
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 04 May 2023
Sec. Structural Interventional Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1162203
Introduction: Coarctation of the aorta (CoA) is usually diagnosed and corrected early in life. Most untreated patients with CoA usually die before 50 years of age. Adult patients with concomitant CoA and severe bicuspid aortic stenosis are relatively rare and present complex management challenges without standard guidelines.
Case summary: A 63-year-old female patient with uncontrolled hypertension was admitted due to chest pain and dyspnea upon exertion (NYHA grades III). Echocardiogram showed a severely calcified and stenotic bicuspid aortic valve (BAV). A severe stenotic calcified eccentric aortic coarctation 20 mm distal to the left subclavian artery (LSA) was discovered by computed tomography (CT) angiography. Following consultation with the cardiac team and patient willingness, we performed a one-stop interventional procedure to repair both defects. First, a cheatham-platinum (CP) stent was implanted via the right femoral access, immediately distal to the LSA. Due to the markedly twisted and angled descending aortic arch, we chose to perform transcatheter aortic valve replacement (TAVR) via the left common carotid artery. The patient was discharged and followed up for 1 year without symptoms.
Discussion: Although surgery is still the main treatment for these diseases, it is not suitable for high-risk surgical patient. Transcatheter intervention for patients with severe aortic stenosis complicated with CoA simultaneously is rarely reported. The success of this procedure depends on the patient's vascular condition, the skills of the heart team, and the availability of the technical platform.
Conclusion: Our case report demonstrates the feasibility and efficacy of a one-stop interventional procedure in an adult patient with concurrent severely calcified BAV and CoA via two different vascular approaches. Transcatheter intervention, in contrast to traditional surgical approaches or two-stop interventional procedures, as a minimally invasive and novel method, offers a wider range of therapeutic methods for such diseases.
Coarctation of the aorta (CoA) is a common defect that accounts for 5%–8% of all congenital heart defects (1, 2). CoA is often associated with concomitant cardiac pathologies, particularly bicuspid aortic valve (BAV), mitral valve abnormality, and ventricular septal defect (3). It has been reported that BAV is present in approximately two-thirds of CoA patients and has been linked to an increased risk for valvular dysfunction and aortic dilation. Most untreated patients with CoA usually die before 50 years of age (4). Adult patients with concomitant CoA and severe bicuspid aortic stenosis are relatively rare and present complex management challenges without standard guidelines (5, 6). Here, we present a case of a one-stop interventional procedure using two different vascular approaches in a patient with CoA and severe bicuspid aortic stenosis.
A 63-year-old female patient was admitted for chest pain, dyspnea on exertion in the previous 8 months, and aggravation for 1 week (NYHA grades III). She reported uncontrolled hypertension despite taking antihypertensive medications such as amlodipine 5 mg and valsartan 80 mg once daily. The physical examination showed a blood pressure of 160/110 mmHg, a rhythmic pulse of 83 beats/min, and a harsh grade 4/6 systolic ejection murmur in the right second intercostal space, which led to the carotids. A blood pressure difference of 10mmHg was observed in both lower extremities when compared to that of an arm. The blood pressure of both lower limbs is 10 mmHg lower than the arm. There were no other significant clinical findings and family history of cardiovascular disease. Blood tests showed elevated N-terminal pro brain natriuretic peptide (NT-proBNP) levels of 2,580 pg/ml (normal range 300–900 pg/ml). There were no significant abnormalities in routine blood test, liver and kidney function, and Troponin I.
The Electrocardiography (EKG) demonstrated a sinus rhythm of 80 beats per minute, a normal axis, and left ventricular hypertrophy. Echocardiogram showed severe aortic stenosis (aortic valve area, 0.2 cm2; mean pressure gradient, 78 mmHg; peak velocity, 5.8 m/s) and a reduced left ventricular ejection fraction of 35% (Figure 1A). Computed tomography (CT) angiography of aorta imaging evaluation revealed severe stenotic calcified eccentric aortic coarctation located 20 mm distal to the LSA. The diameter of the narrowest part was approximately 3 mm with significant thoracic collaterals. The descending aorta is observed to be significantly twisted and angled due to eccentric stenosis. Severe asymmetric diffuse leaflet calcifications were observed in bicuspid aortic valve (BAV) type 0, as well as marked calcifications of the ascending aorta and aortic arch (Figures 2A,B). A pre-operative left heart catheterization revealed no evidence of coronary artery disease.
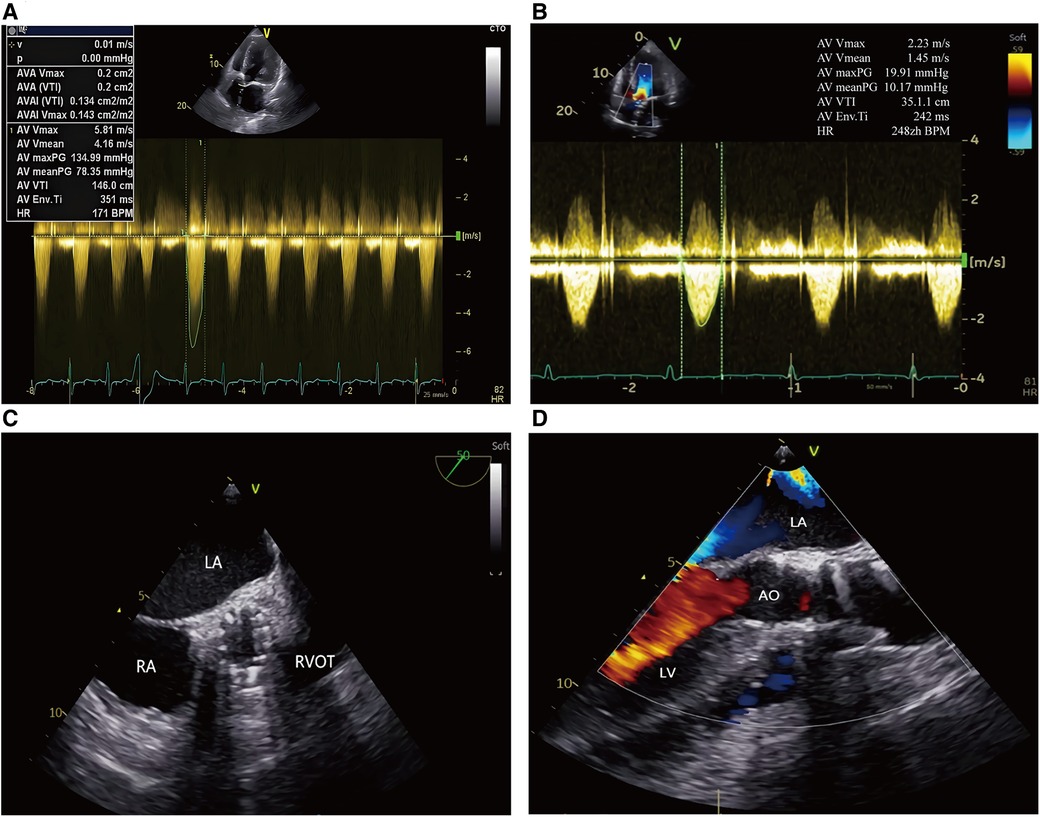
Figure 1. Trans-esophageal echocardiography in TAVR procedure. (A, B) Continuous Doppler showing the peak transvalvular pressure gradient: pre and post TAVR; (C) Postoperative echocardiography showed the prosthetic aortic valve with good morphology; (D) Postoperative echocardiography showed no significant aortic regurgitation after TAVR. LA, left atrium; RA, right atrium; LV, left ventricle; RVOT, right ventricular outflow tract; AO, aorta.
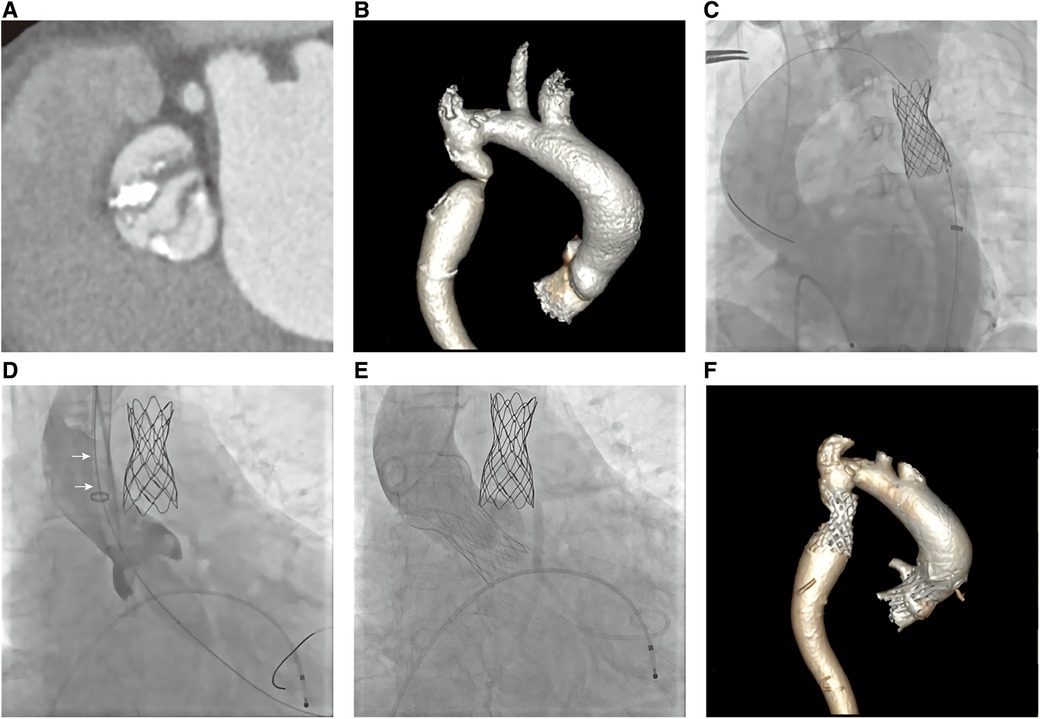
Figure 2. Ct aortogram and invasive aortic angiography. (A, B) CTA showed severe calcified bicuspid aortic valve stenosis (type 0) and stenotic calcified eccentric aortic coarctation 20 mm distal to the left subclavian artery; (C)A CP stent was implanted via the right femoral artery; (D) Aortic valve balloon dilatation via the left carotid artery (arrow: 20F sheath); (E) Prosthetic valve was implanted; (F) CT follow-up revealed both devices were properly positioned.
Based on the association of a bicuspid aortic valve and a coarctation of the aorta, the patient was diagnosed with a complex aortic coarctation and severe aortic valve stenosis. After consulting with the cardiac team and obtaining the patient's consent, we decided to perform a one-stop interventional procedure for repairing both defects. The operation was performed in the cardiac catheter lab under general anesthesia. Firstly, we implanted a 45 mm enlarged Cheatham-Platinum (CP) stent installed on a 22*45 mm BIB (balloon in balloon) catheter via the right femoral access, immediately distal to the LSA (Figure 2C), and achieved complete pressure gradient resolution across the CoA (from 35 mmHg to 3 mmHg) (Figure 3A). To avoid possible damage or displacement of the implanted CP stent during the delivery of the artificial valve due to the markedly twisted and angled descending aortic arch, alternate access for TAVR was considered appropriate through the left carotid artery, which was straight, wide, and free of obvious plaque.
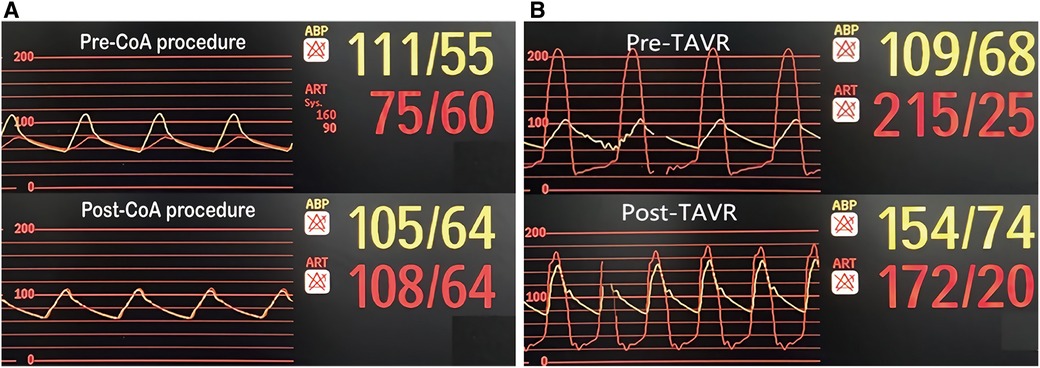
Figure 3. Pressure gradient variation. (A) Pre and post CoA interventional procedure; (B) Pre and post TAVR.
The carotid incision was sutured with purse string to prevent bleeding. First, a 6F sheath was implanted, and the straight guide wire was inserted into the left ventricle through an Amplatze 1.0 catheter and exchanged with a 6F pigtail catheter. The 20F sheath was then replaced for balloon pre-expansion and prosthetic valve implantation. We used a 20 mm balloon to dilate the aortic valve while performing an angiographic evaluation. The findings revealed that there was no obvious balloon lumbar sign and aortic regurgitation, and that the left and right coronary arteries were well-developed (Figure 2D). A 24 mm prosthetic valve (Vitaflow Aortic Valve System, Shanghai Microport medical group) was smoothly implanted through the left carotid artery. Due to the concise and direct approach, the feedback of delivery manipulation is excellent, and the final valve deployment was in the optimal position (Figure 2E). The aortic transvalvular pressure difference decreased from 100mmHg pre-TAVR to 18 mmHg post-TAVR (Figures 1B, 3B). Trans-esophageal echocardiography (TEE) revealed no significant aortic regurgitation and auscultation indicated a markedly reduced murmur (Figures 1C, D; Supplementary Video S1). The patient was discharged on postoperative day 9. At 11 months follow-up, the patient was asymptomatic with (Table 1) well-controlled blood pressure, and echocardiography showed a well-seated prosthetic valve with a mean gradient of 7 mmHg and no valve leakage, as well as no gradient across the coarctation stent. A radiological follow-up revealed that both devices were correctly positioned (Figure 2F).
CoA is defined as a discrete constriction of the aortic lumen, typically occurring at the ductus arteriosus distal insertion site to the LSA. The pathophysiology of CoA is unclear, but it is revealed to occur in 5%–8% of patients with congenital heart disease, including two thirds of patients with BAV, while isolated CoA occurs in <0.05% of live births (3, 7). When one of these lesions is found, screening for other coexisting congenital heart abnormalities is necessary.
The natural history of unrepaired coarctation of the aorta includes the development of systemic hypertension and subsequent morbidity and death from cardiovascular disease. Long-term survivors are at risk for aortic and mitral valve dysfunction, aortic aneurysm, aortic dissection, endocarditis, systemic hypertension, heart failure, and sudden death (8). The combination of symptomatic severe aortic stenosis and CoA in more than 60-year-old patients is a rare clinical entity (9, 10). Progressive aortic valve dysfunction in patients with CoA place patients at increased risk for morbidity and mortality. However, there are no specific guidelines for adult patients concurrent with symptomatic severe aortic stenosis and CoA.
The surgical techniques described separately for each abnormality can be combined in such complex surgeries. Different techniques have been used: either Aortic valve replacement and surgical correction of the coarctation in one single step, or a staged approach (11, 12). Although surgery is still the main treatment for these diseases, it is not suitable for high-risk surgical patient.
Transcatheter intervention is the treatment of choice for single aortic disease because of good results and the less invasive nature of this technique. It is associated with similar short and mid-term hemodynamic improvements and fewer acute complications than surgery (13). However, transcatheter intervention for patients with severe aortic stenosis complicated with CoA simultaneously is rarely reported.
Our patient herein reported having CoA with multiple comorbidities, a poorly functioning heart, and a severe aortic stenosis, for which transcatheter intervention was indicated. We propose a few factors to be considered before the interventional procedure:
(1) Patients must be thoroughly evaluated before operation, including cardiac function, ascending and descending aortic and aortic root width, aortic coarctation shape, vascular access, etc. It is important to implement a cardiac team including interventional cardiologists, radiologists, and cardiac surgeons devoted to meticulously assessing these patients for optimal treatment.
(2) Access to interventions. Because CoA often leads to significant tortuosity of descending aorta, the TAVR delivery system is difficult to pass through. If the CoA is treated first with a CP stent, even if the stent improves the aortic tortuosity, it must still risk high resistance and CP stent displacement when passing through the TAVR delivery system. Therefore, different pathways for TAVR and CoA interventional therapy should be selected. In this case, the left/right carotid artery is the best choice of access for TAVR. The carotid artery with a diameter greater than 6 mm without obvious stenosis (greater than 50%), heavy artery calcification, or tortuosity is generally considered appropriate for TAVR. Furthermore, the operation time following 20F sheath implantation should be as short as possible to reduce the risk of vascular hemorrhage and cerebral ischemia. Our team completed it within 18 min.
(3) The sequence of interventional therapy for aortic stenosis and coarctation. It is difficult to predict the hemodynamic effect of correcting an abnormality on other lesions. Our strategy is to first correct abnormalities that are less disruptive to hemodynamics, such as CoA in this case. Due to the existence of collateral circulation, the pressure difference before and after CoA was only 35 mmHg. The use of CP stents had little effect on overall hemodynamics. The severe aortic stenosis which had a great impact on hemodynamics (transvalvular pressure gradient of 100 mmHg) was then treated. However, if a patient has an acute decompensated circulatory disorder with poor response to medications, severe aortic stenosis with a greater impact on hemodynamics is the key to the disease. At this time, it is reasonable to first treat the severe aortic stenosis with TAVR as soon as possible, followed by CoA.
The simultaneous occurrence of severe aortic stenosis and CoA without dilation of ascending aorta in patients over the age of 60 is rare. The management is complex and must be individualized. Patients must be fully evaluated and prepared for operation, and the comprehensive assessment of the cardiac team can provide patients with the best treatment strategy. Our case report demonstrates the feasibility and efficacy of a one-stop interventional procedure in an adult patient with concurrent severely calcified BAV and CoA via two different vascular approaches. Transcatheter intervention, in contrast to traditional surgical approaches or two-stop interventional procedures, as a minimally invasive and novel method, offers a wider range of therapeutic methods for such diseases.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Research Ethics Commissions of Tongji Hospital Tongji Medical College of Huazhong University of Science and Technology (TJ- IRB20211102). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HZ and QZ came up with the idea. XH drafted the manuscript. WL and XH collected and summarized the medical records. HZ, QZ, and MD revised the manuscript. All authors contributed to the article and approved the submitted version.
Our work reported here was supported by the National Natural Science Foundation of China (No. 81800411) and Chinese Society of Cardiology (CSC) special-fund for clinical research (CSCF 2020B03). The funding bodies were not involved in the design of the study; collection, analysis, or interpretation of the data; or writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1162203/full#supplementary-material.
Supplementary Table 1
Comparison of echocardiography values among this patient, normal population and AS patient.
Supplementary Video 1
Postoperative echocardiography showed no significant aortic regurgitation after TAVR.
1. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, et al. Task force on the management of grown-up congenital heart disease of the European society of cardiology (ESC); association for European paediatric cardiology (AEPC); ESC committee for practice guidelines (CPG). ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. (2010) 31:2915–57. doi: 10.1093/eurheartj/ehq249
2. Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart. (2017) 103(15):1148–55. doi: 10.1136/heartjnl-2017-311173
3. Sinning C, Zengin E, Kozlik-Feldmann R, Blankenberg S, Rickers C, von Kodolitsch Y, et al. Bicuspid aortic valve and aortic coarctation in congenital heart disease-important aspects for treatment with focus on aortic vasculopathy. Cardiovasc Diagn Ther. (2018) 8:780–8. doi: 10.21037/cdt.2018.09.20
4. Campbell M. Natural history of coarctation of the aorta. Br Heart J. (1970) 32:633–40. doi: 10.1136/hrt.32.5.633
5. Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. (2011) 306(10):1104–12. doi: 10.1001/jama.2011.1286
6. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143(5):e35–71. doi: 10.1161/CIR.0000000000000932
7. Matsumoto MM, Milner R. Three stage hybrid approach for congenital aortic coarctation and bicuspid aortic valve with severe aortic stenosis in an adult patient. EJVES Short Rep. (2019) 45:26–9. doi: 10.1016/j.ejvssr.2019.10.003
8. Clair M, Fernandes SM, Khairy P, Graham DA, Krieger EV, Opotowsky AR, et al. Aortic valve dysfunction and aortic dilation in adults with coarctation of the aorta. Congenit Heart Dis. (2014) 9(3):235–43. doi: 10.1111/chd.12109
9. Onohara D, Sato A, Tasaki Y, Yamada T. Co-existence of severe coarctation of the aorta and aortic valve stenosis in a 65-year-old woman: a case report. Ann Thorac Cardiovasc Surg. (2014) 20(Suppl):750–53. doi: 10.5761/atcs.cr.13-00216
10. Arroyo-Rodríguez AC, Molina-Cancino DL, Arias-Navarro E, Del Carmen Ojeda-Peña A, Sandoval-Navarrete S. Complex aortic coarctation and a bicuspid aortic valve with severe stenosis in a 68 year-old woman. Arch Cardiol Mex. (2018) 88(2):153–5. doi: 10.1016/j.acmx.2017.11.005
11. Cardoso G, Abecasis M, Anjos R, Marques M, Koukoulis G, Aguiar C, et al. Aortic coarctation repair in the adult. J Card Surg. (2014) 29:512–8. doi: 10.1111/jocs.12367
12. Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, et al. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. (2013) 62(11):1020–5. doi: 10.1016/j.jacc.2013.06.016
13. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, et al. 2018 Aha/acc guideline for the management of adults with congenital heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. (2019) 139:e698–800. doi: 10.1161/CIR.0000000000000603
Keywords: aortic coarctation, one'stop, case report, interventional procedure, bicuspid aortic valve
Citation: He X, Dhuromsingh M, Liu W, Zhou Q and Zeng H (2023) One-stop interventional procedure for bicuspid aortic stenosis in a patient with coexisting aortic coarctation: a case report. Front. Cardiovasc. Med. 10:1162203. doi: 10.3389/fcvm.2023.1162203
Received: 9 February 2023; Accepted: 12 April 2023;
Published: 4 May 2023.
Edited by:
Alessandro Iadanza, Siena University Hospital, ItalyReviewed by:
Tommaso Piva, Azienda Ospedaliero Universitaria Ospedali Riuniti, Italy© 2023 He, Dhuromsingh, Liu, Zhou and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Zhou dGhpc2lzemhvdUAxNjMuY29t Hesong Zeng emVuZ2hzQHRqaC50am11LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.