- 1Department of Urology, Institute of Urology, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
Objectives: The objective of this study was to explore the association between nocturia and hypertension in a large, nationally representative adult sample.
Methods: We used data from 2005 to 2016 National Health and Nutritional Examination Surveys (NHANES). A total of 29,505 participants aged 20 years old or older were included. A participant was considered to have nocturia if he or she had two or more voiding episodes at night. Multivariable logistic regression models were used to explore the association between nocturia and hypertension.
Results: Participants with nocturia were associated with a higher risk of hypertension (OR, 1.36; 95% CI, 1.28–1.45). Interaction tests revealed no significant effect of sex, age, race, or body mass index on the association of nocturia with hypertension. As the severity of nocturia increases, the risk of hypertension increases (P for trend <0.0001). In addition, nocturia was also related to different grades of hypertension (II vs. I: OR, 1.34, 95% CI, 1.16–1.55; III vs. I: OR, 1.67, 95% CI, 1.32–2.13).
Conclusion: In this cross-sectional study, our results suggest that nocturia is associated with an increased risk for hypertension.
1. Introduction
Nocturia was defined as a person who had one or more interruptions in sleep due to the need to urinate (1). The definition is currently controversial. One voiding episode at night is common, and two or more voiding episodes nightly were considered to be more clinically meaningful. Nocturia is a clinical manifestation of lower urinary tract symptoms and can result in sleep loss, which is related to daytime sleepiness and a decline in quality of life. According to the data from the 2005–2012 NHANES, the prevalence of ≥2 voids/night was approximately 34.9% in women and 30.5% in men (2). In addition, the prevalence of ≥1 void/night increases with age in men (20–39 years, 56.8%; 40–59 years, 70.2%; ≥60 years, 82.7%) and women (20–39 years, 68.9%; 40–59 years, 74.3%; ≥60 years, 84.7%) (3). Although at least one urination overnight is sufficient to define nocturia, two or more voidings at night have been reported to be associated with more adverse clinical outcomes (4). A cross-sectional study including 14,114 participants reported that nocturia (≥2 voids/night) was related to cardiovascular disease (CVD), and the risk of CVD increased with increasing frequency of voiding at night (5). Among them, participants who urinated more than four times per night had a 74% increased risk of CVD (5). Another study enrolled 15,988 subjects aged 20 years old or older and reported that nocturia (≥2 voids/night) was a predictor of mortality and that the mortality risk increased with the increased number of voiding episodes nightly (6). Dutoglu et al. reported that ≥2 voids at night was related to insomnia, polypharmacy, decreased walking speed and recurrent falls for older women (4). The occurrence of nocturia is a condition with a variety of factors, including drinking too much water at night, decreased bladder capacity, increased postvoid residual volume, overactive bladder, sleep disorders, hypertension, diabetes and medications (e.g., diuretics, calcium channel blockers) (7, 8). Appropriate lifestyle interventions and treatment of underlying diseases are sufficient to reduce the incidence of nocturia to some extent.
Hypertension is a medical condition that significantly increases the risk of heart, brain, kidney and other diseases. According to WHO statistics, as of 2019, there were approximately 1.28 billion people aged 30–79 years with high blood pressure worldwide, which is 2 times that of 1990. High blood pressure is related to a large burden of cardiovascular disease and premature death. In 2015, approximately 7.8 million deaths were related to systolic blood pressure ≥140 mmHg globally, which was mainly attributed to ischemic heart disease, ischemic stroke and hemorrhagic stroke (9). There are also multiple risk factors for hypertension, including high sodium intake, low potassium intake, smoking, alcohol consumption, psychological stress, sleep disorders, obesity, unhealthy diet, and noise exposure (10). If the risk of hypertension is reduced, it will help reduce the global economic burden.
The relationship between nocturia and hypertension has been reported previously. Rahman et al. conducted a systematic review and meta-analysis including 25 studies to explore the association between nocturia and hypertension. The results revealed that ≥1 voiding episode at night was related to a 1.2-fold higher risk of hypertension, while ≥2 voiding episodes at night were associated with a 1.3-fold increased risk of hypertension (11). A cross-sectional analysis of data from a prospective national cohort enrolled 22,674 obstructive sleep apnea (OSA) patients, including 11,332 participants with hypertension and 11,342 subjects without hypertension. The results reported that OSA patients with nocturia (≥1 void/night) were more likely to have hypertension and that the risk of hypertension increased with increasing nocturia severity for OSA patients with 2 or more voiding episodes at night (12).
These findings suggest different degrees of an association between nocturia and hypertension. However, there is a paucity of cross-sectional studies with large samples of natural populations on this potential association that avoided confounding factors. Moreover, no study has explored the association between nocturia and different degrees of hypertension. Therefore, we conducted this study to comprehensively explore the relationship between nocturia and hypertension in adults 20 years old or older among the large population of the United States.
2. Methods
2.1. Data and population
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative cross-sectional survey conducted in the United States to assess the health and nutrition status of adults and children. The data of our study were obtained by consolidating demographic data, examination data, laboratory data and questionnaire data from NHANES 2005–2016, which can be downloaded from its website (https://www.cdc.gov/nchs/nhanes/index.htm). The Research Ethics Review Board at the National Center for Health Statistics (NCHS) approved the survey protocol.
In total, 60,936 participants were investigated in the NHANES 2005–2016. We excluded participants who were under 20 years old (N = 26,756). Participants without questionnaire information about nocturia (N = 4,633) and hypertension (N = 42) were also excluded. Finally, 29,505 participants aged 20 years old or older with complete information about nocturia, hypertension and model covariates were enrolled in this study.
2.2. Exposure and outcome
Whether the participants had nocturia was assessed by using a questionnaire. “During the past 30 days, how many times per night did you most typically get up to urinate from the time going to bed at night to waking up in the morning?” The responses were divided into 0 void/night, 1 void/night, 2 voids/night, 3 voids/night, 4 voids/night and 5 voids/night. Participants with ≥2 voids/night were regarded as having nocturia in our study.
Whether the participants had high blood pressure was assessed in several ways. First, if a person responded “yes” to the question “Have you ever been told by a doctor or other health professional that you have hypertension or high blood pressure?”, he was considered to have hypertension. Second, if a participant self-reported using an antihypertensive drug, he was regarded as having hypertension. Finally, if a participant had systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg in the physical examination, he was also regarded as a patient with hypertension. When data were missing for one assessment method, we used another method, and a participant who met any one of the above three methods was considered to have high blood pressure.
Among 29,505 participants, 26,050 subjects underwent 3 blood pressure measurements. We took the average of the results of the 3 blood pressure measurements as the participants' blood pressure value, according to which the participants' blood pressure status were divided into 4 levels (nonhypertension: systolic pressure <140 mmHg and diastolic pressure <90 mmHg; hypertension grade I: systolic pressure 140 mmHg–159 mmHg and/or diastolic pressure 90 mmHg–99 mmHg; hypertension grade II: systolic pressure 160 mmHg–179 mmHg and/or diastolic pressure 100 mmHg–109 mmHg; hypertension grade III: systolic pressure ≥180 mmHg and/or diastolic pressure ≥110 mmHg). Among them, there were 21,386 participants without hypertension, 3,451 participants with hypertension grade I, 922 participants with hypertension grade II and 291 participants with hypertension grade III.
2.3. Covariates
Based on previous studies, potential confounding variables that may affect the association between nocturia and hypertension were included in the multivariable models. Those covariates included gender, age, race, education level, marital status, family poverty income ratio (PIR), body mass index (BMI), smoking, alcohol consumption, diabetes, depression, cardiovascular disease (CVD), cancer, cholesterol and triglycerides level. The race of participants was divided into 4 categories, including Non-Hispanic white people, Non-Hispanic black people, Mexican American and others. Age (20–39, 40–59, ≥60), education level (less than high school, high school or equivalent and college or above), marital status (married, separated and never married), family PIR (<1.3, 1.3–3.5, >3.5), BMI (<25 kg/m2, 25–29.9 kg/m2, ≥30 kg/m2), smoking (never smoker, former smoker, current smoker), and alcohol consumption (nondrinker, low to moderate drinker, heavy drinker) were divided into 3 categories. Diabetes, depression, CVD (congestive heart failure, coronary heart disease, heart disease, angina pectoris or stroke) and cancer status were divided into 2 categories. Depression was assessed by using the Nine-item Patient Health Questionnaire (PHQ-9) depression scale, and a PHQ-9 score ≥10 was considered to indicate depression. Cholesterol and triglyceride levels were regarded as continuous variables.
2.4. Statistical analysis
For the baseline characteristics, categorical variables are presented as the number of cases and percentage, while continuous variables are presented as the mean ± standard deviation. The baseline characteristics of participants were categorized by hypertension status, and the differences between the two groups were calculated by the chi-square test for categorical variables and the t test for continuous variables. A multivariable logistic regression model was used to explore the association between nocturia and hypertension. The degree of association was represented by the odds ratio (OR) and 95% confidence interval (CI). Multivariable models included the nonadjusted model 1, minimally adjusted model 2 (sex, age and race were adjusted), and fully adjusted model 3 (covariates of model 2 combined with education level, marital status, family PIR, BMI, smoking status, alcohol consumption, diabetes, depression, CVD, cancer, cholesterol and triglycerides level were adjusted). Interaction and stratified analyses were performed according to sex, age (20–39, 40–59, ≥60), race (Non-Hispanic white people, Non-Hispanic black people, Mexican American and others), and BMI (<25 kg/m2, 25–29.9 kg/m2, ≥30 kg/m2). Multinomial logistic regression was conducted between nocturia and different degrees of hypertension. The software packages R (http://www.R-project.org, The R Foundation) and Empowerstats (http://www.empowerstats.com) were used to perform all statistical analyses. P < 0.05 was regarded as statistically significant.
3. Results
3.1. Demographic characteristics
The baseline characteristics of the study participants based on blood pressure status are presented in Table 1. A total of 29,505 participants aged 20 years or older, including 14,501 males and 15,004 females, were enrolled in the study. The prevalence of hypertension and nonhypertension was 36.11% and 63.89%, respectively. Participants with hypertension were more likely to be female, ≥60 years old, non-Hispanic white, married, and have less education, lower family PIR, higher BMI, higher level of triglycerides, more alcohol consumption and smoking. In addition, the hypertension group was more likely to have diabetes, depression, CVD, and cancer.
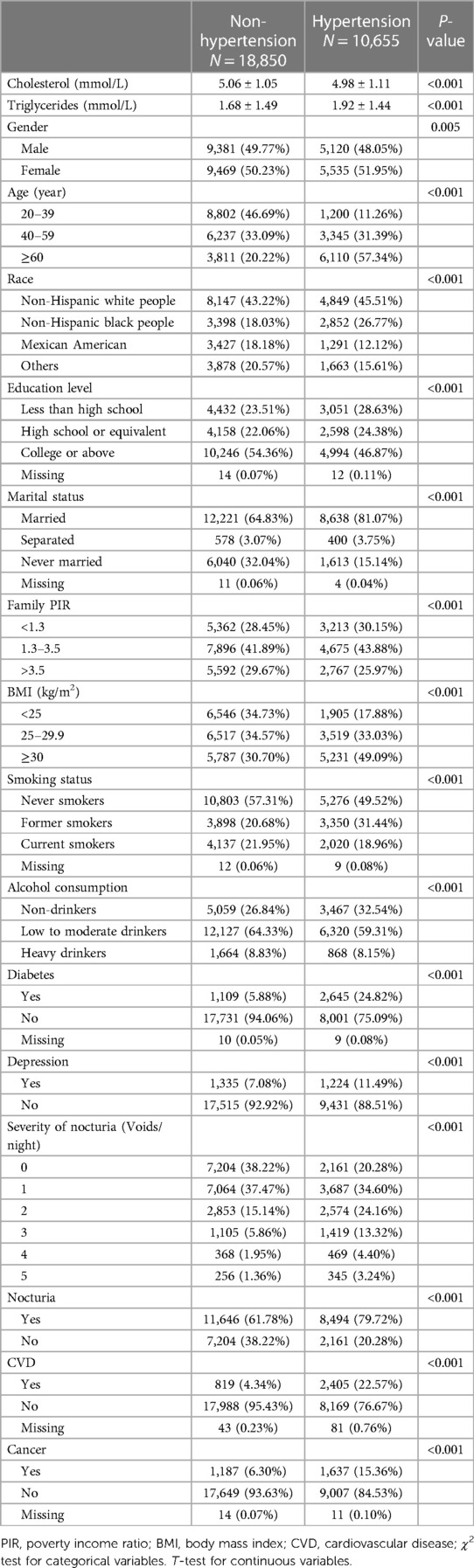
Table 1. Characteristics of participants in national health and nutritional examination surveys 2005–2016 (Chengdu, China. 2022).
3.2. Association between nocturia and hypertension
The results of multivariable logistic regression analysis are presented in Table 2. Our study revealed that nocturia (≥2 voids/night) was associated with an increased risk of hypertension (Model 1, OR, 2.56, 95% CI, 2.43–2.69; Model 2, OR, 1.69, 95% CI, 1.60–1.79). After full adjustment, participants with nocturia had a 36% higher risk of hypertension (Model 3, OR, 1.36, 95% CI, 1.28–1.45). In addition, as the frequency of voiding episodes at night increases, so does the risk of hypertension. When no variables were adjusted in Model 1, the OR values of 1 void/night vs. 0 void/night, 2 voids/night vs. 0 void/night, 3 voids/night vs. 0 voids/night, 4 voids/night vs. 0 void/night, and ≥5 voids/night vs. 0 void/night were 1.74, 3.01, 4.28, 4.25, and 4.49, respectively. The association remained stable after adjusting for gender, age, and race in Model 2. Even though all covariables included were adjusted in Model 3, participants with 1 void/night, 2 voids/night, 3 voids/night, and 4 voids/night were associated with 22%, 45%, 68%, and 53% higher risks of hypertension, respectively, than those with 0 voids/night. The risk even increased to 83% for 5 voids/night vs. 0 voids/night. The P-value of trend testing was <0.0001 for all models, which further indicated that with the increase in the severity of nocturia, the risk of hypertension increases.
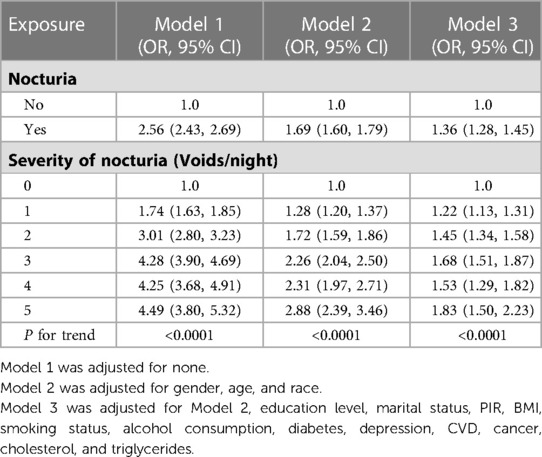
Table 2. Association between nocturia and hypertension among participants in national health and nutritional examination surveys 2005–2016 (Chengdu, China. 2022).
There was still a positive relationship between nocturia and the risk of hypertension when stratifying by sex, age, race and BMI (Table 3). No significant difference was found by the interactions testing the association between nocturia and hypertension among different groups of sex, age, race, and BMI (P for interaction = 0.16, 0.53, 0.58, 0.96), which indicated that the association was not affected by these baseline characteristics.
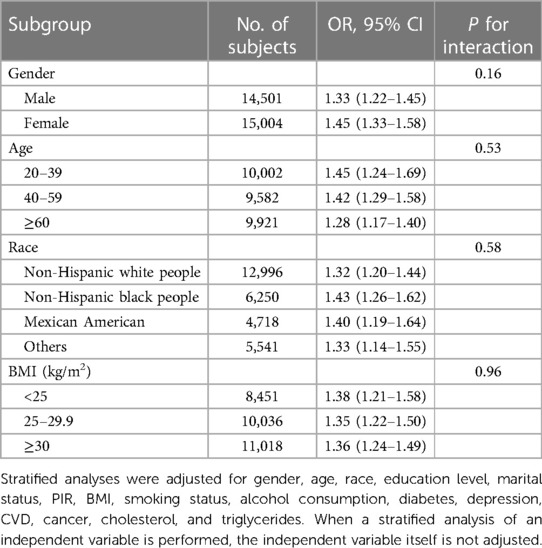
Table 3. Stratified analyses of association between nocturia and hypertension according to gender, age, race and BMI (Chengdu, China. 2022).
To explore the relationship between nocturia and different degrees of hypertension, multinomial logistic regression was conducted between nocturia and hypertension grades I, II and III (Table 4). The results revealed that in participants with hypertension grade II, those with nocturia had a 34% higher risk of hypertension grade II than those with hypertension grade I (OR, 1.34, 95% CI, 1.16–1.55). In participants with hypertension grade III, those with nocturia had a 67% higher risk of hypertension grade III than those with hypertension grade I (OR, 1.67, 95% CI, 1.32–2.13).
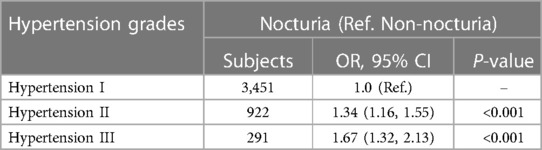
Table 4. Multinomial logistic regression models evaluating the association of nocturia and different grades of hypertension (Chengdu, China. 2022).
4. Discussion
In this study, we enrolled 29,505 participants aged 20 years or older in the US to explore the association between nocturia and hypertension. The results revealed that participants with nocturia (voiding ≥2/night) were more likely to have hypertension, and this association did not appear to be affected by sex, age, race or BMI. In addition, the higher the frequency of voiding episodes at night, the more severe the symptoms of nocturia, and the severity of nocturia was also positively associated with the risk of hypertension. Moreover, when hypertension was divided into different degrees, positive associations were also detected between nocturia and different hypertension grades. To our knowledge, this is the first cross-sectional study with a large national population to explore the association between nocturia and hypertension.
A population-based study in Finland including 6,000 subjects reported that nocturia was related to age and that the prevalence rates of nocturia increased at a constant rate with age (13). Because the incidence of prostatic hyperplasia is increased in men over 50 years of age, nocturia prevalence rises steeply after 50 years old. A multicountry sample survey including 73,607 participants showed that the incidence of hypertension increases with age (14). The mechanism underlying age-related hypertension has not been fully elucidated, but aging was reported to be associated with arterial stiffness, elevated sympathetic nervous system activity and renal sodium transport systems, which was closely related to the occurrence of hypertension (15, 16). Therefore, the above two points explain to some extent why the relationship between nocturia and hypertension was not related to age in our study.
A systematic review and meta-analysis conducted by Rahman et al. reported that the association between nocturia and a higher risk of hypertension was more robust in females than in males (11). The results of our study also revealed that there were 33% and 45% higher risks of hypertension in subjects with nocturia for males and females, respectively, although the difference was not statistically significant. Possibly because nocturia has been regarded for a long time as the main male symptom caused by BPH, studies on nocturia in female patients have rarely been reported (17). Further investigation is required to understand the mechanism of this relationship.
African Americans are known to have a higher prevalence of hypertension than white and they have a somewhat higher incidence of enuresis and nocturia. Previous studies have shown that the prevalence of nocturia and hypertension is ethnically linked, with higher rates, especially in non-Hispanic black people (3, 18, 19). A large community-based cross-sectional study conducted by Victor et al. tested the hypothesis that nocturia is a common symptom of black people with hypertension, which can be used as a screening tool for high blood pressure (20). Although non-Hispanic black people with nocturia had the highest risk of hypertension in our study, the difference among different races was not statistically significant. There is no evidence that racial disparities in the risk of hypertension and nocturia can be explained by genetic factors. Sociodemographic, environmental and behavioral factors are therefore likely to be the main contributors. Further studies are needed to explore the racial and ethnic differences in the association between nocturia and hypertension. The prevalence of nocturia and hypertension was both reported to increase with increasing body mass index (21, 22). However, the association between nocturia and hypertension was reported to be unrelated to BMI (11), which was consistent with our findings. This indicated that nocturia has the potential to be used as a screening tool to identify hypertension among people with different weights.
There are multiple causes of nocturia. In males with symptoms of BPH there seems to be at least 2 groups of nocturians. Those with a small functional bladder and those with a slightly higher blood pressure during daytime, an accumulation of sodium and then a nighttime natriuresis. The sleep apnoea is related to hypertension and increased BNP and decreased O2 tension during the apnoea leading to natriuresis and nocturia (23). Sleep deprivation leads to attenuation of the sleep dipping in BP and increased diuresis. Attenuated dipping is related to an attenuation of the increase in AVP in the beginning of the night, mostly in men. Sleep deprivation leads to attenuation of the sleep dipping in BP and increased diuresis. Attenuated dipping is related to an attenuation of the increase in AVP in the beginning of the night, mostly in men (24). With age, there is a shift from AVP increase during the night (first third) to BNP. About females, sequelae after delivery with urologic cystocele. They tend to restore some of the cystocele during night and then have to void.
There may be a bidirectional influence between nocturia and hypertension. Patients with hypertension tend to have symptoms of nocturnal urination, which is related to physiological and pathological changes caused by high blood pressure. Hypertension can result in nocturia by altering the glomerular filtration rate and tubular transport (25). Patients with hypertension may have symptoms of peripheral edema, which can lead to an increase in nocturia when reabsorbed at night. Congestive heart failure is one of the complications of hypertension and leads to increased atrial tone, resulting in the release of atrial natriuretic peptides, which increases urine output (26). Elevated systolic blood pressure was accompanied by a decrease in serum levels of the antidiuretic vasopressin at night, causing an increase in nocturia (27). However, there have been few studies on the mechanism of the effect of nocturia on hypertension. A systematic review and meta-analysis conducted by Wang et al. involving 85,838 subjects reported that short sleep was associated with a 16% higher risk of hypertension (28). In addition, poor sleep quality was also reported to be related to an increased risk of new-onset hypertension among people aged 18 years or older (29). However, having 2 or more voiding episodes at night could disturb sleep, resulting in a deterioration in sleep quality or a decrease in sleep duration, which is associated with an increase in nocturnal blood pressure and the development of hypertension during the daytime. On the one hand, short sleep can disturb circadian rhythmicity and autonomic nervous system balance, resulting in heightened sympathetic nervous system activity (30), which plays a role in the occurrence of elevated blood pressure. On the other hand, people who are sleep-deprived are in a state of stress, which can promote salt intake and reduce salt excretion from the kidney (31). However, excessive salt intake has been demonstrated to be associated with the risk of hypertension. Moreover, chronic sleep deprivation can increase the risk of obesity and diabetes by influencing glucose metabolism and promoting excessive food intake through neuroendocrine regulation (32), which has been proven to be a risk factor for high blood pressure.
This study had some limitations. First, the assessment of nocturia and hypertension for participants was based on self-questionnaires, which were subjective and not entirely accurate. Second, the grade of hypertension was defined by the results of blood pressure measurement; however, some participants may have taken antihypertensive drugs, which will have an impact on the results. Third, the study is a cross-sectional design, which can only explore the relevant associations and cannot infer causal relationships.
5. Conclusion
Nocturia was found to be associated with an increased risk of hypertension. As the severity of nocturia increases, the risk of hypertension increases. Thus, it is necessary to take appropriate measures to alleviate the symptoms of nocturia, thereby reducing the risk of hypertension. For example, the treatment of prostatic hyperplasia, sleep disorders and other diseases that can easily lead to nocturia. For patients with high blood pressure, we should ask whether they have symptoms of nocturia to provide more precise prevention and treatment. Further studies are also needed to investigate its mechanism.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Research Ethics Review Board at the National Center for Health Statistics (NCHS). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JC and ZL: contributed equally in this study. Conception and study design: JC, ZL and QD Methodology: JC, ZL and LY Acquisition of data: JC, ZL and ZP Analysis and interpretation of data: JC, ZL, JZ and KM Writing, review or revision of the manuscript: JC, ZL, LY and QD Technical and material support: QD, ZP, JZ and KM All authors contributed to the article and approved to the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (grant no: 2021YFC2009304), the Project of Science and Technology Department of Chengdu (grant no: 2021-YF05-00717-SN) and the Project of Science and Technology Department of Sichuan Province (grant no: 2021YFS0117). Sichuan Provincial Central Guidance Local Science and Technology Development Project: Molecular pathological mechanism of phthalate ester compounds inducing prostatic hyperplasia (grant no: 2022ZYD0058).
Acknowledgments
Thanks to all contributors to the NHANES database, and its policy of open access to all.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An international urogynecological association (IUGA)/international continence society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. (2010) 21(1):5–26. doi: 10.1007/s00192-009-0976-9
2. Moon S, Chung HS, Yu JM, Ko KJ, Choi DK, Kwon O, et al. The association between obesity and the nocturia in the U.S. population. Int Neurourol J. (2019) 23(2):169–76. doi: 10.5213/inj.1938062.031
3. Soysal P, Cao C, Xu T, Yang L, Isik AT, Turan Kazancioglu R, et al. Trends and prevalence of nocturia among US adults, 2005–2016. Int Urol Nephrol. (2020) 52(5):805–13. doi: 10.1007/s11255-019-02361-5
4. Dutoglu E, Soysal P, Smith L, Arik F, Kalan U, Kazancioglu RT, et al. Nocturia and its clinical implications in older women. Arch Gerontol Geriatr. (2019) 85:103917. doi: 10.1016/j.archger.2019.103917
5. Moon S, Yu SH, Chung HS, Kim YJ, Yu JM, Kim SJ, et al. Association of nocturia and cardiovascular disease: data from the national health and nutrition examination survey. Neurourol Urodyn. (2021) 40(6):1569–75. doi: 10.1002/nau.24711
6. Kupelian V, Fitzgerald MP, Kaplan SA, Norgaard JP, Chiu GR, Rosen RC. Association of nocturia and mortality: results from the third national health and nutrition examination survey. J Urol. (2011) 185(2):571–7. doi: 10.1016/j.juro.2010.09.108
7. van Doorn B, Bosch JL. Nocturia in older men. Maturitas. (2012) 71(1):8–12. doi: 10.1016/j.maturitas.2011.10.007
8. Varilla V, Samala RV, Galindo D, Ciocon J. Nocturia in the elderly: a wake-up call. Clevel Clin J Med. (2011) 78(11):757–64. doi: 10.3949/ccjm.78a.11025
9. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. Global burden of hypertension and systolic blood pressure of at least 110–115 mm Hg, 1990–2015. JAMA. (2017) 317(2):165–82. doi: 10.1001/jama.2016.19043
10. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
11. Rahman SN, Cao DJ, Monaghan TF, Flores VX, Vaysblat M, Moy MW, et al. Phenotyping the association between nocturia and hypertension: a systematic review and meta-analysis. J Urol. (2021) 205(6):1577–83. doi: 10.1097/JU.0000000000001433
12. Destors M, Tamisier R, Sapene M, Grillet Y, Baguet JP, Richard P, et al. Nocturia is an independent predictive factor of prevalent hypertension in obstructive sleep apnea patients. Sleep Med. (2015) 16(5):652–8. doi: 10.1016/j.sleep.2014.10.019
13. Tikkinen KA, Tammela TL, Huhtala H, Auvinen A. Is nocturia equally common among men and women? A population based study in Finland. J Urol. (2006) 175(2):596–600. doi: 10.1016/S0022-5347(05)00245-4
14. Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA. (2003) 289(18):2363–9. doi: 10.1001/jama.289.18.2363
15. Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals DR. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol Regul Integr Comp Physiol. (2002) 282(3):R909–16. doi: 10.1152/ajpregu.00335.2001
16. Frame AA, Wainford RD. Mechanisms of altered renal sodium handling in age-related hypertension. Am J Physiol Renal Physiol. (2018) 315(1):F1–6. doi: 10.1152/ajprenal.00594.2017
17. Drangsholt S, Peyronnet B, Arcila-Ruiz M, Sussman RD, Palmerola R, Pape DR, et al. Nocturia in female patients: current clinical features, treatment patterns and outcomes at a tertiary referral centre. Arab J Urol. (2019) 17(1):82–6. doi: 10.1080/2090598X.2019.1589792
18. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med. (2005) 165(18):2098–104. doi: 10.1001/archinte.165.18.2098
19. Markland AD, Vaughan CP, Johnson TM 2nd, Goode PS, Redden DT, Burgio KL. Prevalence of nocturia in United States men: results from the national health and nutrition examination survey. J Urol. (2011) 185(3):998–1002. doi: 10.1016/j.juro.2010.10.083
20. Victor RG, Li N, Blyler CA, Mason OR, Chang LC, Moy NPB, et al. Nocturia as an unrecognized symptom of uncontrolled hypertension in black men aged 35–49 years. J Am Heart Assoc. (2019) 8(5):e010794. doi: 10.1161/JAHA.118.010794
21. Kuwabara M, Kuwabara R, Niwa K, Hisatome I, Smits G, Roncal-Jimenez CA, et al. Different risk for hypertension, diabetes, dyslipidemia, and hyperuricemia according to level of body mass Index in Japanese and American subjects. Nutrients. (2018) 10(8):1011. doi: 10.3390/nu10081011
22. Fitzgerald MP, Litman HJ, Link CL, McKinlay JB. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. (2007) 177(4):1385–9. doi: 10.1016/j.juro.2006.11.057
23. Brown J, Yazdi F, Jodari-Karimi M, Owen JG, Reisin E. Obstructive sleep apnea and hypertension: updates to a critical relationship. Curr Hypertens Rep. (2022) 24(6):173–84. doi: 10.1007/s11906-022-01181-w
24. Kamperis K, Hagstroem S, Radvanska E, Rittig S, Djurhuus JC. Excess diuresis and natriuresis during acute sleep deprivation in healthy adults. Am J Physiol Renal Physiol. (2010) 299(2):F404–11. doi: 10.1152/ajprenal.00126.2010
25. Shipley RE, Study RS. Changes in renal blood flow, extraction of inulin, glomerular filtration rate, tissue pressure and urine flow with acute alterations of renal artery blood pressure. Am J Physiol. (1951) 167(3):676–88. doi: 10.1152/ajplegacy.1951.167.3.676
26. Chen HH, Lainchbury JG, Matsuda Y, Harty GJ, Burnett JC Jr. Endogenous natriuretic peptides participate in renal and humoral actions of acute vasopeptidase inhibition in experimental mild heart failure. Hypertension. (2001) 38(2):187–91. doi: 10.1161/01.HYP.38.2.187
27. Asplund R. Diuresis pattern, plasma vasopressin and blood pressure in healthy elderly persons with nocturia and nocturnal polyuria. Neth J Med. (2002) 60(7):276–80. 12430573
28. Wang L, Hu Y, Wang X, Yang S, Chen W, Zeng Z. The association between sleep duration and hypertension: a meta and study sequential analysis. J Hum Hypertens. (2021) 35(7):621–6. doi: 10.1038/s41371-020-0372-y
29. Yuan Y, Heizhati M, Wang L, Li M, Lin M, Gan L, et al. Poor sleep quality is associated with new-onset hypertension in a diverse young and middle-aged population. Sleep Med. (2021) 88:189–96. doi: 10.1016/j.sleep.2021.10.021
30. Kreier F, Yilmaz A, Kalsbeek A, Romijn JA, Sauerwein HP, Fliers E, et al. Hypothesis: shifting the equilibrium from activity to food leads to autonomic unbalance and the metabolic syndrome. Diabetes. (2003) 52(11):2652–6. doi: 10.2337/diabetes.52.11.2652
31. Torres SJ, Turner AI, Nowson CA. Does stress induce salt intake? Br J Nutr. (2010) 103(11):1562–8. doi: 10.1017/S000711451000098X
Keywords: nocturia, hypertension, epidemiology, clinical study, NHANES
Citation: Chen J, Liu Z, Yang L, Zhou J, Ma K, Peng Z and Dong Q (2023) Relationship between nocturia and hypertension: findings from the NHANES 2005–2016. Front. Cardiovasc. Med. 10:1165092. doi: 10.3389/fcvm.2023.1165092
Received: 13 February 2023; Accepted: 14 June 2023;
Published: 6 July 2023.
Edited by:
Guido Iaccarino, University of Naples Federico II, ItalyReviewed by:
Basil Nwaneri Okeahialam, University of Jos, NigeriaJens Djurhuus, Aarhus University, Denmark
© 2023 Chen, Liu, Yang, Zhou, Ma, Peng and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Dong ZHFpYW5nNjY2QDE2My5jb20=
†These authors have contributed equally to this work
 Junhao Chen1,2,†
Junhao Chen1,2,† Zhenghuan Liu
Zhenghuan Liu Kai Ma
Kai Ma Zhufeng Peng
Zhufeng Peng Qiang Dong
Qiang Dong