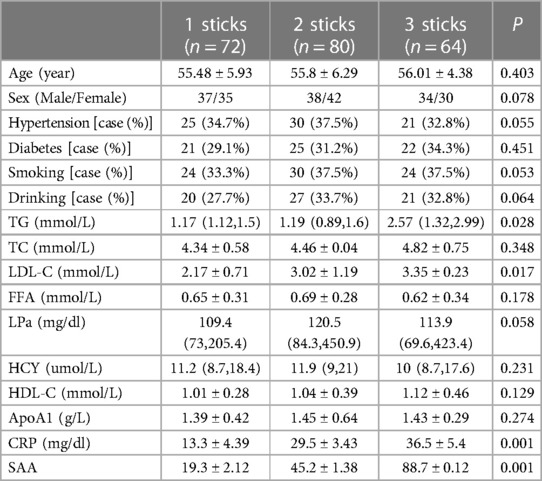
Table 2. Association between the number of coronary branches with lesions and clinical parameters in CHD patients.
It was observed that the contents of TNF-α, VCAM-1, ICAM-1, MCP-1, and IL-6, measured by ELISA, were significantly raised in the CHD group in comparison with the controls (P < 0.001) (Table 3).
METTL14 levels were observed to be markedly increased in the CHD group relative to the controls (P < 0.001) (Table 4).
Patients with CHD were grouped as described above based on the number of branches containing coronary lesions. The groups were defined as the 1-branch lesion (n = 72), 2-branch lesion (n = 80), and 3-branch lesion (n = 64) groups. The control was the 0-branch lesion group. The results are shown in Table 5.
There was no correlation between METTL14 and age, sex, diabetes, hypertension, alcohol consumption, smoking, TG, TC, LDL-C, FFAs, LPa, HCY, HDL-C, or ApoA1 (P > 0.05). A significant association, however, was observed with SAA (P < 0.01) (Table 6).
METTL14 levels were significantly linked to those of VCAM-1, ICAM-1, SAA, and IL-6, as well as the Gensini scores in the CHD group (Table 7).
The cut-off values and sensitivity and specificity according to the maximum Jorden index were determined from the ROC curves. The results showed that METTL14 had a cut-off value of 342.37, a sensitivity of 77%, a specificity of 84%, and an AUC of 0.881 (0.679, 0.894).
In the regression analysis, CHD was set as the dependent variable with inflammatory factor and METTL14 levels as independent variables. The analysis showed that MCP-1, VCAM-1, IL-6, SAA, and METTL14 were independent risk factors for CHD (P < 0.05) (Table 8).
Although the specific risk factors for CHD have not been fully elucidated, it is known that factors such as hypertension, hyperlipidemia, diabetes, and inflammation are associated with its development (13–15). A characteristic feature of CHD is the presence of atherosclerotic plaques, formed by a combination of lipid, calcium, and inflammation-associated cells (16, 17). The presence of plaque narrows the lumen of the artery, and, in the case of coronary arteries, can lead to the development of angina, either persistent or episodic. Plaque rupture can lead to the development of blood clots which can cause myocardial infarction through blockage of the vessels. Atherosclerosis is also linked with inflammation. It is thus possible to assess the severity and progression of CHD by measuring the levels of specific inflammatory indicators (18). Here, it was found that the presence of hypertension and diabetes, as well as the levels of TC, CRP, and SAA, were significantly associated with CHD. This suggests that both hyperlipidemia and the inflammatory response are closely associated with CHD pathogenesis, as has been found in earlier studies (17, 18).
Recent evidence has indicated that epigenetic modifications are associated with both the initiation and subsequent promotion of atherosclerosis, playing important parts in the development of atherosclerotic plaque. This suggests the potential significance of using markers of epigenetic modifications as indicators or biomarkers for CHD risk and progression (19). This appears to be the first investigation of the role of the methyltransferase METTL14 in CHD, and demonstrated that METTL14 levels were markedly raised in the sera of patients with CHD. However, the METTL14 levels were not found to be linked to either the TC or TG levels, which were used in the inclusion criteria.
Studies have shown that the m6A methylation process affects various types of cells, including those associated with blood vessels, such as vascular endothelial and smooth muscle cells, as well as macrophages, and that changes in methylation levels contribute to the pathogenesis of atherosclerosis. METTL14 is documented to methylate pri-miR-19a and promote the processing of the mature miR-19a, stimulating the proliferation and invasion of atherosclerotic vascular endothelial cells, indicating that the METTL14/m6A/miR-19a axis may represent a novel target for anti-atherosclerosis treatment (20). In addition, METTL14 reduction inhibits the endothelial cell inflammatory response, thereby preventing atherosclerotic plaque formation (21). A study using mass spectrometry to analyze m6A levels in non-atherosclerotic arterial and carotid atherosclerotic tissues found that m6A methylesterase and demethylase levels were altered in atherosclerotic tissues (22). More importantly, knockdown of METTL14 inhibited the m6A modification of FOXO1 and decreased FOXO1 expression to suppress the endothelial inflammatory response and atherosclerotic plaque formation (21). It has also been shown that METTL14 promotes inflammatory responses in atherosclerosis-associated macrophages via NF-κB/IL-6 signaling (23). WTAP promotes myocardial I/R injury through promoting ER stress and cell apoptosis by regulating m6A modification of ATF4 mRNA (24). NCBP3, a novel hypoxia-specific response protein, functions as a scaffold for the coordination of METTL3 and eIF4A2 for enhancing gene translation by m6A RNA methylation in cardiomyocytes subjected to hypoxic stress (25). Moreover, METTL14 promotes the renal ischemia-reperfusion injury (IRI) via suppressing YAP1 pathway (26). UCHL5 modified by METTL14/YTHDF1 axis could facilitate the inflammation and vascular remodeling in atherosclerosis by activating the NLRP3 inflammasome (27).
Here, plasma concentrations of inflammatory factors were analyzed by ELISA, showing that CHD patients had significantly elevated concentrations of TNF-α, MCP-1, ICAM-1, VCAM-1, and IL-6, which is consistent with previous findings (28–31). Notably, METTL14 levels were found to be significantly associated with those of VCAM-1, IL-6, ICAM-1, and SAA, suggesting a close relationship between METTL14 and the inflammatory response. The relationship between SAA and METTL14 has not yet been reported and the precise mechanisms underlying this association require further elucidation in future work. METTL14 was also observed to correlate with the Gensini score and higher numbers of coronary artery branches containing lesions, suggesting a relationship between METTL14 and CHD stenosis and severity. The above studies provide a stronger theoretical basis for the relationship between METTL14 and inflammation. Although studies have identified the role of METTL14 in CHD and its relationship with inflammatory markers, the biological effects of METTL14 on the initiation and progression of CHD have not been investigated. Combined with previous and our results, it is speculated that it is possible to delay the progression of CHD by intervening or inhibiting potential molecular targets. Nevertheless, there are still several shortcomings in this study. First, it was a single-center study with a small sample size, with some variation in the clinical baseline data of the study population, so a subsequent large-sample study is needed to further confirm these results. In addition, there are many risk factors for CHD and only a single inclusion criterion was used. Thus, although we observed a significant link between METTL14 levels and CHD risk, the use of METTL14 as a CHD biomarker requires further verification with large-sample and multi-center studies. Specific targeting is challenging in disease treatment and it is possible that the combination of transcription factors with targets may be useful, providing a stronger theoretical basis for the prevention of CHD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
FG and GL: conceived and supervised the study. FG: designed experiments. FG and MH: performed experiments. FG and BH: analyzed the data. FG: wrote the manuscript; and FG and GL: revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the National Natural Sciences Foundation of China (grant no 82002199).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tian Y, Deng P, Li B, Wang J, Li J, Huang Y, et al. Treatment models of cardiac rehabilitation in patients with coronary heart disease and related factors affecting patient compliance. Rev Cardiovasc Med. (2019) 20:27–33. doi: 10.31083/j.rcm.2019.01.53
2. Chen D, Liang M, Jin C, Sun Y, Xu D, Lin Y. Expression of inflammatory factors and oxidative stress markers in serum of patients with coronary heart disease and correlation with coronary artery calcium score. Exp Ther Med. (2020) 20(3):2127–33. doi: 10.3892/etm.2020.8958
3. Ndrepepa G, Holdenrieder S, Cassese S, Fusaro M, Xhepa E, Laugwitz KL, et al. A comparison of gamma-glutamyl transferase and alkaline phosphatase as prognostic markers in patients with coronary heart disease. Nutr Metab Cardiovasc Dis. (2018) 28(1):64–70. doi: 10.1016/j.numecd.2017.09.005
4. Hou P, Xue HP, Mao XE, Li YN, Wu LF, Liu YB. Inflammation markers are associated with frailty in elderly patients with coronary heart disease. Aging (Albany NY). (2018) 10(10):2636–45. doi: 10.18632/aging.101575
5. Qin Y, Li L, Luo E, Hou J, Yan G, Wang D, et al. Role of m6A RNA methylation in cardiovascular disease (review). Int J Mol Med. (2020) 46:1958–72. doi: 10.3892/ijmm.2020.4746
6. Zhang B, Jiang H, Dong Z, Sun A, Ge J. The critical roles of m6A modification in metabolic abnormality and cardiovascular diseases. Genes Dis. (2021) 8:746–58. doi: 10.1016/j.gendis.2020.07.011
7. Kumari R, Ranjan P, Suleiman ZG, Goswami SK, Li J, Prasad R, et al. mRNA modifications in cardiovascular biology and disease: with a focus on m6A modification. Cardiovasc Res. (2022) 118:1680–92. doi: 10.1093/cvr/cvab160
8. Zhao K, Yang CX, Li P, Sun W, Kong XQ. Epigenetic role of N6-methyladenosine (m6A) RNA methylation in the cardiovascular system. J Zhejiang Univ Sci B. (2020) 21:509–23. doi: 10.1631/jzus.B1900680
9. Meng L, Lin H, Huang X, Weng J, Peng F, Wu S. METTL14 suppresses pyroptosis and diabetic cardiomyopathy by downregulating TINCR lncRNA. Cell Death Dis. (2022) 13:38. doi: 10.1038/s41419-021-04484-z
10. Wang L, Wang J, Yu P, Feng J, Xu GE, Zhao X, et al. METTL14 is required for exercise-induced cardiac hypertrophy and protects against myocardial ischemia-reperfusion injury. Nat Commun. (2022) 13:6762. doi: 10.1038/s41467-022-34434-y
11. Liu Y, Luo G, Tang Q, Song Y, Liu D, Wang H, et al. Methyltransferase-like 14 silencing relieves the development of atherosclerosis via m(6)A modification of p65 mRNA. Bioengineered. (2022) 13:11832–43. doi: 10.1080/21655979.2022.2031409
12. Zencirci AE, Zencirci E, Degirmencioglu A, Karakus G, Ugurlucan M, Gunduz S, et al. The relationship between gensini score and ST-segment resolution in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Kardiol Pol. (2014) 72(6):494–503. doi: 10.5603/KP.a2013.0355
13. Luscher TF, von Eckardstein A, Simic B. Therapeutic targets to raise HDL in patients at risk or with coronary artery disease. Curr Vasc Pharmacol. (2012) 10(6):720–4. doi: 10.2174/157016112803520972
14. de Araújo Gonçalves P, Garcia-Garcia HM, Carvalho MS, Dores H, Sousa PJ, Marques H, et al. Diabetes as an independent predictor of high atherosclerotic burden assessed by coronary computed tomography angiography: the coronary artery disease equivalent revisited. Int J Cardiovasc Imaging. (2013) 29(5):1105–14. doi: 10.1007/s10554-012-0168-4
15. Mahalle N, Kulkarni MV, Naik SS. Is hypomagnesaemia a coronary risk factor among Indians with coronary artery disease? J Cardiovasc Dis Res. (2012) 3(4):280–6. doi: 10.4103/0975-3583.102698
16. Li H, Sun K, Zhao R, Hu J, Hao Z, Wang F, et al. Inflammatory biomarkers of coronary heart disease. Front Biosci (Schol Ed). (2018) 10(1):185–96. doi: 10.2741/s508
17. Qian H, Luo Z, Xiao C, Chen J, Li D, Xu H, et al. Red cell distribution width in coronary heart disease: prediction of restenosis and its relationship with inflammatory markers and lipids. Postgrad Med J. (2018) 94(1115):489–94. doi: 10.1136/postgradmedj-2018-135806
18. Asztalos BF, Horvath KV, Schaefer EJ. High-density lipoprotein particles, cell-cholesterol efflux, and coronary heart disease risk. Arterioscler Thromb Vasc Biol. (2018) 38(9):2007–15. doi: 10.1161/ATVBAHA.118.311117
19. Wu S, Zhang S, Wu X, Zhou X. M(6)A RNA methylation in cardiovascular diseases. Mol Ther. (2020) 28(10):2111–9. doi: 10.1016/j.ymthe.2020.08.010
20. Jian D, Wang Y, Jian L, Tang H, Rao L, Chen K, et al. METTL14 aggravates endothelial inflammation and atherosclerosis by increasing FOXO1 N6-methyladeosine modifications. Theranostics. (2020) 10(20):8939–56. doi: 10.7150/thno.45178
21. Quiles-Jimenez A, Gregersen I, Mittelstedt Leal de Sousa M, Abbas A, Kong XY, Alseth I, et al. N6-methyladenosine in RNA of atherosclerotic plaques: an epitranscriptomic signature of human carotid atherosclerosis. Biochem Biophys Res Commun. (2020) 533(4):631–7. doi: 10.1016/j.bbrc.2020.09.057
22. Aslibekyan S, Agha G, Colicino E, Do AN, Lahti J, Ligthart S, et al. Association of methylation signals with incident coronary heart disease in an epigenome-wide assessment of circulating tumor necrosis factor alpha. JAMA Cardiol. (2018) 3(6):463–72. doi: 10.1001/jamacardio.2018.0510
23. Zheng Y, Li Y, Ran X, Wang D, Zheng X, Zhang M, et al. METTL14 mediates the inflammatory response of macrophages in atherosclerosis through the NF-κB/IL-6 signaling pathway. Cell Mol Life Sci. (2022) 79(6):311. doi: 10.1007/s00018-022-04331-0
24. Wang J, Zhang J, Ma Y, Zeng Y, Lu C, Yang F, et al. WTAP promotes myocardial ischemia/reperfusion injury by increasing endoplasmic reticulum stress via regulating m(6)A modification of ATF4 mRNA. Aging (Albany NY). (2021) 13:11135–49. doi: 10.18632/aging.202770
25. Ye F, Wang X, Tu S, Zeng L, Deng X, Luo W, et al. The effects of NCBP3 on METTL3-mediated m6A RNA methylation to enhance translation process in hypoxic cardiomyocytes. J Cell Mol Med. (2021) 25:8920–8. doi: 10.1111/jcmm.16852
26. Xu Y, Yuan XD, Wu JJ, Chen RY, Xia L, Zhang M, et al. The N6-methyladenosine mRNA methylase METTL14 promotes renal ischemic reperfusion injury via suppressing YAP1. J Cell Biochem. (2020) 121:524–33. doi: 10.1002/jcb.29258
27. Yang X, Wang C, Zhu G, Guo Z, Fan L. METTL14/YTHDF1 axis-modified UCHL5 aggravates atherosclerosis by activating the NLRP3 inflammasome. Exp Cell Res. (2023) 427:113587. doi: 10.1016/j.yexcr.2023.113587
28. Leocadio PCL, Menta P, Dias MTS, Fraga JR, Goulart AC, Santos IS, et al. Low serum levels of CCL2 are associated with worse prognosis in patients with acute coronary syndrome: 2-year survival analysis. Biomed Pharmacother. (2019) 109:1411–6. doi: 10.1016/j.biopha.2018.10.087
29. Santos JCD, Cruz MS, Bortolin RH, Oliveira KM, Araujo JNG, Duarte VHR, et al. Relationship between circulating VCAM-1, ICAM-1, E-selectin and MMP9 and the extent of coronary lesions. Clinics. (2018) 73:e203. doi: 10.6061/clinics/2018/e203
30. Wang Y, Sun X, Xia B, Le C, Li Z, Wang J, et al. The role of OX40l and ICAM-1 in the stability of coronary atherosclerotic plaques and their relationship with sudden coronary death. BMC Cardiovasc Disord. (2019) 19(1):272. doi: 10.1186/s12872-019-1251-8
31. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. (2017) 6(10):e005077. doi: 10.1161/JAHA.116.005077
Keywords: coronary heart disease, m6A methylation, METTL14, inflammatory markers, atherosclerosis
Citation: Guo F, He M, Hu B and Li G (2023) Levels and clinical significance of the m6A methyltransferase METTL14 in patients with coronary heart disease. Front. Cardiovasc. Med. 10:1167132. doi: 10.3389/fcvm.2023.1167132
Received: 16 February 2023; Accepted: 14 June 2023;
Published: 27 June 2023.
Edited by:
Mark Slevin, Manchester Metropolitan University, United KingdomReviewed by:
Mirjana Macvanin, University of Belgrade, Serbia© 2023 Guo, He, Hu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Li bGlnYW5nMjAyMTAyMDNAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.