
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 17 April 2023
Sec. Hypertension
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1166554
Background: High visit-to-visit blood pressure variability (BPV) and hypertension are risk factors for mild cognitive impairment (MCI) and probable dementia (PD). Few articles assessed the effect of BPV on the MCI and PD in intensive blood pressure treatment and the different functions of three types of visit-to-visit BPV: systolic blood pressure variability (SBPV), diastolic blood pressure variability (DBPV) and pulse pressure variability (PPV).
Methods: We performed a post hoc analysis of the SPRINT MIND trial. The primary outcomes were MCI and PD. BPV was measured by average real variability (ARV). The Kaplan-Meier curves were used to clarify the difference in tertiles of BPV. We fit Cox proportional hazards models to our outcome. We also did an interaction analysis between the intensive and standard groups.
Results: We enrolled 8,346 patients in the SPRINT MIND trial. The incidence of MCI and PD in the intensive group was lower than that in the standard group. 353 patients had MCI and 101 patients had PD in the standard group while 285 patients had MCI and 75 patients had PD in the intensive group. Tertiles with higher SBPV, DBPV and PPV in the standard group had a higher risk of MCI and PD (all p < 0.05). Meanwhile, higher SBPV and PPV in the intensive group were associated with an increased risk of PD (SBPV: HR(95%) = 2.1 (1.1–3.9), p = 0.026; PPV: HR(95%) = 2.0 (1.1–3.8), p = 0.025 in model 3) and higher SBPV in the intensive group was associated with an increased risk of MCI(HR(95%) = 1.4 (1.2–1.8), p < 0.001 in model 3). The difference between intensive and standard blood pressure treatment was not statistically significant when we considered the effect of the higher BPV on the risk of MCI and PD (all p for interaction >0.05).
Conclusion: In this post hoc analysis of the SPRINT MIND trial, we found that higher SBPV and PPV were associated with an increased risk of PD in the intensive group, and higher SBPV was associated with an increased risk of MCI in the intensive group. The effect of higher BPV on the risk of MCI and PD was not significantly different in intensive and standard blood pressure treatment. These findings emphasized the need for clinical work to monitor BPV in intensive blood pressure treatment.
An estimated 6.5 million Americans age 65 and older are living with Alzheimer's dementia (AD) in 2022. This number could grow to 13.8 million by 2060, barring the development of medical breakthroughs to prevent, slow or cure AD (1). Mild cognitive impairment (MCI) represented the transitional stage from the cognitive changes of normal aging to very early dementia (2) and was suggested as an individual risk factor for subsequent AD with an estimated conversion rate of 10%–15% per year (3).
Visit-to-visit blood pressure variability (BPV) represents a dynamic and characteristic physiologic feature of the cardiovascular system function (4) and is a monitoring marker for patients with hypertension (5). Systolic blood pressure variability (SBPV), diastolic blood pressure variability (DBPV) and pulse pressure variability (PPV) are three different types of visit-to-visit BPV. A meta-analysis of longitudinal studies has identified a dose-response relationship between SBPV and dementia and a long-term plan for reducing SBPV can be a target for preventing mild cognitive impairment (MCI) or probabel dementia (PD) (6). Previous articles also considered DBPV as a key risk marker for cognition decline in later life and several meta-analyses have demonstrated the association between higher DBPV and PD (7–9). However, PPV was not included among the main cognition decline risk factors because of its interdependence with other risk factors and of its dynamic nature (10).
The SPRINT-MIND trial has demonstrated that compared with standard blood pressure treatment (SBP target <140 mm Hg), intensive blood pressure (SBP target <120 mm Hg) can significantly reduce the occurrence of MCI as well as the combined occurrence of MCI or PD (11). The effect of three types of visit-to-visit BPV on the risk of MCI and PD is also unclear when take the intensive blood pressure treatment into account. The aim of the study is thus to assess the association of SBPV, DBPV and PPV with MCI and PD in both standard and intensive blood pressure treatment.
We performed a post hoc analysis of the SPRINT (Systolic Blood Pressure Intervention Trial) MIND (Memory and Cognition in Decreased Hypertension) trial. Data were obtained from the National Institutes of Health Biologic Specimen and Data Repository Information Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/sprint/).
The design, rationale, protocols, and main results of the SPRINT MIND trial have been published previously (12, 13). The SPRINT MIND trial was conducted in 102 clinical sites in the United States and enrolled 9,361 participants[mean age, 67.9 years; 3,332 women (35.6%)]. All participants had no diagnosis of dementia, prevalent diabetes mellitus, or history of stroke. Participants were randomly assigned to a systolic blood pressure target of either less than 140 mm Hg (the standard group) or less than 120 mm Hg (the intensive group). The purpose of the SPRINT MIND trial was to describe the effect of intensive blood pressure treatment on the rate of MCI and PD compared with standard blood pressure treatment. In our study, we defined the exposure period as the first 600 days of the SPRINT MIND trial. We used the data of blood pressure measured during the exposure period to calculate the BPV. This was consistent with the previous post hoc analyses of the SPRINT MIND trial (14). Moreover, we could ensure the sample size since no patients had MCI or PD in the first 600 days. The outcomes were recorded during the subsequent SPRINT MIND follow-up period.
Figure 1 shows the delineation of our study population. 9,361 participants were included in this study, while we excluded 737 participants who had missing records of blood pressure and MCI or PD. Among these participants, there were 14 patients without the records of blood pressure, 732 patients without the records of MCI and PD, and 9 patients without all of the records of blood pressure, MCI and PD. In addition, 254 participants were excluded because their blood pressure records were less than 4 times and 24 participants were excluded because the follow-up days were less than 600 days. At last, 8,346 participants were enrolled.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured at baseline, monthly for the first 3 months and every 3 months thereafter. The mean counts of blood pressure measurement was 10.8 ± 2.5. Pulse pressure (PP) was calculated as follows: PP = SBP − DBP. Mena et al. (15) concluded that the average real variability (ARV) index had a greater predictive value than the standard deviation (SD) and was more useful for determining therapeutic measures aimed at the treatment of BPV. So, we used ARV for blood pressure records with at least four follow-up data to assess the systolic blood pressure variability (SBPV), diastolic blood pressure variability (DBPV) and pulse pressure variability (PPV). Meanwhile, the statistical results measured by SD were also showed in Supplementary Tables S1, S2. Besides, MMD (the difference of maximum minus minimum) meant the delta in blood pressure and Michiaki Nagai have demonstrated the association between MMD and cardiovascular disease (16, 17). So, we also included the statistical results measured by MMD in Supplementary Tables S3, S4. ARV represented the average of absolute differences between successive types and was calculated using the following formula:
where N denotes the number of valid BP types, and k is the order of types.
The outcomes of this study were probable dementia (PD) and mild cognitive impairment (MCI). All participants were classified into 3 categories: no cognitive impairment, mild cognitive impairment, or probable dementia. MCI represents the transitional stage from the cognitive changes of normal aging to very early dementia (4). The diagnoses of MCI and PD were using standardized diagnostic criteria by 2 adjudicators independently (18, 19). The SPRINT MIND trial followed a 3-step process to ascertain the cognitive status among global cognitive function, learning and memory and processing speed (6). The critical criteria that distinguish MCI from dementia were the preservation of independence in functional abilities (ADLs and IADLs) and lack of significant impairment in social or occupational functioning (20).
We used frequencies and percentages to reflect the categorical variables and means ± standard deviations or medians (intertertile ranges) to express the continuous variables based on the data distribution. Chi-square analysis was used to evaluate the differences in categorical variables. We can identify any significant differences between groups via the two-tailed t-test (normal distribution) or the Mann–Whitney U test (skewed distribution). The Kolmogorov–Smirnov test assessed the normal distribution of data.
The Kaplan-Meier curves were used to indicate the difference in the risk of MCI and PD among different BPV tertiles separately and the logrank test was used for comparison between different Kaplan-Meier curves. Cox proportional hazards models were used to describe the effect of SBPV, DBPV or PPV on the rate of MCI and PD. We used a variety of models: Model 1 adjusted age, BMI, sex and race. Model 2 adjusted age, BMI, sex, race, smoke, estimated glomerular filtration rate, Framingham 10-year cardiovascular disease risk score, subclinical cardiovascular disease and total cholesterol. Model 3 further adjusted the mean blood pressure (SBP, DBP or PP) based on the adjustment of Model 2. All models met the proportional hazard assumption. We also performed interaction analyses between different blood pressure treatment arms (standard blood pressure treatment vs. intensive blood pressure treatment). All analyses were performed using the statistical software package R (The R Foundation; http://www.R-project.org). Statistical significance was set at p < 0.05.
8,346 patients were eventually enrolled in the analysis, including 4,170 in the intensive blood pressure treatment group (the intensive group) and 4,176 in the standard blood pressure treatment group (the standard group). The average age of the participants was 67.7 ± 9.2 years and there were 2,926 (35.1%) women in the study. Supplementary Figures S1, S2 demonstrated the frequency and intervals of BP measurements during the exposure period. As shown in Table 1, the visit-to-visit SBP, DBP and PP of the intensive group were significantly lower than those of the standard group (all p < 0.05). Notably, SBPV, DBPV and PPV in the intensive group were also significantly lower than that in the standard group (SBPV: 12.4 ± 5.2 mmHg vs. 11.5 ± 4.9 mmHg, p < 0.001; DBPV: 7.4 ± 2.8 mmHg vs. 7.0 ± 2.7 mmHg, p < 0.001; PPV: 8.4 ± 3.5 mmHg vs. 7.6 ± 3.3 mmHg, p < 0.001). There was no significant difference in other variables between the two groups, including demographic and laboratory data (all p > 0.05).
In terms of outcome, the incidence of MCI and PD in the intensive group was lower than that in the standard group (MCI: 6.8% vs. 8.4%, p = 0.005; PD: 1.8% vs. 2.4%, p = 0.049).
As to the antihypertension (AH) medications, in the whole population, the average of the types of AH medications in the standard group was 1.9 ± 1.0 and patients in the intensive group took an average of 2.6 ± 1.0 types of AH medications. The types of AH medications for the different BPV tertiles were demonstrated in Supplementary Table S3.
As was shown in Figure 2, in standard blood pressure treatment, tertiles with higher SBPV, DBPV, and PPV had a higher risk of MCI (SBPV: p < 0.0001; DBPV: p = 0.0257; PPV: p <0.0001) and PD (SBPV: p < 0.0001; DBPV: p = 0.0038; PPV: p =0.0003) respectively. As was shown in Figure 3, in intensive blood pressure treatment, tertiles with higher SBPV and PPV had a higher risk of MCI (SBPV: p = 0.0037; PPV: p = 0.0018) and PD (SBPV: p = 0.0004; PPV: p < 0.0001). However, there was no significant difference in the risk of MCI or PD among different DBPV tertiles (p = 0.3452 for MCI; p = 0.4682 for PD) in the intensive blood pressure treatment group.
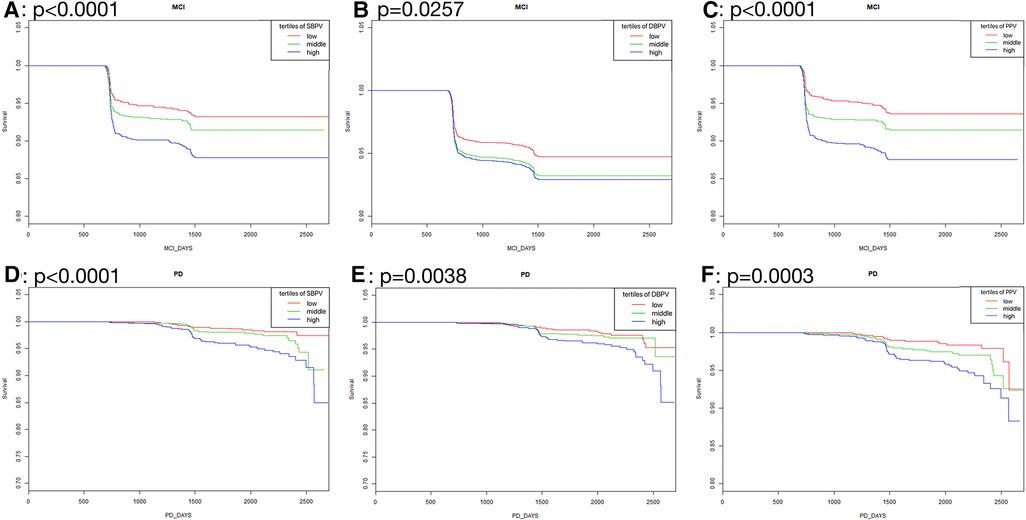
Figure 2. Kaplan-Meier curves analysis among tertiles of BPV in standard blood pressure treatment. (A–C) Showed the rates free of mild cognitive impairment in follow-up after stratification by SBPV, DBPV and PPV in standard blood pressure treatment; (D–F) showed the rates free of probable dementia in follow-up after stratification by SBPV, DBPV and PPV in standard blood pressure treatment.
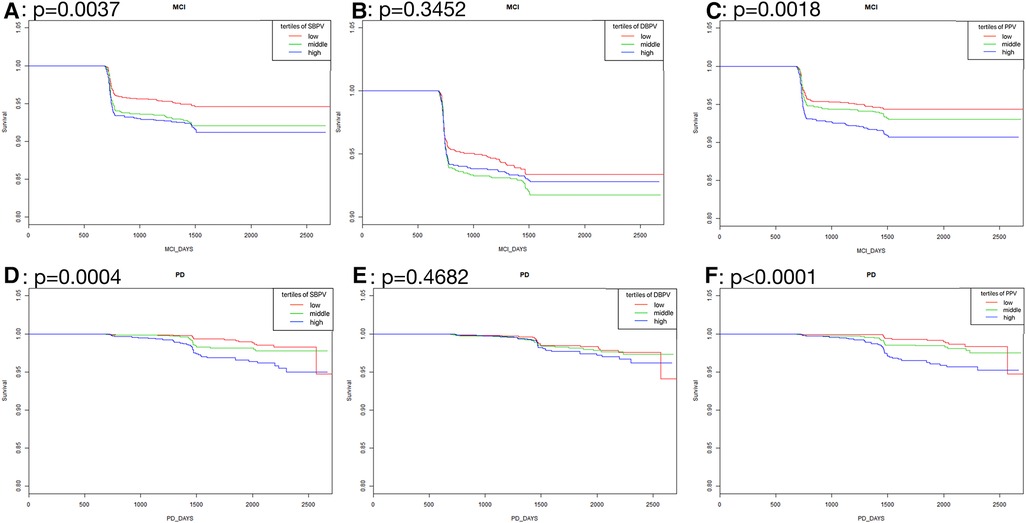
Figure 3. Kaplan-Meier curves analysis among tertiles of BPV in intensive blood pressure treatment. (A–C) Showed the rates free of mild cognitive impairment in follow-up after stratification by SBPV, DBPV and PPV in intensive blood pressure treatment; (D–F) showed the rates free of probable dementia in follow-up after stratification by SBPV, DBPV and PPV in intensive blood pressure treatment.
We used three COX regression models to analyze the association between BPV and MCI. As was shown in Table 2, in the standard group, the risks of MCI in T3 of SBPV and PPV were significantly higher than in T1 in Model 1 and Model 2. In Model 3, which considered visit-to-visit mean blood pressure, the risks of MCI in T3 of these three types were significantly higher than that in T1.(SBPV: HR(95%) = 1.7 (1.3–2.2), p < 0.001; DBPV: HR(95%) = 1.3 (1.0–1.7), p = 0.048; PPV: HR(95%) = 1.6 (1.2–2.2), p = 0.001).
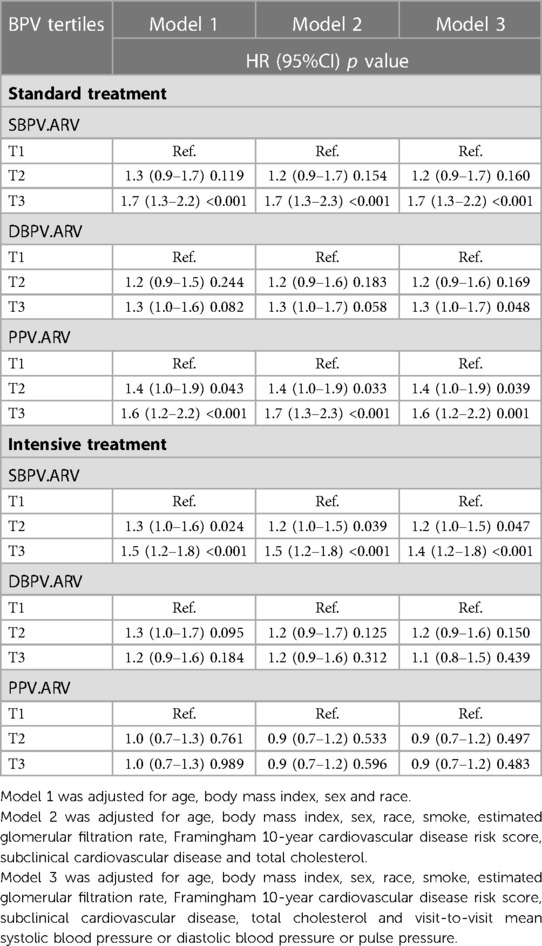
Table 2. Association between BPV (SBPV, DBPV and PPV) and MCI in different blood pressure treatment arms.
In the intensive group, only the highest tertile of SBPV remained a significant difference in the risk of MCI compared with the lowest in Model 1 and Model 2 (SBPV: HR(95%) = 1.5 (1.2–1.8), p < 0.001 in Model 2; DBPV: HR(95%) = 1.2 (0.9–1.6), p = 0.312 in Model 2; PPV: HR(95%) = 0.9 (0.7–1.2), p = 0.596 in Model 2). The adjustment of visit-to-visit mean blood pressure in model 3 did not change the association between higher SBPV and the growing risk of MCI (HR(95%) = 1.4 (1.2–1.8), p < 0.001).
In the standard group, elevated BPV was significantly associated with an increased risk of MCI in Model 3. In the intensive group, only higher SBPV was statistically significant with the increased risk of MCI.
We adjusted the same models for the association between different BPV and PD. As was shown in Table 3, in the standard group, the risk of PD in T3 of SBPV and DBPV were significantly higher than in T1 in Model 1 (SBPV: HR(95%) = 2.5 (1.4–4.4), p = 0.002; DBPV: HR(95%) = 2.0 (1.2–3.3), p = 0.008). In Model 2 with multiple covariates adjusted, the risks of MCI in T3 of SBPV and PPV were significantly higher than that in T1 (SBPV: HR(95%) = 2.4 (1.4–4.3), p = 0.003; PPV: HR(95%) = 1.8 (1.0–3.4), p = 0.049). In Model 3, which considered visit-to-visit mean blood pressure, the risks of PD in T3 of all these three types were significantly higher than in T1.(SBPV: HR(95%) = 2.4 (1.4–4.4), p = 0.003; DBPV: HR(95%) = 1.8 (1.1–3.0), p = 0.030; PPV: HR(95%) = 2.0 (1.1–3.7), p = 0.026).
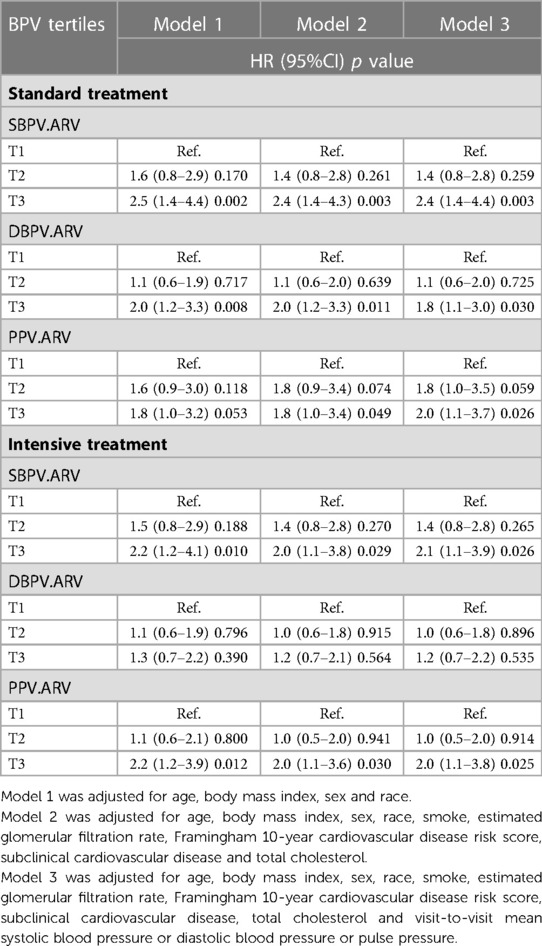
Table 3. Association between BPV (SBPV, DBPV and PPV) and PD in different blood pressure treatment arms.
In the intensive group, the highest tertiles of SBPV and PPV, compared with the lowest, increased the risk of PD in Model 1. After adjusting the covariates in Model 2, the highest tertiles of SBPV and PPV remained a significant difference with the risk of MCI compared with the lowest (SBPV: HR(95%) = 2.0 (1.1–3.8), p = 0.029; DBPV: HR(95%) = 1.2 (0.7–2.1); p = 0.564; PPV: HR(95%) = 2.0 (1.1–3.6), p = 0.030). The adjustment of visit-to-visit mean blood pressure in model 3 did not change the association between higher SBPV or PPV and the growing risk of MCI (SBPV: HR(95%) = 2.1 (1.1–3.9), p = 0.026; PPV: HR(95%) =2.0 (1.1–3.8), p = 0.025).
In the standard group, elevated BPV was significantly associated with an increased risk of PD in Model 3. In the intensive group, higher SBPV and PPV were statistically significant with the increased risk of PD.
As was shown in Table 4, the interaction analysis was used in all three different BPV (SBPV, DBPV and PPV) types (all p for interaction >0.05). It demonstrated that compared with standard blood pressure treatment, intensive blood pressure treatment made no difference in the effect of the higher tertile of BPV on the risk of MCI and PD.
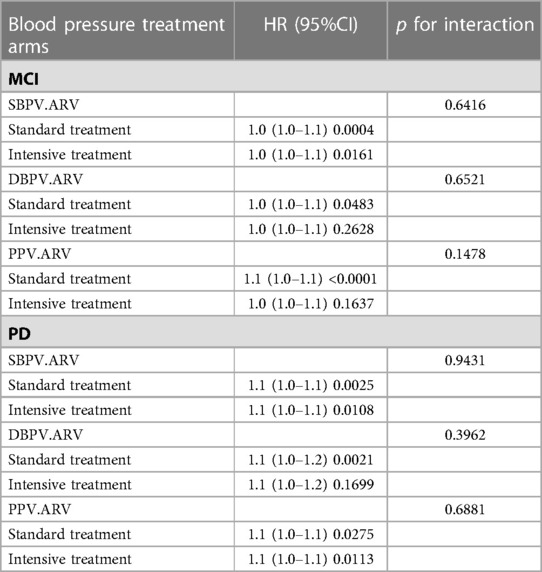
Table 4. Interaction analysis between different blood pressure treatment arms (standard blood pressure treatment vs. Intensive blood pressure treatment) on the risk of MCI or PD by BPV (SBPV, DBPV and PPV).
In this post hoc analysis of the SPRINT MIND trial, we investigated the association of three types of BPV with MCI and PD in intensive and standard blood pressure treatment. In the standard group, we found that the increase of SBPV, DBPV and PPV showed a positive relationship with the higher risk of MCI and PD. In the intensive group, we found higher SBPV and PPV were associated with an increased risk of PD and that higher SBPV was associated with an increased risk of MCI. Moreover, the difference between intensive and standard blood pressure treatment was not statistically significant when we considered the effect of the higher BPV on the risk of MCI and PD. These findings emphasized the need for clinical work to monitor BPV in intensive blood pressure treatment.
In the standard group, previous studies assessed the different effects of three types of BPV on different domains of cognition. Tan Lai Zhou (21) evaluated the effect of higher SBPV and DBPV on cognitive impairment by memory function, information processing speed and executive function in 40- to 75-year-old individuals from The Maastricht Study. They did a further investigation into three different cognitive domains. They concluded that greater DBPV was associated with both lower information processing speed and executive function and was marginally associated with lower memory function. In contrast, greater SBPV was marginally associated with a lower memory function. In addition, after a cohort study of the association of SBPV and DBPV with the increased risk of incident dementia in 6,506 elderly individuals followed-up for 8 years, Annick Alpérovitch (22) presented that an increase of 1 standard deviation in the coefficient of SBPV or DBPV was associated with an increased risk of dementia of about 10%. However, Laure Rouch (7) showed that PPV was no longer associated with cognition function in a total of 3,319 subjects from the S.AGES cohort. The possible reason was that their participants were with chronic pain, type 2 diabetes mellitus or atrial fibrillation, which might affect the basic level of pulse pressure.
Moreover, this study further explored the relationship between various BPV and MCI or PD in intensive blood pressure treatment. Previous research has found that SBPV was associated with the development of probable dementia in intensive blood pressure treatment (14). Our study found that in the intensive group, the increase of SBPV significantly increased the risk of PD and MCI, and the increase of PPV significantly increased the risk of PD. However, our study did not find a relationship between DBPV and PD or MCI. There was a mechanism that could explain the different effects of SBPV, DBPV or PPV on the risk of MCI and PD in intensive blood pressure treatment. Smith EE (23) have shown that BPV could be responsible for cerebral small vessel disease and which was one of the possible mechanisms of MCI and PD (24). Meanwhile, white matter hyperintensities (WMH) represented the progression of cerebral small vessel disease (25) and Van Middelaar T (26) indicated that higher SBPV and PPV were associated with more progression of WMH while DBPV was not associated with WMH progression. Although the SPRINT MIND trial has shown that intensive blood pressure treatment was associated with slower progression of white matter hyperintensities (6), it did not change the difference in the association between three types of BPV and the progression of white matter hyperintensities. Therefore, according to the progression of WMH, there might be a strong association of SBPV or PPV with cerebral small vessel disease, while DBPV had no such correlation.
Compared with standard blood pressure treatment, we found that the strong relationship between higher BPV and increased risk of MCI and PD did not change after implementing intensive blood pressure treatment. Our findings were consistent with the previous study where Adam de Havenon (17) showed that SBPV remained a risk factor for developing cognitive impairment despite the excellent blood pressure treatment in the SPRINT MIND trial. We further investigated DBPV and PPV measured by ARV and found the relationship was consistent in all three types of BPV. Large numbers of previous studies that showed BPV was a risk factor for incident dementia independent of mean BP levels were based on standard blood pressure treatment (1, 8, 27). Our study made a supplement that BPV was still an independent risk factor for MCI and PD in intensive blood pressure treatment. Various studies have found different mechanisms to explain the independent effect of elevated BPV on the increased risk of MCI and PD. Firstly, higher BPV was associated with endothelial injury (28), and injury in cerebral microvessels may compromise the function of the blood-brain barrier and increase vascular permeability and protein extravasation in cerebral parenchyma (29). Secondly, BPV could be responsible for cerebral small vessel diseases, including white matter lesions, microbleeds, silent infarcts, and brain atrophy, resulting in MCI and PD (18, 30). Moreover, elevated short-term BPV is associated with lower cerebrovascular reactivity (CVR), independent of mean BP levels (31). CVR was a marker of cerebrovascular dysfunction or prodromal disease, which represented the ability of the brain's vessels to dilate and constrict in response to vasoactive stimuli (32).
The strengths of our study included the use of three types of BPV and the well-characterized, large study population, which made it possible to adjust for a large variety of covariates. The adjustment of visit-to-visit mean blood pressure in model 3 increased the credibility of the article.
We acknowledged that there were some limitations of our study. First of all, it was a post hoc analysis of a trial that conformed to neither the population nor the randomization model of statistical inference. Secondly, the trial excluded persons with type 2 diabetes, previous stroke, advanced kidney disease, or symptomatic heart failure which limited our results to patients without corresponding basic diseases. Thirdly, the assessment of cognitive function was based on the endpoint events (MCI or PD), which were unable to evaluate either different cognitive dimensions or subtypes of MCI and PD.
In this post hoc analysis of the SPRINT MIND trial, we found that, in standard blood pressure treatment, elevated BPV, regardless of using any of the three BPV types, was significantly associated with an increased risk of MCI. In intensive blood pressure treatment, higher SBPV and PPV were associated with an increased risk of PD, and higher SBPV was associated with an increased risk of MCI. The effect of higher BPV on the risk of MCI and PD was not significantly different in intensive and standard blood pressure treatment. These findings indicated visit-to-visit BPV as a risk factor for MCI and PD, independent of mean blood pressure. Moreover, it was of great value for clinical work to monitor BPV, especially SBPV or PPV, in both standard and intensive blood pressure treatment.
Publicly available datasets were analyzed in this study. This data can be found here: the National Institutes of Health Biologic Specimen and Data Repository Information Coordinating Center at https://biolincc.nhlbi.nih.gov/studies/sprint.
The studies involving human participants were reviewed and approved by the Ethical Committee of Beijing Anzhen Hospital. The patients/participants provided their written informed consent to participate in this study.
HG and ZY: wrote the manuscript. YT and JY: applied for the database and performed the statistical analysis. XM and ZZ: revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1166554/full#supplementary-material.
1. 2022 Alzheimer's disease facts and figures. Alzheimers Dement. (2022) 18(4):700–89. doi: 10.1002/alz.12638
2. Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, et al. Natural history of mild cognitive impairment in older persons. Neurology. (2002) 59(2):198–205. doi: 10.1212/wnl.59.2.198
3. Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. (2001) 58(12):1985–92. doi: 10.1001/archneur.58.12.1985
4. Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens. (2018) 20(7):1133–7. doi: 10.1111/jch.13304
5. Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. Br Med J. (2016) 354:i4098. doi: 10.1136/bmj.i4098
6. Jia P, Lee HWY, Chan JYC, Yiu KKL, Tsoi KKF. Long-term blood pressure variability increases risks of dementia and cognitive decline: a meta-analysis of longitudinal studies. Hypertension. (2021) 78(4):996–1004. doi: 10.1161/HYPERTENSIONAHA.121.17788
7. Rouch L, Cestac P, Sallerin B, Piccoli M, Benattar-Zibi L, Bertin P, et al. Visit-to-visit blood pressure variability is associated with cognitive decline and incident dementia: the S.AGES cohort. Hypertension. (2020) 76(4):1280–8. doi: 10.1161/HYPERTENSIONAHA.119.14553
8. Peters R, Xu Y, Eramudugolla R, Sachdev PS, Cherbuin N, Tully PJ, et al. Diastolic blood pressure variability in later life may be a key risk marker for cognitive decline. Hypertension. (2022) 79(5):1037–44. doi: 10.1161/HYPERTENSIONAHA.121.18799
9. de Heus RAA, Tzourio C, Lee EJL, Opozda M, Vincent AD, Anstey KJ, et al. Association between blood pressure variability with dementia and cognitive impairment: a systematic review and meta-analysis. Hypertension. (2021) 78(5):1478–89. doi: 10.1161/HYPERTENSIONAHA.121.17797
10. Yang X, Hidru TH, Han X, Zhang X, Liu Y, Wang B, et al. Link between elevated long-term resting heart rate variability and pulse pressure variability for all-cause mortality. J Am Heart Assoc. (2020) 9(6):e014122. doi: 10.1161/JAHA.119.014122
11. SPRINT MIND Investigators for the SPRINT Research Group; Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, et al. Effect of intensive vs standard blood pressure treatment on probable dementia: a randomized clinical trial. JAMA. (2019) 321(6):553–61. doi: 10.1001/jama.2018.21442
12. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, et al. The design and rationale of a multicenter clinical trial comparing two strategies for treatment of systolic blood pressure: the systolic blood pressure intervention trial (SPRINT). Clin Trials. (2014) 11(5):532–46. doi: 10.1177/1740774514537404 Erratum in: Clin Trials. 2017;14(2):222.24902920
13. SPRINT Research Group; Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MVet al.A randomized trial of intensive versus standard blood-pressure treatment. N Engl J Med. (2015) 373(22):2103–16. doi: 10.1056/NEJMoa1511939
14. De Havenon A, Anadani M, Prabhakaran S, Wong KH, Yaghi S, Rost N. Increased blood pressure variability and the risk of probable dementia or mild cognitive impairment: a post hoc analysis of the SPRINT MIND trial. J Am Heart Assoc. (2021) 10(18):e022206. doi: 10.1161/JAHA.121.022206
15. Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. (2005) 23(3):505–11. doi: 10.1097/01.hjh.0000160205.81652.5a
16. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit-to-visit blood pressure variations: new independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens. (2011) 5(3):184–92. doi: 10.1016/j.jash.2011.03.001
17. Cheng Y, Li J, Ren X, Wang D, Yang Y, Miao Y, et al. Visit-to-visit office blood pressure variability combined with framingham risk score to predict all-cause mortality: a post hoc analysis of the systolic blood pressure intervention trial. J Clin Hypertens. (2021) 23(8):1516–25. doi: 10.1111/jch.14314
18. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jr JC, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7(3):263–9. doi: 10.1016/j.jalz.2011.03.005
19. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. (2011) 7(3):270–9. doi: 10.1016/j.jalz.2011.03.008
20. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. (2014) 312(23):2551–61. doi: 10.1001/jama.2014.13806
21. Zhou TL, Kroon AA, van Sloten TT, van Boxtel MPJ, Verhey FRJ, Schram MT, et al. Greater blood pressure variability is associated with lower cognitive performance. Hypertension. (2019) 73(4):803–11. doi: 10.1161/HYPERTENSIONAHA.118.12305
22. Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues JF, Richard-Harston S, et al. Blood pressure variability and risk of dementia in an elderly cohort, the three-city study. Alzheimers Dement. (2014) 10(5 Suppl):S330–7. doi: 10.1016/j.jalz.2013.05.1777
23. Smith EE, Beaudin AE. New insights into cerebral small vessel disease and vascular cognitive impairment from MRI. Curr Opin Neurol. (2018) 31(1):36–43. doi: 10.1097/WCO.0000000000000513
24. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effectsofantihypertensive- drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. (2010) 375:906–15. doi: 10.1016/S0140-6736(10)60235-8
25. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. (2019) 18(7):684–96. doi: 10.1016/S1474-4422(19)30079-1
26. Van Middelaar T, Richard E, van Charante EP M, van Gool WA, van Dalen JW. Visit-to-visit blood pressure variability and progression of white matter hyperintensities among older people with hypertension. J Am Med Dir Assoc. (2019) 20(9):1175–7.e1. doi: 10.1016/j.jamda.2019.04.003
27. Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, et al. Blood pressure variability and cerebral small vessel disease: a systematic review and meta-analysis of population-based cohorts. Stroke. (2020) 51:82–9. doi: 10.1161/STROKEAHA.119.026739
28. Diaz KM, Veerabhadrappa P, Kashem MA, Thakkar SR, Feairheller DL, Sturgeon KM, et al. Visit-to-visit and 24-h blood pressure variability: association with endothelial and smooth muscle function in African Americans. J Hum Hypertens. (2013) 27:671–7. doi: 10.1038/jhh.2013.33
29. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci. (2011) 12(12):723–38. doi: 10.1038/nrn3114
30. Saji N, Toba K, Sakurai T. Cerebral small vessel disease and arterial stiffness: tsunami effect in the brain? Pulse. (2016) 3(3–4):182–9. doi: 10.1159/000443614
31. Sible IJ, Jang JY, Dutt S, Yew B, Alitin JPM, Li Y, et al. Older adults with higher blood pressure variability exhibit cerebrovascular reactivity deficits. Am J Hypertens. (2023) 36(1):63–8. doi: 10.1093/ajh/hpac108
Keywords: systolic blood pressure variability, diastolic blood pressure variability, pulse pressure variability, mild cognitive impairment, probable dementia
Citation: Guo H, Tan Y, Yao Z, Zhang Z, Yan J and Meng X (2023) Effect of visit-to-visit blood pressure variability on mild cognitive impairment and probable dementia in hypertensive patients receiving standard and intensive blood pressure treatment. Front. Cardiovasc. Med. 10:1166554. doi: 10.3389/fcvm.2023.1166554
Received: 15 February 2023; Accepted: 28 March 2023;
Published: 17 April 2023.
Edited by:
Michiaki Nagai, Hiroshima City Asa Hospital, JapanReviewed by:
Yuda Turana, Atma Jaya Catholic University of Indonesia, Indonesia© 2023 Guo, Tan, Yao, Zhang, Yan and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Meng bWVuZ3hpYW9mZW5nX2hrenl5QDE2My5jb20=
Specialty Section: This article was submitted to Hypertension, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.