
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 22 November 2023
Sec. Structural Interventional Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1161834
 Jiazheng Li†‡
Jiazheng Li†‡ Zhanjiang Cao†
Zhanjiang Cao† Tong Zhang
Tong Zhang Keqiang Zhao
Keqiang Zhao Junlai Zhao
Junlai Zhao Yu Yang
Yu Yang Chao Jiang
Chao Jiang Zipeng Li
Zipeng Li Rongrong Zhu*
Rongrong Zhu* Weiwei Wu*
Weiwei Wu*
Objective: To compare the ultrasound guidance and traditional methods in femoral artery puncture.
Methods: We searched the databases to evaluate the rate of success on first attempt and the incidence of hematoma. The random effects model was used for performing a meta-analysis to estimate the odds ratio (ORs), mean difference (MD), and 95% confidence interval (CI).
Results: A total of nine articles including 2,361 patients were included in this meta-analysis. The rate of success on first attempt were 79.6% (1,289/1,619) and 54.1% (883/1,644) in patients of the ultrasound group and traditional method group, respectively [OR = 3.14 (95% CI = 2.30–4.28), combined OR value Z = 7.23 (P < 0.00001)]. The rates of incidence of hematoma in the ultrasound group and traditional puncture group patients were 1.4% (16/1,168) and 3.8% (45/1,193), respectively (OR = 0.41, 95% CI = 0.17–1.00, p = 0.05).
Conclusion: Ultrasound-guided femoral artery puncture has certain advantages compared with traditional puncture with regard to success on first attempt and the incidence of hematoma. Moreover, ultrasound-guided puncture reduces the incidence of hematoma in the retrograde puncture group patients.
Peripheral artery disease (PAD) affects approximately 200 million people worldwide (1), which is estimated to impact a large number of people (2). PAD associated with a 5-year significant morbidity is approximately 33.2% (3). If no timely treatment is provided to patients with PAD, the disease may progress to critical limb ischemia (CLI). The amputation rate of patients diagnosed with CLI within 1 year is 30% (4).
Traditionally, drugs and open surgery were used to treat diseases. In recent years, endovascular treatments have been increasingly adopted (5–7). Successful placement of the needle in the common femoral artery is an important surgical step, which is closely related to complications related to many vascular-access related complications (8). Improper positioning of the femoral artery puncture increases the risk of complications. Puncture below the bifurcation of the common femoral artery is more likely to lead to the formation of pseudoaneurysms (9–11). Conversely, puncture of the artery above the inguinal ligament is associated with a high incidence of retroperitoneal hemorrhage (12–15).
Traditionally, people used methods such as palpation of body surface markers and fluoroscopy to determine the location of the puncture. In recent years, ultrasound-guided puncture has been used increasingly because it provides the surgeon a more rapid access to the puncture site and causes fewer complications at the site (16). The purpose of this meta-analysis is to evaluate whether ultrasound guidance is associated with an increase in the rate of success on first attempt and a lower rate of hematoma.
This report conforms to the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA). We performed a comprehensive search of the CNKI, VIP, Wanfang, PubMed, Cochrane, and Embase databases for articles evaluating the efficacy of ultrasound guidance vs. traditional guidance of femoral arterial access. The last search was run on 28 September 2021. The following search strategy was used in articles published in the Chinese language: (chaosheng[Title/Abstract]) and ((gudongmai[Title/Abstract]) or (guqiandongmai[Title/Abstract]))) and ((toushi[Title/Abstract]) or (mangchuan[Title/Abstract])). The following search strategy was used in articles published in the English language. The search strategy was ((‘femoral artery’/exp OR (‘arteries, femoral’:ab,ti OR ‘artery, femoral’:ab,ti OR ‘femoral arteries’:ab,ti OR ‘common femoral artery’:ab,ti OR ‘arteries, common femoral’: ab, ti OR ‘artery, common femoral’:ab,ti OR ‘common femoral arteries’:ab,ti OR ‘femoral arteries, common’:ab,ti OR ‘femoral artery, common’:ab,ti)) AND (‘echography’/exp OR (‘ultrasonography, interventional’:ab,ti OR ultrasonography:ab,ti OR ‘diagnostic ultrasound’:ab,ti OR ‘diagnostic ultrasounds’:ab,ti OR ‘ultrasound, diagnostic’:ab,ti OR ‘ultrasounds, diagnostic’:ab,ti OR ‘ultrasound imaging’:ab,ti OR ‘imaging, ultrasound’:ab,ti OR ‘imagings, ultrasound’:ab,ti) OR (echotomography:ab,ti OR ‘ultrasonic imaging’:ab,ti OR ‘imaging, ultrasonic’:ab,ti OR ‘sonography, medical’:ab,ti OR ‘medical sonography’:ab,ti OR ‘ultrasonographic imaging’:ab,ti OR ‘imaging, ultrasonographic’:ab,ti OR ‘imagings, ultrasonographic’:ab,ti OR ultrasonographic:ab,ti) OR (echography:ab,ti OR ‘diagnosis, ultrasonic’:ab,ti OR ‘diagnoses, ultrasonic’:ab,ti OR ‘ultrasonic diagnoses’:ab,ti OR ‘ultrasonic diagnosis’:ab,ti OR ‘echotomography, computer’:ab,ti OR ‘computer echotomography’:ab,ti OR ‘tomography, ultrasonic’:ab,ti OR ‘ultrasonic tomography’:ab,ti) OR (‘ultrasound, interventional’:ab,ti OR ‘interventional ultrasound’:ab,ti OR ‘interventional ultrasonography’:ab,ti OR ‘ultrasonography, intravascular’:ab,ti OR ‘intravascular ultrasonography’:ab,ti)) AND (‘palpation’/exp OR ‘fluoroscopy’/exp OR (traditional:ab,ti OR anatomical:ab,ti OR fluoroscopic:ab,ti OR fluroscopy:ab,ti))) AND (‘health care quality’/exp OR (random:ab,ti OR ‘clinical trial’)).
Two authors (ZC and JL) independently assessed the eligibility of all retrieved studies. A third and a fourth author (WW and RZ) reviewed their findings. The investigators reached a consensus and the differences were resolved. The literature included in the meta-analysis was based on the following criteria: (1) randomized controlled trials; (2) the effect of ultrasound-guided femoral artery puncture was counted with the traditional femoral artery puncture as the control; (3) report on the rates of success on first attempt, or complications of the puncture. The study selected an initial search that identified 453 relevant articles, 430 of which were excluded after screening for titles or abstracts. After a careful reading of the remaining 23 articles, it was found that nine of them (3,313 patients) finally met the selection criteria and were, therefore, included in the current meta-analysis (Figure 1). The characteristics of all included studies are summarized in Table 1.
Two authors (JL and ZC) independently extracted the following data from the included articles: first author, year of publication, study design, success rate of first puncture, success rate of total puncture, time of puncture, and complications. The seven main parts of the Cochrane Risk of Bias tool were used to evaluate the quality of all the articles: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases.
Heterogeneity was assessed using the I2 statistic; values of <25%, 25%–50%, and >50% were considered low, moderate, and high heterogeneity, respectively. An I2 > 50% (p < 0.05) represented significant heterogeneity across the included studies. Potential publication bias was estimated by using the Begger's and Egger's tests.
The rate of success on first attempt, total puncture success rate, puncture time, and complications in ultrasound puncture and traditional puncture were compared. The rate of success on first attempt: The number of patients with successful first common femoral artery puncture accounted for the proportion of the total number of patients in this group. Total success rate of puncture: the proportion of patients with common femoral artery cannulation after puncture in the group. Operation time: the recording of time from local anesthesia injection to vascular sheath implantation. Number of punctures: Each withdrawal of the needle is recorded as one time.
Because of the heterogeneity of the research, the random effects model was used to conduct a meta-analysis of the results. For continuous variables, if the mean and standard deviation were expressed in the same unit, they were combined into a mean difference with a 95% confidence interval. Odds ratio and 95% CI were used for categorical variables. Multivariate-adjusted ORs from cohort studies were pooled using generic inverse variance weighting. A subgroup analysis based on study design was conducted. Sensitivity analyses for the rate of success on first attempt were performed to test the reliability of the results by removing one study at a time and repeating the meta-analysis. A two-sided p < 0.05 indicated statistical significance. Analyses were performed using RevMan (version 5.3; Cochrane Information Management System; http://ims.cochrane.org/revman) and Stata software (version 14.0).
Nine articles involving 2,361 patients were included in this study (16–24). An insufficient blinding strategy in 9 RCTs increased the risk of bias. With regard to the blinding of Outcome Assessment, the difference in standards made the difference in results (Figure 2; Table 2).
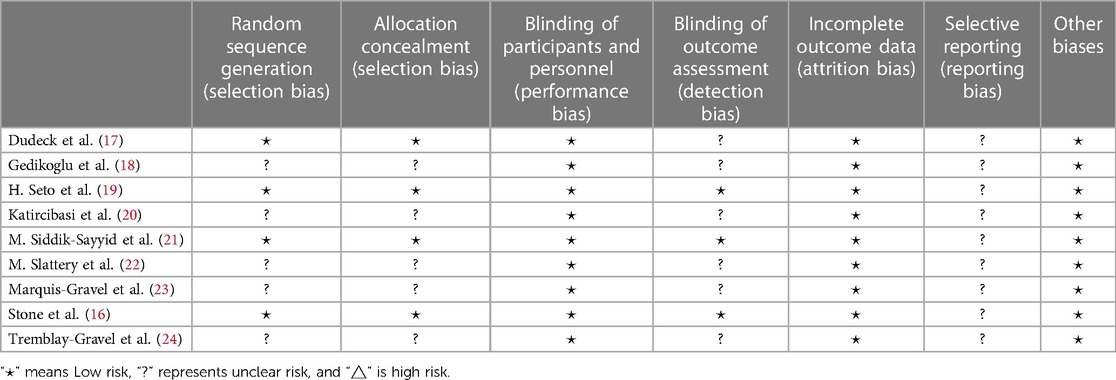
Table 2. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Eight studies reported the rate of success on first attempt. The rates of success on first attempt in the ultrasound group patients were 79.6% (1,289/1,619) vs. 54.1% (883/1,644) in the traditional method group patients. The overall OR was 3.14 (95% CI 2.30–4.28), and the Z-score for the overall effect was Z = 7.23 (P < 0.00001), suggesting a significant difference between the two methods (Figure 3A). The heterogeneity in the studies reporting the first-pass success was high (I2 = 64%, p = 0.007). After the third study (19) was excluded, heterogeneity reduced significantly (Figure 3B), and the relevant reasons for this will be analyzed in the Discussion section. Sensitivity analysis showed that the estimates did not change significantly after other studies were excluded, implying that the result was relatively reliable (Figure 3C). The result of the Begger's test did not show significant publication bias (p = 0.386). After Egger's test, p was 0.044. The Trim and full Analysis showed that the result was relatively stable. Funnel charts were symmetrical (Figure 3D). In general, there was no publication bias for these inspection methods.
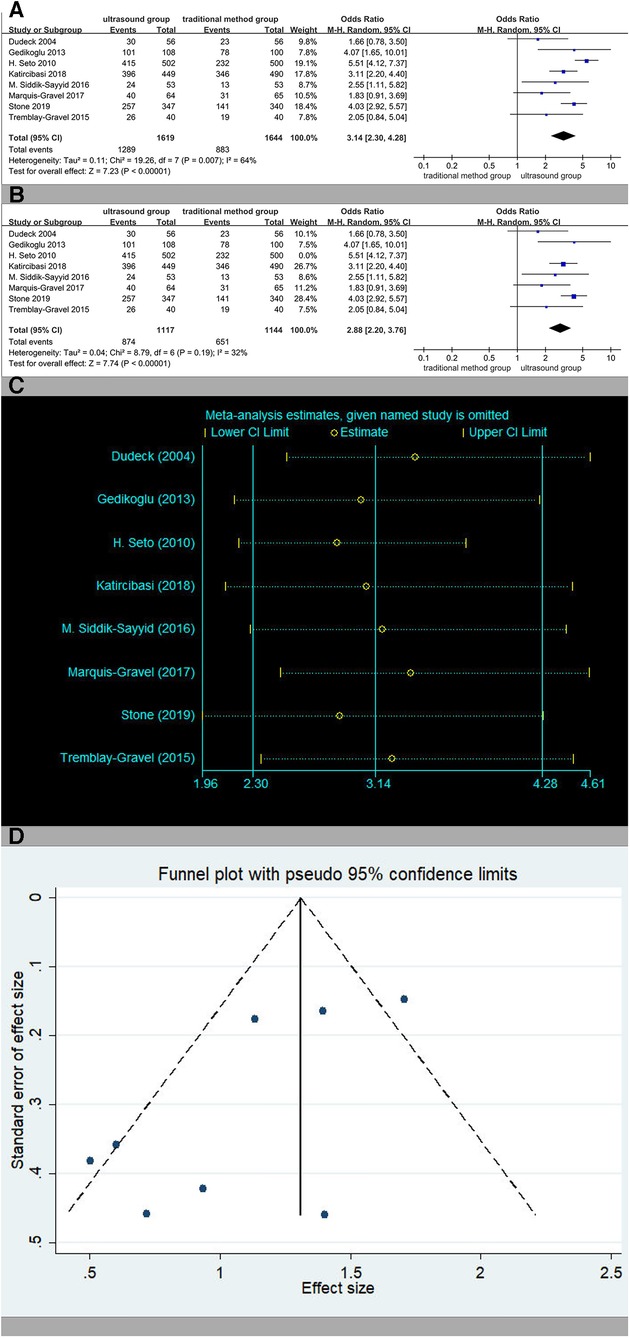
Figure 3. (A) The rate of success on first attempt. (B) The rate of success on first attempt except (19). (C) Sensitivity analysis of the rate of success on first attempt. (D) Funnel charts of the rate of success on first attempt.
Subgroup analyses based on traditional methods vs. ultrasound are presented in Figure 4. Ultrasound was more effective than the traditional palpation and fluoroscopy method (OR 3.60, 95% CI 2.87–4.53, p < 0.00001). There were significant differences when compared with anatomic landmarks (OR 2.88, 95% CI 2.20–3.76, p < 0.00001), too. There was no significant heterogeneity.
Five studies reported the total success rate. The total success rates in the ultrasound group patients were 94.1% (591/628) vs. 88.1% (541/614) in the traditional method group patients. The overall OR was 2.23 (95% CI 1.45–3.45), and the Z-score for the overall effect was Z = 3.63 (P = 0.0003), suggesting a significant difference between the two methods (Figure 5A). There was no significant heterogeneity (I2 = 0%, p = 0.49). No significant publication bias was observed (Begger's test p = 0.734, Egger's test p = 0.902). The results of the sensitivity analysis are shown in Figure 5B.
Six studies reported the rate of venipuncture. The rates of venipuncture in the ultrasound group patients were 3.8% (55/1,458) vs. 12.0% (179/1,491) in the traditional method group patients. The overall OR was 0.26 (95% CI 0.17–0.39), and the Z-score for the overall effect was Z = 6.66 (p < 0.00001), suggesting a significant difference between the two methods (Figure 6A). The heterogeneity in the studies was low (I2 = 20%, p = 0.28). Sensitivity analysis showed that the estimates did not change significantly after each study was excluded, implying that those results were relatively reliable (Figure 6B). No significant publication bias existed in the rate of venipuncture (Begger's test p = 0.452, Egger's test p = 0.140).
Five studies reported the rate of hematoma (Figure 7A). The rates of hematoma in the ultrasound group patients were 1.4% (16/1,168) vs. 3.8% (45/1,193) in the traditional method group patients. The overall OR was 0.41 (95% CI 0.17–1.00). It was numerically less in the ultrasound group patients, although this was not statistically significant (p = 0.05). No significant publication bias was observed (Begger's test p = 0.462, Egger test p = 0.564). The results of the sensitivity analysis are shown in Figure 7B.
In the subgroup analyses based on antegrade or retrograde access, ultrasound was more effective than the traditional method (OR 0.37, 95% CI 0.21–0.65, p = 0.0005). When retrograde access was separated from antegrade access, there was less heterogeneity in the results (Figure 8).
Time to access the artery was significantly less in the ultrasound group patients (Figure 9A). No significant publication bias was observed (Begger's test p = 0.806, Egger's test p = 0.489). The results of the sensitivity analysis are shown in Figure 9B.
In the ultrasound group patients, the number of attempts was obviously less (Figure 10A). No significant publication bias was observed (Begger's test p = 0.308, Egger's test p = 0.307). The results of the sensitivity analysis are shown in Figure 10B.
Three studies were included in the analysis of the incidence of bleeding (Figure 11A) and the incidence of pseudoaneurysm (Figure 11B), respectively, with no statistical difference. The results of the sensitivity analysis are shown in Figures 11C,D.
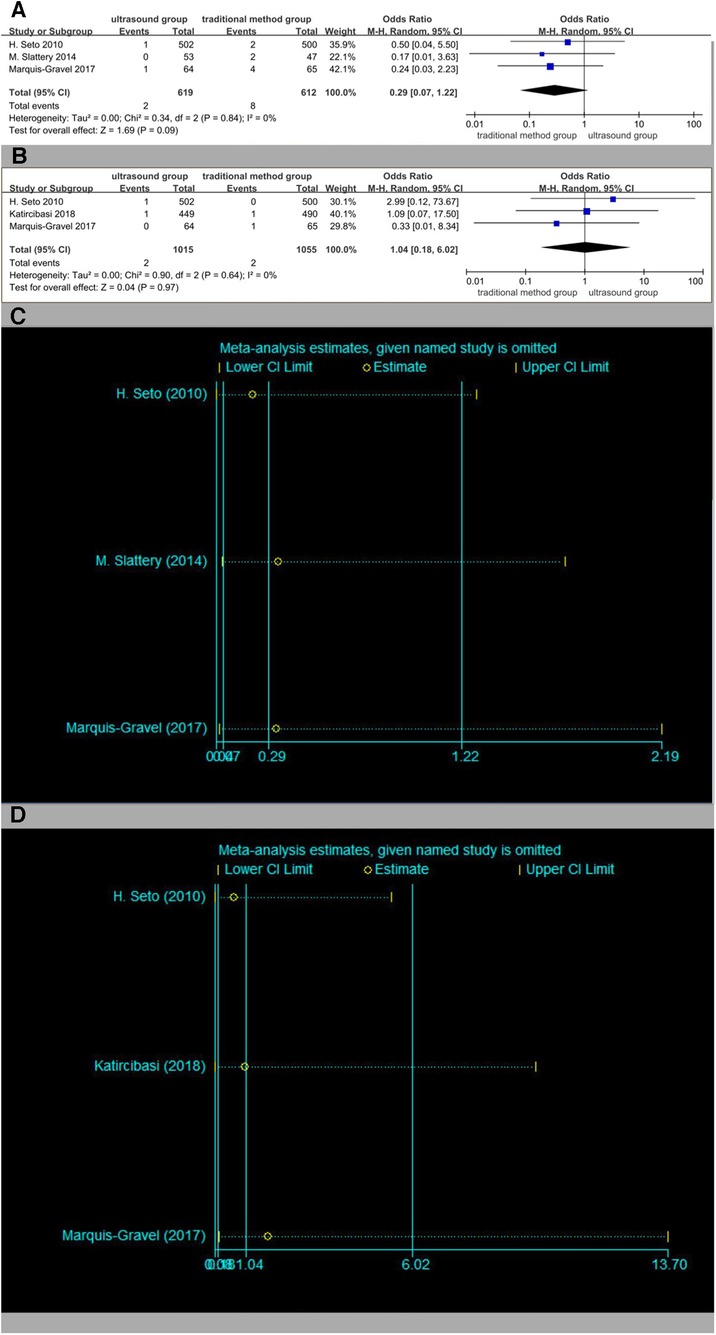
Figure 11. (A) Forest plot of bleeding. (B) Forest plot of pseudoaneurysm. (C) Sensitivity analysis of bleeding. (D) Sensitivity analysis of pseudoaneurysm.
The results showed that the rate of success on first attempt in the ultrasound group patients was 25% higher than that in the traditional puncture group patients, and the number of punctures also reduced by 0.6 times. The rate of hematoma caused by traditional puncture is a common problem when the pathway is established, and its incidence is about 1.7 times higher than that of ultrasound-guided puncture.
When analyzing the relevant data on the rate of success on first attempt, one of the articles (19) brought more heterogeneity. The article does not report on operator skill level by objective proficiency measures. The analysis shows that although operating proficiency has nothing to do with the total success rate, it is related to the operating time (19). The heterogeneity of the operator's proficiency may be the reason for the low success rate of ultrasound puncture for the first time in this article. The higher the number of punctures during the catheterization process, the more likely it is to damage the blood vessel wall and cause hematoma.
Hematoma is the most frequent local complication after puncture. The analysis in this article showed that the incidence of traditional punctures in patients with hematomas was slightly higher than that of ultrasound-guided puncture in patients with hematomas. A subgroup analysis of the incidence of postoperative hematoma based on antegrade or retrograde access can significantly reduce the heterogeneity of the incidence of hematoma in the subgroups. After subgroup analysis, it was found that the incidence of ultrasound-guided hematoma in patients who underwent retrograde puncture was significantly lower than that in those who underwent traditional puncture. The reasons for this include the higher rate of success on first attempt, less damage to blood vessels, and the easy-to-apply modified Seldinger method by which it is easier to puncture the anterior surface. A comparison of the previous fluoroscopy-guided antegrade (25) and ultrasound-guided antegrade (26) revealed that ultrasound-guided antegrade is less likely to cause hematoma, as it may be easier with ultrasound to successfully avoid puncturing the posterior wall of the artery and causing minor damage to the blood vessels (26). Therefore, it is considered that there is no significant difference in the rate of hematoma between the ultrasound-guided puncture method and the traditional method in the antegrade puncture group patients and the traditional puncture group patients, which may be related to the smaller sample size.
Although hematomas occurred in both patient groups, the ultrasound group patients had inguinal hematomas, and the traditional puncture group patients had retroperitoneal hematomas. Retroperitoneal hematomas may evolve into retroperitoneal hemorrhage. Retroperitoneal hemorrhage is often extremely dangerous (27). The occurrence of retroperitoneal hematoma is often associated with a higher puncture position (10, 11). In previous randomized controlled trials, the severity of hematoma was not distinguished, resulting in higher heterogeneity on the rate of hematoma. Therefore, if similar studies are to be carried out in the future, the type and size of hematomas should be further refined.
The difference between this study and previous studies is that the included randomized controlled trials have significantly increased, avoiding the previous situation where data from a single center accounted for the vast majority of patients (28). In this paper, the rate of success on first attempt is used as the primary endpoint because it is related to hematoma and to the catheterization time, which prolongs the overall time of the operation.
Because of the differences in the original documents, this study has limitations. The heterogeneity of the access time and the number of attempts are relatively high. There is no subgroup analysis of lesions in the vessel being punctured in the previous randomized controlled trials. The difficulty involved in puncture varies between patients with femoral artery diseases and those who need interventional treatment because of other vascular diseases. Bleeding is also a common complication, but the classification methods mentioned in each article are not uniform. They are often divided into major bleeding and non-bleeding, and therefore, a comprehensive grouping method can be considered (29).
Ultrasound guidance is effective for puncture in patients with conditions such as obesity, artery anatomical abnormalities, hypotension, and weak arterial pulsation (30). Ultrasound can also clearly determine the calcification of the blood vessel wall, and it is easier to puncture the healthy blood vessel area by using ultrasound than by using the anatomical positioning method, thus reducing the possibility of hematoma. The use of ultrasound adds part of the cost to patients, but if a local hematoma occurs, the required treatment cost is approximately 1,399$ (31). Ultrasound avoids greater risks with a small investment. Compared with fluoroscopy guidance, ultrasound-guided puncture does not require additional radiation (22). The disadvantage of ultrasound guidance is that its training cycle is long, and the puncture time of operators with different proficiency levels varies significantly (19), which is an obstacle to the popularization of ultrasound.
Ultrasound-guided femoral artery puncture may have certain advantages compared with traditional puncture with regard to success on first attempt. In this study, we found that the possibility of hematoma occurring under ultrasound guidance was lower, but the difference was not obvious. Ultrasound was more effective in the retrograde group than in the traditional method group. The difference between the two methods necessitates a randomized controlled experiment with a larger sample size.
JL and ZC independently extracted the following data from the included articles: first author, year of publication, study design, success rate of first puncture, success rate of total puncture, time of puncture, and complications. Cochrane bias seven major part of the risk assessment tool used to evaluate the quality of all the articles: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. (2013) 382(9901):1329–40. doi: 10.1016/S0140-6736(13)61249-0
2. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. (2015) 116(9):1509–26. doi: 10.1161/CIRCRESAHA.116.303849
3. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord. (2005) 5:14. doi: 10.1186/1471-2261-5-14
4. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG; TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. (2007) 45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037
5. El-Sayed HF. Bypass surgery for lower extremity limb salvage: vein bypass. Methodist Debakey Cardiovasc J. (2012) 8(4):37–42. doi: 10.14797/mdcj-8-4-37
6. Wiseman JT, Fernandes-Taylor S, Saha S, Havlena J, Rathouz PJ, Smith MA, et al. Endovascular versus open revascularization for peripheral arterial disease. Ann Surg. (2017) 265(2):424–30. doi: 10.1097/SLA.0000000000001676
7. Vartanian SM, Conte MS. Surgical intervention for peripheral arterial disease. Circ Res. (2015) 116(9):1614–28. doi: 10.1161/CIRCRESAHA.116.303504
8. Criado FJ, Twena M, Halsted M, Abul-Khoudoud O. Percutaneous femoral puncture for endovascular treatment of occlusive arterial lesions. Am J Surg. (1998) 176(2):119–21. doi: 10.1016/S0002-9610(98)00162-7
9. Altin RS, Flicker S, Naidech HJ. Pseudoaneurysm and arteriovenous fistula after femoral artery catheterization: association with low femoral punctures. AJR Am J Roentgenol. (1989) 152(3):629–31. doi: 10.2214/ajr.152.3.629
10. Gabriel M, Pawlaczyk K, Waliszewski K, Krasiński Z, Majewski W. Location of femoral artery puncture site and the risk of postcatheterization pseudoaneurysm formation. Int J Cardiol. (2007) 120(2):167–71. doi: 10.1016/j.ijcard.2006.09.018
11. Kim D, Orron DE, Skillman JJ, Kent KC, Porter DH, Schlam BW, et al. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Cathet Cardiovasc Diagn. (1992) 25(2):91–7. doi: 10.1002/ccd.1810250203
12. Ellis SG, Bhatt D, Kapadia S, Lee D, Yen M, Whitlow PL. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv. (2006) 67(4):541–5. doi: 10.1002/ccd.20671
13. Farouque HM, Tremmel JA, Raissi Shabari F, Aggarwal M, Fearon WF, Ng MK, et al. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol. (2005) 45(3):363–8. doi: 10.1016/j.jacc.2004.10.042
14. Sherev DA, Shaw RE, Brent BN. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc Interv. (2005) 65(2):196–202. doi: 10.1002/ccd.20354
15. Tiroch KA, Arora N, Matheny ME, Liu C, Lee TC, Resnic FS. Risk predictors of retroperitoneal hemorrhage following percutaneous coronary intervention. Am J Cardiol. (2008) 102(11):1473–6. doi: 10.1016/j.amjcard.2008.07.039
16. Stone P, Campbell J, Thompson S, Walker J. A prospective, randomized study comparing ultrasound versus fluoroscopic guided femoral arterial access in noncardiac vascular patients. J Vasc Surg. (2020) 72(1):259–67. doi: 10.1016/j.jvs.2019.09.051
17. Dudeck O, Teichgraeber U, Podrabsky P, Lopez Haenninen E, Soerensen R, Ricke J. A randomized trial assessing the value of ultrasound-guided puncture of the femoral artery for interventional investigations. Int J Cardiovasc Imaging. (2004) 20(5):363–8. doi: 10.1023/B:CAIM.0000041949.59255.3f
18. Gedikoglu M, Oguzkurt L, Gur S, Andic C, Sariturk C, Ozkan U. Comparison of ultrasound guidance with the traditional palpation and fluoroscopy method for the common femoral artery puncture. Catheter Cardiovasc Interv. (2013) 82(7):1187–92. doi: 10.1002/ccd.24955
19. Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (femoral arterial access with ultrasound trial). JACC Cardiovasc Interv. (2010) 3(7):751–8. doi: 10.1016/j.jcin.2010.04.015
20. Tuna Katırcıbaşı M, Güneş H, Çağrı Aykan A, Aksu E, Özgül S. Comparison of ultrasound guidance and conventional method for common femoral artery cannulation: a prospective study of 939 patients. Acta Cardiol Sin. (2018) 34(5):394–8. doi: 10.6515/ACS.201809_34(5).20180524A
21. Siddik-Sayyid SM, Aouad MT, Ibrahim MH, Taha SK, Nawfal MF, Tfaili YJ, et al. Femoral arterial cannulation performed by residents: a comparison between ultrasound-guided and palpation technique in infants and children undergoing cardiac surgery. Paediatr Anaesth. (2016) 26(8):823–30. doi: 10.1111/pan.12935
22. Slattery MM, Goh GS, Power S, Given MF, McGrath FP, Lee MJ. Comparison of ultrasound-guided and fluoroscopy-assisted antegrade common femoral artery puncture techniques. Cardiovasc Intervent Radiol. (2015) 38(3):579–82. doi: 10.1007/s00270-014-0998-7
23. Marquis-Gravel G, Tremblay-Gravel M, Lévesque J, Généreux P, Schampaert E, Palisaitis D, et al. Ultrasound guidance versus anatomical landmark approach for femoral artery access in coronary angiography: a randomized controlled trial and a meta-analysis. J Interv Cardiol. (2018) 31(4):496–503. doi: 10.1111/joic.12492
24. Tremblay-Gravel M, Marquis-Gravel G, Lévesque J, Donald A. Comparison of anatomical versus ultrasound-guided techniques for femoral artery access in patients undergoing coronary angiography: a randomized single-blinded trial. Can J Cardiol. (2015) 31(10 Suppl. 1):S25–6. doi: 10.1016/j.cjca.2015.07.067
25. Fırat A, İgüs B. Combined percutaneous direct puncture of occluded artery—antegrade intervention for recanalization of below the knee arteries. Diagn Interv Radiol. (2019) 25(4):320–7. doi: 10.5152/dir.2019.18580
26. Hwang JH, Park SW, Kwon YW, Min J, Chee HK, Shin JK. Ultrasonography-guided antegrade common femoral artery approach: factors associated with access time. J Vasc Access. (2021) 22(3):364–9. doi: 10.1177/1129729820942053
27. Liu SY, Zeng B, Deng JB. Massive retroperitoneal hemorrhage secondary to femoral artery puncture: a case report and review of literature. Medicine (Baltimore). (2017) 96(50):e8724. doi: 10.1097/MD.0000000000008724
28. Rashid MK, Sahami N, Singh K, Winter J, Sheth T, Jolly SS. Ultrasound guidance in femoral artery catheterization: a systematic review and a meta-analysis of randomized controlled trials. J Invasive Cardiol. (2019) 31(7):E192–8.31257213
29. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. (2011) 123(23):2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
30. Zochios VA, Wilkinson J, Dasgupta K. The role of ultrasound as an adjunct to arterial catheterization in critically ill surgical and intensive care unit patients. J Vasc Access. (2014) 15(1):1–4. doi: 10.5301/jva.5000190
Keywords: femoral artery, ultrasound-guided puncture, traditional method, success on first attempt, hematoma
Citation: Li J, Cao Z, Zhang T, Zhao K, Zhao J, Yang Y, Jiang C, Li Z, Zhu R and Wu W (2023) Meta-analysis of ultrasound-guided and traditional femoral artery puncture. Front. Cardiovasc. Med. 10:1161834. doi: 10.3389/fcvm.2023.1161834
Received: 8 February 2023; Accepted: 26 October 2023;
Published: 22 November 2023.
Edited by:
Alfonso Ielasi, IRCCS Ospedale Galeazzi Sant'Ambrogio, ItalyReviewed by:
Emily Spangler, University of Alabama at Birmingham, United States© 2023 Li, Cao, Zhang, Zhao, Zhao, Yang, Jiang, Li, Zhu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongrong Zhu emh1cnJ2YXNAMTYzLmNvbQ== Weiwei Wu d2Vpd2VpLnd1QGJ0Y2guZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡ORCID Jiazheng Li orcid.org/0000-0003-2294-0805
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.