- 1Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Anesthesiology, Tibet Autonomous Region People’s Hospital, Lhasa, China
- 3Medical Research Center, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: The risk of perioperative cardiac complications (PCCs) in patients living in high-altitude areas may increase with more adverse clinical outcomes due to the special geographical environment, which has not yet been studied. We aimed to determine the incidence and analyze risk factors for PCCs in adult patients undergoing major noncardiac surgery in the Tibet Autonomous Region.
Methods: This prospective cohort study enrolled resident patients from high-altitude areas receiving major noncardiac surgery in Tibet Autonomous Region People's Hospital in China. Perioperative clinical data were collected, and the patients were followed up until 30 days after surgery. The primary outcome was PCCs during the operation and within 30 days after the surgery. Logistic regression was used to build the prediction models for PCCs. A receiver operating characteristic (ROC) curve was used to evaluate the discrimination. A prognostic nomogram was constructed to generate a numerical probability of PCCs for patients undergoing noncardiac surgery in high-altitude areas.
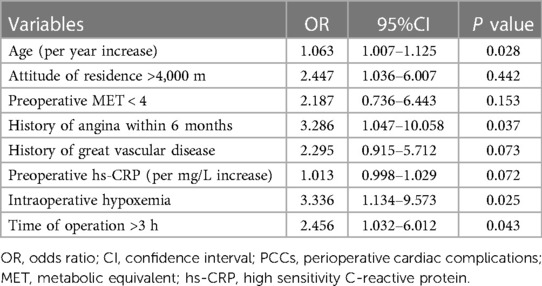
Results: Among the 196 patients living in high-altitude areas involved in this study, 33 (16.8%) suffered PCCs perioperatively and within 30 days after surgery. Eight clinical factors were identified in the prediction model, including older age (P = 0.028), extremely high altitude above 4,000 m (P = 0.442), preoperative metabolic equivalent (MET) < 4 (P = 0.153), history of angina within 6 months (P = 0.037), history of great vascular disease (P = 0.073), increased preoperative high sensitivity C-reactive protein (hs-CRP) (P = 0.072), intraoperative hypoxemia (P = 0.025) and operation time >3 h (P = 0.043). The area under the curve (AUC) was 0.766 (95% confidence interval: 0.785–0.697). The score calculated from the prognostic nomogram predicted the risk of PCCs in high-altitude areas.
Conclusion: The incidence of PCCs in resident patients living in high-altitude areas who underwent noncardiac surgery was high, and the risk factors included older age, high altitude above 4,000 m, preoperative MET < 4, history of angina within 6 months, history of great vascular disease, increased preoperative hs-CRP, intraoperative hypoxemia, and operation time >3 h. The prognostic nomogram of this study could help to assess the PCCs for patients in high-attitude areas undergoing noncardiac surgery.
Clinical Trial Registration: ClinicalTrials.gov ID: NCT04819698.
Introduction
Cardiac complications are uncommon but are reported as the primary cause of postoperative death (1–3). Older age, history of cardiovascular disease, peripheral and major vascular disease, cerebral infarction, renal insufficiency, and diabetes have been recommended as preoperative risk comorbidities for patients experiencing perioperative cardiac complications (PCCs) (2, 4, 5).
The hypoxic environment in high-altitude areas leads to a decrease in arterial partial pressure of oxygen (PaO2) and pulse oxygen saturation (SpO2). Long-term exposure to chronic hypoxia can cause pulmonary arterioles to contract, which leads to pulmonary artery hypertension (PAH) and increased right ventricular load (6, 7). In addition to cardiac overload, the incidences of hyperhemoglobinemia, hyperlipemia, and hypercoagulability are also higher than those found in patients living in plain areas (8). Furthermore, reduced oxygen supply and hyperlipidemia contribute to coronary heart disease and other cardiac events (9). Living at high altitude also increases the risks of deep vein thrombosis and pulmonary embolism after surgery, which is believed to be related to the pathological state of high-altitude polycythemia, venous stasis and hypercoagulability (10–13). Therefore, these patients may have an increased risk of adverse cardiac events during the perioperative period. However, few studies have elaborated the incidence and risk factors for limited case resources in high-attitude areas thus far.
The Tibet Autonomous Region of China is known as the “roof of the world”, with an average altitude of more than 4,000 m. In this study, we prospectively explored the incidence of PCCs for patients in the Tibet Autonomous Region undergoing noncardiac surgery and analyzed the risk factors. This study will help optimize perioperative care management and enhance the prognosis of patients in high-altitude areas.
Materials and methods
Study population
This study is a prospective cohort study of adult patients living in high-altitude areas receiving major noncardiac surgeries in Tibet Autonomous Region People's Hospital, China, between June 2021 and August 2022. The altitude of Tibet Autonomous Region People's Hospital is 3,568 m. The study was approved by the Medical Ethics Committee of the People's Hospital of Tibet Autonomous Region (Ethics No: ME-TBHP-20-26) and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
All patients who met the following criteria and voluntarily participated in this study were considered for inclusion consecutively, and the follow-up contents and methods were kept the same. The inclusion criteria were as follows: patients received general anesthesia for intermediate- to high-risk noncardiac surgery; lived in high-altitude areas above 3,000 m for more than 5 years; and were ≥50 years old. The types of surgery were considered based on the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines of perioperative cardiovascular evaluation (14, 15), including abdominal and retroperitoneal surgery (e.g., included laparoscopic surgery), vascular surgery (e.g., included large vessels and peripheral vessels), orthopedic surgery, neurosurgery, head and neck surgery, and thoracic surgery. Exclusion criteria included patient refusal, emergency operation, low-risk surgery, operation under local anesthesia, cardiac surgery, and American Society of Anesthesiologists (ASA) classification V.
Preoperative evaluation and data collection
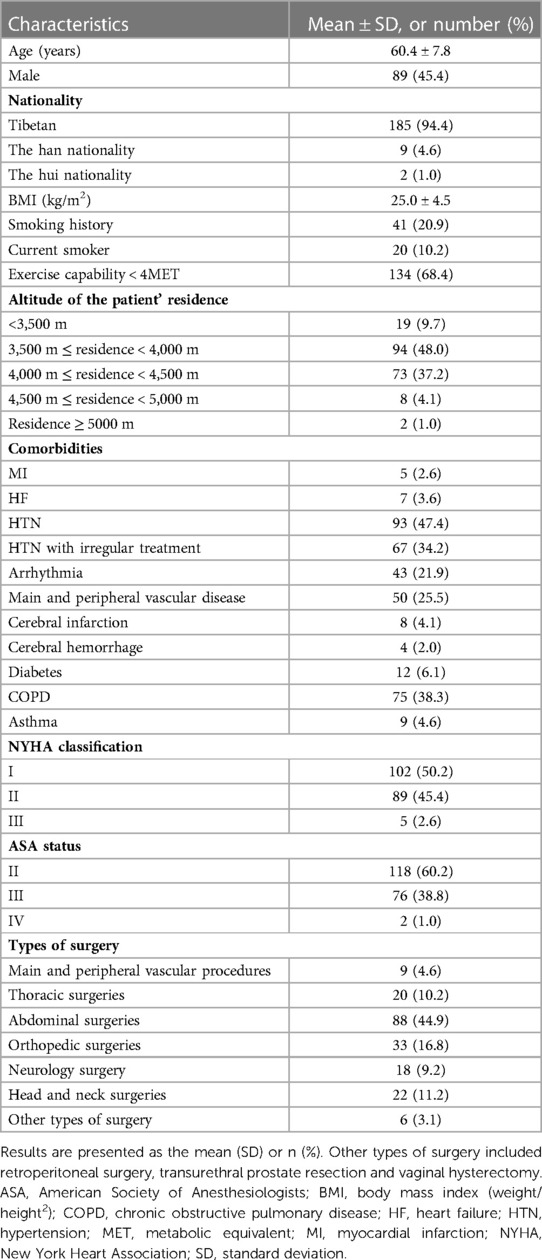
The general information of patients was prospectively collected, including sex, age, nationality, smoking history, altitude of habitation, previous medical history, history of peripheral vascular disease, cerebrovascular disease, cardiovascular disease, hypertension and treatment, diabetes and treatment, hyperthyroidism, chronic obstructive pulmonary disease (COPD) and asthma. Hypertension (HTN) with irregular treatment in this study means that patients did not regularly take the antihypertensive drugs prescribed by the doctor or never took any antihypertensive drugs after the diagnosis of hypertension. The metabolic equivalent (MET), New York Heart Association (NYHA), and ASA classification were evaluated and recorded.
All patients completed routine preoperative physical examinations, including body mass index (BMI), preoperative heart rate (HR), blood pressure (BP), and SpO2. The results of preoperative laboratory examination, such as hemoglobin level and coagulation index, 12-lead electrocardiogram (ECG), and transthoracic echocardiography, were noted. Preoperative pulmonary hypertension (PAH) was diagnosed as pulmonary artery pressure >35 mmHg by transthoracic echocardiography (16, 17).
Procedures
No preoperative medication was administered. After entering the operation room, SpO2, BP, HR, 3-lead ECG, and end tidal carbon dioxide (EtCO2) were continuously monitored. Surgical procedures were performed under general anesthesia with total intravenous anesthesia (TIVA) or sevoflurane inhalation. The bispectral index (BIS) was kept between 40 and 60. A lung protective ventilation strategy was performed during the operation with a tidal volume of 6 to 8 ml/kg of ideal body weight, titrimetric positive end expiratory pressure (PEEP) and recruitment maneuver. Vasopressors, fluids, or a combination of both were used if systolic arterial pressure decreased above 20% of the base value. Monitoring of patients' temperature was mandatory to avoid hypothermia and hyperthermia. Maintenance of normothermia was implemented perioperatively through active warming devices. Perioperative multimodal analgesia and postoperative nausea and vomiting prevention were performed. The total volume of fluid infusion, blood loss, blood transfusion, urine volume, operation time, operative site, and anesthesia protocol were all recorded. Hypotension was defined as systolic arterial pressure below 80% of the baseline. Prolonged intraoperative hypoxemia was defined as PaO2 < 60 mmHg (8.00 kPa) or SpO2 < 90% over 10 min.
HR, BP, SpO2, and 3-lead ECG monitoring were carried out for at least 24 h after surgery. If there were any typical or atypical cardiac symptoms, the patients received echocardiography and enzymography of the myocardium immediately. Patients were followed up 30 days after the surgery.
Outcomes
The primary outcome was the occurrence of PCCs during the operation and within 30 days after the surgery. PCCs were defined as acute coronary syndrome (ACS), heart failure (HF), new-onset severe arrhythmia, nonfatal cardiac arrest, and cardiac death (15, 18–22). ACS included ST-elevation myocardial infarction (STEMI) and non-ST-elevation acute coronary syndrome (NSTE-ACS). NSTE-ACS was further subdivided into non-ST-elevation MI and unstable angina. New-onset severe arrhythmia included malignant arrhythmia, severe sinus bradycardia, paroxysmal supraventricular tachycardia (PSVT), atrial fibrillation (AF), frequent ventricular premature contractions (VPCs), and severe atrioventricular block (AVB) that needed to be treated with drugs or electrical conversion. The definitions of PCCs and adjudication for confirmation are described in detail in Supplementary Table S1. Myocardial enzymes were detected if necessary to diagnose myocardial injury after noncardiac surgery (MINS). MINS is usually recognized when the concentration of cardiac troponin (cTn) exceeds the 99th percentile value of the upper limit of the reference range of the normal population, which was defined in this study as a peak cTnI level > 0.06 µg/L. The type of PCCs and the exact occurrence time were recorded if any of these complications were present from admission to the operating theatre to 30 days after the surgery. If successive PCCs of more than one type occurred, the first cardiac complication was recorded.
Statistical analysis
Categorical variables were presented as numbers and percentages (%), and continuous variables were presented as the mean ± standard deviation (SD) if the data distribution was normal or else presented as the median and interquartile range (IQR). First, the recorded preoperative and intraoperative factors were analyzed by univariate analysis, such as the chi-square test, t test, or Mann‒Whitney U test, according to different data distributions. Factors with significant differences (P < 0.1) and factors with clinical significance based on previous literature or clinical experience were all included in logistic regression analysis to screen out independent risk factors for PCCs. The receiver operating characteristic (ROC) curve was used to evaluate the discrimination of the indicators to predict PCCs. The area under the curve (AUC) was also calculated (23). Calibration was evaluated using Hosmer and Lemeshow's goodness of fit test and calibration plot. A two-sided P value < 0.05 was considered statistically significant except for the Hosmer and Lemeshow goodness of fit test, in which a two-sided P value < 0.1 was considered significant. A prognostic nomogram was constructed to generate a numerical probability of PCCs at high attitude. Statistical analysis was performed using R (Austria; version 3.5.2) with the “pROC” and “rms” packages (24, 25).
Results
Demographics
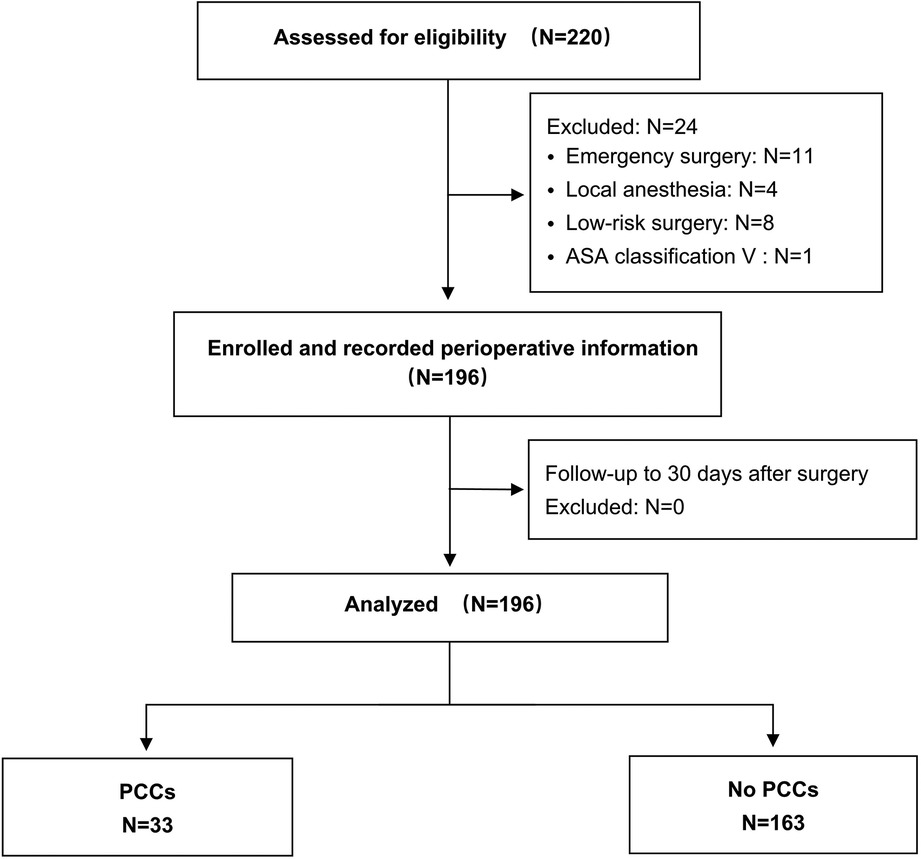
In this study, a total of 220 consecutive patients were assessed for eligibility from June 1st, 2021, to August 31st, 2022, of whom 24 were excluded before surgery because they were judged ineligible. In total, 196 patients were enrolled, underwent surgery and were followed up until 30 days after surgery (Figure 1). Among these patients, the median age was 60.4 ± 7.8 years. Most of them were Tibetans (94.4%), the majority of whom (90.3%) had lived in high-altitude areas above 3,500 m for a long time. The demographic characteristics and types of surgery are summarized in Table 1.
Incidence of PCCs
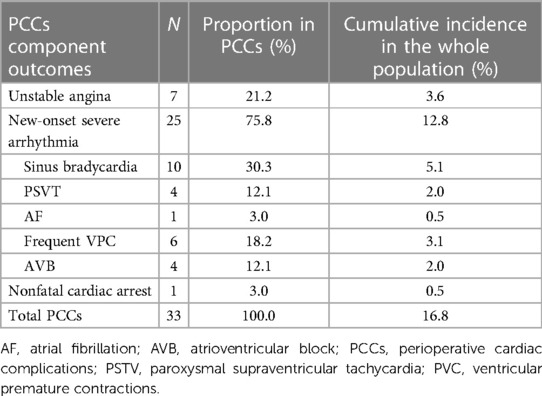
A total of 33 patients (16.8%) had cardiac complications within 30 days after surgery (14 patients intraoperatively, 14 patients postoperatively, and 5 patients experienced complications both intraoperatively and postoperatively). Among the 14 patients with postoperative cardiac complications, 8 patients (57.1%) had PCCs within the first 3 days after surgery. The details of PCCs incidence are shown in Table 2.
Arrhythmia (n = 25, 75.8%) was the most common PCCs in this study, including sinus bradycardia, PSVT, AF, frequent VPC and AVB. Among these 25 patients, 22 patients had cardiac enzyme detection after the operation, and 2 patients with PSVT (2/4) and 1 patient with AF (1/1) were in line with MINS. Seven patients experienced unstable angina during the perioperative period, of whom 5 patients underwent myocardial enzyme examination and were diagnosed with MINS. No patient had perioperative heart failure. One patient suffered nonfatal cardiac arrest, accompanied by elevated myocardial enzymes. One patient with PCCs (intraoperative bradycardia) died 10 days after the operation due to postoperative stroke. Among 163 patients without PCCs, a total of 29 patients were tested for myocardial enzymes, all within the normal range of the level of cTnI.
Independent risk factors of PCCs and ROC analysis
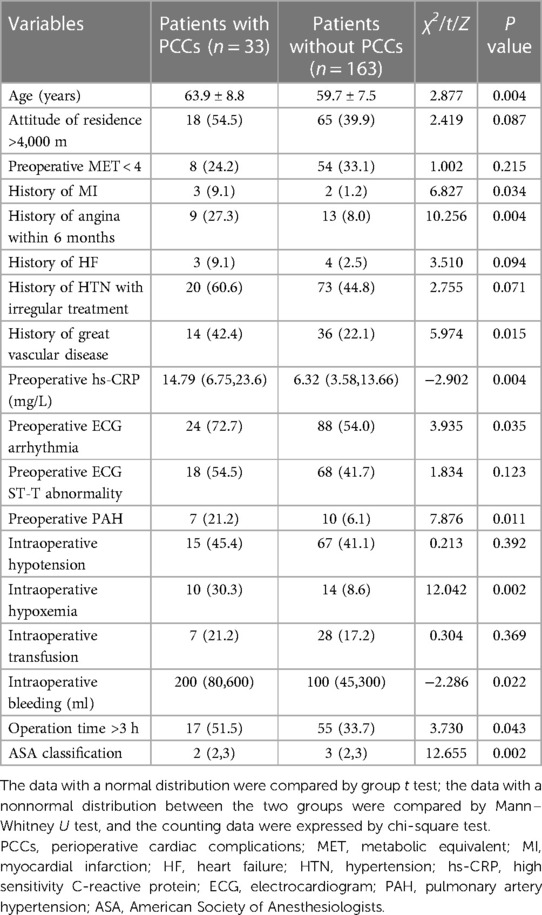
Table 3 shows 14 possible risk factors for PCCs (P < 0.1) in univariate analysis. In addition, we added 4 other clinically important risk factors that may be related to the outcome, namely, preoperative MET < 4, preoperative ECG ST-T abnormality, intraoperative hypotension, and intraoperative transfusion. After analysis by logistic regression, 8 clinical factors were identified in the prediction model, including older age (P = 0.028), extremely high altitude above 4000 m (P = 0.442), preoperative MET < 4 (P = 0.153), history of angina within 6 months (P = 0.037), history of great vascular disease (P = 0.073), increased preoperative high sensitivity C-reactive protein (hs-CRP) (P = 0.072), intraoperative hypoxemia (P = 0.025) and operation time >3 h (P = 0.043) (Table 4). The AUC of the ROC analysis was 0.766 (95% CI: 0.785–0.697, P = 0.196) (Figure 2).
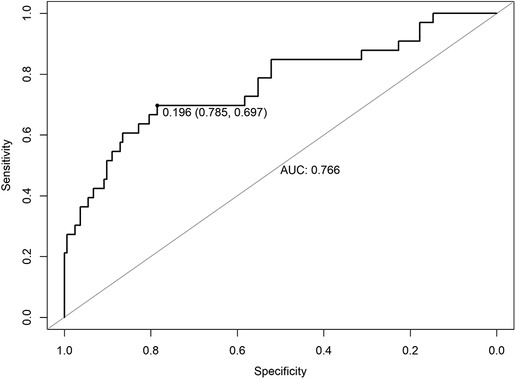
Figure 2. ROC of risk factors for PCCs for patients undergoing major noncardiac surgery in high-attitude areas. ROC, receiver operating characteristic; AUC, area under the curve.
Prognostic nomogram
A prognostic nomogram integrated into the model was constructed (Figure 3). The total score was calculated according to the corresponding score assigned to each variable. The total score could correspond to the risk of PCCs in each patient. The nomogram had a concordance index of 0.766 and was well calibrated (Supplementary Figure S1).
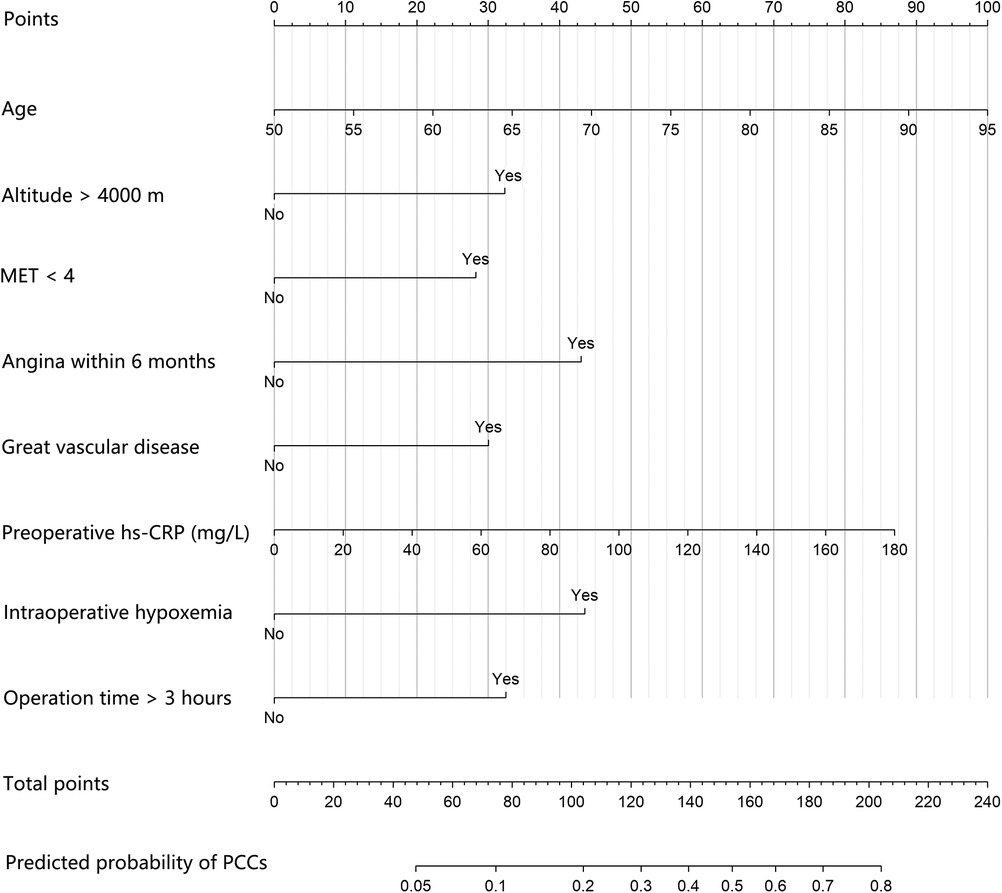
Figure 3. The prognostic nomogram of PCCs for patients undergoing major noncardiac surgery in high-attitude areas. An individual's value is located on each variable axis, and a line is drawn upward to determine the points received for each variable. Corresponding points for each clinical factor are as follows: 50 to 95 years old correspond to 0 to 100 points, respectively; 33 points for high altitude and 0 points for not; 28 points for preoperative MET < 4 and 0 points for not; 43 points for history of angina within 6 months and 0 points for not; 30 points for history of great vascular disease and 0 points for not; 0 to 180 mg/L preoperative hs-CRP correspond to 0 to 87 points, respectively; 44 points for intraoperative hypoxemia and 0 points for not; 33 points for operation time >3 h and 0 points for not. The sum of these points is located on the total point axis, and a line is drawn downward to the survival axes to determine the likelihood of PCCs. PCCs, perioperative cardiac complications; MET, metabolic equivalent; hs-CRP: high sensitivity C-reactive protein.
Discussion
The incidence of PCCs explored in this study was 16.8% in patients who had been living in high-altitude areas for a long time and underwent noncardiac surgery. The prediction model consisting of 8 perioperative risk factors had high discrimination and accuracy in predicting PCCs, which were older age, high altitude above 4,000 m, preoperative MET < 4, history of angina within 6 months, history of great vascular disease, increased preoperative hs-CRP, intraoperative hypoxemia, and operation time >3 h.
As previously reported, the incidence of PCCs in patients undergoing noncardiac surgery varies greatly from 0.9% to 8.6% according to different study populations (26–28). However, there is limited evidence showing the incidence of PCCs in high-altitude areas. The present study found that the incidence of PCCs in patients undergoing noncardiac surgery in Tibet was 16.8%, much higher than that in plain areas. Extremely high altitudes above 4,000 m were an independent risk factor for PCCs among the population in this study. Surely, the hypoxic environment at high altitude leads to a series of alterations in the metabolism and physiological functions of people living there, such as hypertension, pulmonary arterial hypertension (PAH), right heart failure, hyperlipidemia, elevated blood viscosity and erythrocytosis but relatively poor oxygen carrying capacity (29–31). Pulmonary artery pressure and the afterload of the heart may further increase during perioperative stress in low-oxygen environments, aggravating the imbalance of myocardial oxygen supply and demand, which is the most direct cause of perioperative arrhythmia and ischemic heart events.
In this study, arrhythmia was the main type of PCCs in plateau areas. Sinus bradycardia already existed in many patients at high altitude before operation, and the incidence of newly emerging sinus bradycardia during the perioperative period was also the highest among arrhythmias. In fact, this is the cardioprotective mechanism of the body under a high-altitude hypoxic environment (32). In addition, intermittent hypoxic conditioning has been shown to reduce myocardial reperfusion injury, myocardial infarction and tachyarrhythmia through β-adrenergic, δ-dopaminergic, and reactive oxygen-nitrogen signaling pathways (33). Therefore, during the perioperative period, bradycardia does not require urgent drug treatment. ACS is less common than arrhythmia in patients living at high attitude (34). Hypoxia is one of the main causes of myocardial ischemia during surgery (35). In addition, we found that more than half of postoperative cardiac complications occurred within the first 3 days after surgery, in agreement with the results in our previous study (15). Therefore, the first 72 h after operation is also a dangerous period for patients in high-altitude areas to have adverse cardiac events.
MET < 4 has been taken as a threshold for poor functional capacity and is associated with a high risk of adverse cardiac events after major noncardiac surgery (36, 37). In this study, we found that 68.4% of patients had preoperative MET < 4, and these patients were more prone to PCCs. However, it has been reported that only 56% of patients receiving noncardiac surgery in plain areas showed poor exercise tolerance in a prospective study (38). The preoperative activity tolerance of patients at high altitude is generally poor, so preoperative assessment of functional capacity is particularly important for patients at high altitude, and it is a simple but dynamic evaluation reflecting cardiopulmonary and systemic functions to assist static evaluations, such as computed tomography (CT) and echocardiography. Such a simple preoperative evaluation is also suitable for plateau areas where medical resources are relatively scarce.
hs-CRP is a nonspecific marker of acute systemic inflammatory reactions synthesized by the liver and one of the most powerful predictors of cardiovascular and cerebrovascular event risk (39, 40). It has been reported that an increase in preoperative hs-CRP levels is significantly related to the risk of adverse events after surgery (41), which is consistent with the results of our study on the plateau. Hypoxia can directly increase the hepatic synthesis of hs-CRP to promote the inflammatory response (42), indicating the more important predictive value of perioperative levels of hs-CRP in high-altitude localities.
In this study, 24 patients (12.2%) had intraoperative hypoxia, which was significantly associated with PCCs. Patients living in high-altitude areas for a long time have chronic hypoxia and may develop emphysema or cor pulmonale before surgery. Such patients are more likely to suffer from hypoxia under surgical stress than those in plain areas intraoperatively. A cohort study evaluating the relationship between the degree of hypoxemia and cardiac metabolic risk in permanent residents at high altitude found that every 5% decrease in resting SpO2 doubled the cardiac metabolic risk (43). These patients should thus be monitored and managed carefully during the perioperative period.
In addition, older age, history of angina within 6 months and severe vascular disease were also detected as risk factors in predicting PCCs in patients receiving noncardiac surgery at high altitude, which was consistent with previous research in the general population (5, 15, 44–46). Although these factors are uncontrollable, they have great predictive value for preoperative evaluation. A preoperative understanding of these patient factors is also important for the prevention of PCCs.
It has become an international consensus that postoperative cTn monitoring should be carried out for high-risk patients undergoing noncardiac surgery, and MINS is effective in predicting serious cardiac complications and mortality (1, 47). However, it seemed less significant and slightly difficult to achieve in plateau areas. We found that the most types of cardiac events after operation in plateau areas were arrhythmia instead of myocardial ischemia. The myocardial enzymes of patients with arrhythmia could be normal. Besides, timely postoperative cTnI measurement for all enrolled patients was difficult due to limited capacity of the laboratory. Therefore, our main criteria for determining perioperative PCCs in plateau areas in this study were mainly based on more accessible clinical symptoms, monitoring data and ECG, though cTnI monitoring for high-risk patients is also critical when available.
Our nomogram provides surgeons and anesthesiologists with the prediction of PCCs in patients receiving noncardiac surgery at high altitude. Therefore, the preoperative risk assessment can be made very quickly by calculating the 8 demographic and perioperative factors included. Through this nomogram, we can optimize perioperative management by improving preoperative oxygen reserve to fully ensure intraoperative oxygen supply and reduce long-term intraoperative SpO2 < 90% and by improving functional status and activity tolerance. Minimally invasive surgery should be adopted, and the operation time should be controlled as much as possible. For high-risk patients, the operation should be postponed or canceled until the patient's body is adjusted to a better condition.
There are several limitations in our study. Although the discrimination and accuracy are relatively high in predicting PCCs in those patients, we have not verified it internally or externally. The main reason is that the number of cases collected is relatively small; thus, a larger sample size needs to be studied in the future. In addition, due to the limited hospital diagnosis and treatment conditions in plateau areas, some data on possible risk factors could not be collected, such as myocardial enzymes and markers of myocardial injury closely related to PCCs. We acknowledge that without taking MINS as the main endpoint, the design scheme of this study may lack rigor, and the generalization of the research conclusion will be affected. Therefore, we still recommended that myocardial enzymes should be routinely monitored after surgery for high-risk patients, if conditions permit.
Conclusion
In this study, we found that the incidence of PCCs in patients who had been living in the Tibet Autonomous Region for a long time and underwent noncardiac surgery was high. The prediction model that included the following 8 perioperative clinical factors had high discrimination and accuracy in predicting PCCs in high-altitude patients: older age, high altitude above 4,000 m, preoperative MET < 4, history of angina within 6 months, history of great vascular disease, increased preoperative hs-CRP, intraoperative hypoxemia, and operation time >3 h. Perioperative evaluation and management should be strengthened for surgical patients in high-altitude areas to reduce the risk of cardiac events. Future trials should address a larger sample size to verify the promotion value of this prediction model.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the People's Hospital of Tibet Autonomous Region (Ethics No. ME-TBHP-20-26). The patients/participants provided their written informed consent to participate in this study.
Author contributions
ZL, L, LS and YH contributed to the study design; HD and YL enrolled patients and complicated data collection; QZ wrote the manuscript; ZL and YZ helped analyze data; all authors reviewed the report and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a grant from the Tibet Natural Science Funding Committee [No. XZ2020ZR-ZYZ06 (z)] and the National High Level Hospital Clinical Research Funding of Peking Union Medical College Hospital (2022-PUMCH-B-007).
Acknowledgments
The authors thank the following contributors who helped with data collection for this study at their respective sites: Cirendeji, Cideji, Junsheng Duan and Lijiao Zhu, all from the Department of Anesthesiology, Tibet Autonomous Region People's Hospital, Lhasa, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1158711/full#supplementary-material.
References
1. Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. (2014) 120:564–78. doi: 10.1097/aln.0000000000000113
2. Devereaux PJ, Sessler DI. Cardiac complications in patients undergoing Major noncardiac surgery. N Engl J Med. (2015) 373:2258–69. doi: 10.1056/NEJMra1502824
3. Sanaiha Y, Juo YY, Aguayo E, Seo YJ, Dobaria V, Ziaeian B, et al. Incidence and trends of cardiac complications in major abdominal surgery. Surgery. (2018) 164:539–45. doi: 10.1016/j.surg.2018.04.030
4. Li P, Lei Y, Li Q, Lakshmipriya T, Gopinath SCB, Gong X. Diagnosing perioperative cardiovascular risks in noncardiac surgery patients. J Anal Methods Chem. (2019) 2019:6097375. doi: 10.1155/2019/6097375
5. Bovonratwet P, Bohl DD, Malpani R, Haynes MS, Rubio DR, Ondeck NT, et al. Cardiac complications related to spine surgery: timing, risk factors, and clinical effect. J Am Acad Orthop Surg. (2019) 27:256–63. doi: 10.5435/jaaos-d-17-00650
6. Hirota K. Hypoxia-dependent signaling in perioperative and critical care medicine. J Anesth. (2021) 35:741–56. doi: 10.1007/s00540-021-02940-w
7. Sydykov A, Mamazhakypov A, Maripov A, Kosanovic D, Weissmann N, Ghofrani HA, et al. Pulmonary hypertension in acute and chronic high altitude maladaptation disorders. Int J Environ Res Public Health. (2021) 18:1692, doi: 10.3390/ijerph18041692
8. Yang H, Yang G, Xu M, Zhao Y, He S, Wang Q, et al. Impact of high altitude on the incidence of postoperative venous thromboembolism and its genetic susceptibility: a meta-analysis and systematic review. Sci Total Environ. (2022) 838:156632. doi: 10.1016/j.scitotenv.2022.156632
9. Mikołajczak K, Czerwińska K, Pilecki W, Poręba R, Gać P, Poręba M. The impact of temporary stay at high altitude on the circulatory system. J Clin Med. (2021) 10:1622 doi: 10.3390/jcm10081622
10. Damodar D, Donnally CJ, 3rd, Sheu JI, Law TY, Roche MW, Hernandez VH. A higher altitude is an independent risk factor for venous thromboembolisms after total hip arthroplasty. J Arthroplasty. (2018) 33:2627–30. doi: 10.1016/j.arth.2018.03.045
11. Tyson JJ, Bjerke BP, Genuario JW, Noonan TJ. Thromboembolic events after arthroscopic knee surgery: increased risk at high elevation. Arthroscopy. (2016) 32:2350–4. doi: 10.1016/j.arthro.2016.04.008
12. Cancienne JM, Diduch DR, Werner BC. High altitude is an independent risk factor for postoperative symptomatic venous thromboembolism after knee arthroscopy: a matched case-control study of medicare patients. Arthroscopy. (2017) 33:422–7. doi: 10.1016/j.arthro.2016.07.031
13. Gupta N, Ashraf MZ. Exposure to high altitude: a risk factor for venous thromboembolism? Semin Thromb Hemost. (2012) 38:156–63. doi: 10.1055/s-0032-1301413
14. Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. (2014) 130:2215–45. doi: 10.1161/cir.0000000000000105
15. Liu Z, Xu G, Xu L, Zhang Y, Huang Y. Perioperative cardiac complications in patients over 80 years of age with coronary artery disease undergoing noncardiac surgery: the incidence and risk factors. Clin Interv Aging. (2020) 15:1181–91. doi: 10.2147/cia.S252160
16. Qian Z, Fan A, Dawa D, Pan B. Retrospective cohort analysis of heart rate variability in patients with high altitude pulmonary hypertension in Tibet. Clin Cardiol. (2020) 43:298–304. doi: 10.1002/clc.23312
17. Salehi A. Pulmonary hypertension: a review of pathophysiology and anesthetic management. Am J Ther. (2012) 19:377–83. doi: 10.1097/MJT.0b013e3181f94c02
18. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr., Chung MK, de Lemos JA, et al. ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American college of cardiology foundation/American heart association task force on practice guidelines: developed in collaboration with the American college of emergency physicians and society for cardiovascular angiography and interventions. Catheter Cardiovasc Interv. (2013) 82:E1-27. doi: 10.1002/ccd.24776
19. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr., Ganiats TG, Holmes DR Jr., et al. AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) 64:e139–228. doi: 10.1016/j.jacc.2014.09.017
20. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Circulation. (2012) 126:2020–35. doi: 10.1161/CIR.0b013e31826e1058
21. Andersen LW, Holmberg MJ, Berg KM, Donnino MW, Granfeldt A. In-Hospital cardiac arrest: a review. Jama. (2019) 321:1200–10. doi: 10.1001/jama.2019.1696
22. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of cardiology (ESC). endorsed by: association for European paediatric and congenital cardiology (AEPC). Eur Heart J. (2015) 36:2793–867. doi: 10.1093/eurheartj/ehv316
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
24. Turck N, Vutskits L, Sanchez-Pena P, Robin X, Hainard A, Gex-Fabry M, et al. A multiparameter panel method for outcome prediction following aneurysmal subarachnoid hemorrhage. Intensive Care Med. (2010) 36:107–15. doi: 10.1007/s00134-009-1641-y
25. Liu Z, Xu G, Zhang Y, Duan H, Zhu Y, Xu L. Preoperative transthoracic echocardiography predicts cardiac complications in elderly patients with coronary artery disease undergoing noncardiac surgery. Clin Interv Aging. (2022) 17:1151–61. doi: 10.2147/cia.S369657
26. Santangelo G, Faggiano A, Toriello F, Carugo S, Natalini G, Bursi F, et al. Risk of cardiovascular complications during non-cardiac surgery and preoperative cardiac evaluation. Trends Cardiovasc Med. (2022) 32:271–84. doi: 10.1016/j.tcm.2021.06.003
27. Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. Jama. (2012) 307:2295–304. doi: 10.1001/jama.2012.5502
28. Appel D, Böger R, Windolph J, Heinze G, Goetz AE, Hannemann J. Asymmetric dimethylarginine predicts perioperative cardiovascular complications in patients undergoing medium-to-high risk non-cardiac surgery. J Int Med Res. (2020) 48:300060520940450. doi: 10.1177/0300060520940450
29. Palubiski LM, O'Halloran KD, O'Neill J. Renal physiological adaptation to high altitude: a systematic review. Front Physiol. (2020) 11:756. doi: 10.3389/fphys.2020.00756
30. Wang SY, Gao J, Zhao JH. Effects of high altitude on renal physiology and kidney diseases. Front Physiol. (2022) 13:969456. doi: 10.3389/fphys.2022.969456
31. Zhang X, Zhang Z, Ye R, Meng Q, Chen X. Prevalence of hypertension and its relationship with altitude in highland areas: a systematic review and meta-analysis. Hypertens Res. (2022) 45:1225–39. doi: 10.1038/s41440-022-00955-8
32. Meerson FZ, Ustinova EE, Orlova EH. Prevention and elimination of heart arrhythmias by adaptation to intermittent high altitude hypoxia. Clin Cardiol. (1987) 10:783–9. doi: 10.1002/clc.4960101202
33. Mallet RT, Manukhina EB, Ruelas SS, Caffrey JL, Downey HF. Cardioprotection by intermittent hypoxia conditioning: evidence, mechanisms, and therapeutic potential. Am J Physiol Heart Circ Physiol. (2018) 315:H216–h232. doi: 10.1152/ajpheart.00060.2018
34. Ollila A, Virolainen J, Vanhatalo J, Vikatmaa P, Tikkanen I, Venermo M, et al. Postoperative cardiac ischemia detection by continuous 12-lead electrocardiographic monitoring in vascular surgery patients: a prospective, observational study. J Cardiothorac Vasc Anesth. (2017) 31:950–6. doi: 10.1053/j.jvca.2016.09.027
35. Rosenberg-Adamsen S, Lie C, Bernhard A, Kehlet H, Rosenberg J. Effect of oxygen treatment on heart rate after abdominal surgery. Anesthesiology. (1999) 90:380–4. doi: 10.1097/00000542-199902000-00008
36. Kristensen SD, Knuuti J, Saraste A, Anker S, Bøtker HE, Hert SD, et al. ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the joint task force on non-cardiac surgery: cardiovascular assessment and management of the European society of cardiology (ESC) and the European society of anaesthesiology (ESA). Eur Heart J. (2014) 35:2383–431. doi: 10.1093/eurheartj/ehu282
37. Gilbert-Kawai E, Montgomery H. Cardiovascular assessment for non-cardiac surgery: european guidelines. Br J Hosp Med (London). (2017) 78:327–32. doi: 10.12968/hmed.2017.78.6.327
38. Reilly DF, McNeely MJ, Doerner D, Greenberg DL, Staiger TO, Geist MJ, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. (1999) 159:2185–92. doi: 10.1001/archinte.159.18.2185
39. Xu M, Yan L, Xu J, Yang X, Jiang T. Predictors and prognosis for incident in-hospital heart failure in patients with preserved ejection fraction after first acute myocardial infarction: an observational study. Medicine (Baltimore). (2018) 97:e11093. doi: 10.1097/md.0000000000011093
40. Zhang X, Huang WJ, Yu ZG. Relationship between the hypersensitive c-reactive protein (hs-CRP) level and the prognosis of acute brainstem infarction. Cell Biochem Biophys. (2015) 72:107–10. doi: 10.1007/s12013-014-0414-6
41. Verwijmeren L, Bosma M, Vernooij LM, Linde EM, Dijkstra IM, Daeter EJ, et al. Associations between preoperative biomarkers and cardiac surgery-associated acute kidney injury in elderly patients: a cohort study. Anesth Analg. (2021) 133:570–7. doi: 10.1213/ane.0000000000005650
42. Tomita H, Takamuro M, Soda W, Hatakeyama K, Tsutsumi H. Increased serum high-sensitivity C-reactive protein is related to hypoxia and brain natriuretic peptide in congenital heart disease. Pediatr Int. (2008) 50:436–40. doi: 10.1111/j.1442-200X.2008.02581.x
43. Miele CH, Schwartz AR, Gilman RH, Pham L, Wise RA, Davila-Roman VG, et al. Increased cardiometabolic risk and worsening hypoxemia at high altitude. High Alt Med Biol. (2016) 17:93–100. doi: 10.1089/ham.2015.0084
44. Pipanmekaporn T, Punjasawadwong Y, Charuluxananan S, Lapisatepun W, Bunburaphong P, Patumanond J, et al. Incidence of and risk factors for cardiovascular complications after thoracic surgery for noncancerous lesions. J Cardiothorac Vasc Anesth. (2014) 28:948–53. doi: 10.1053/j.jvca.2014.02.002
45. Goldman L, Caldera DL, Southwick FS, Nussbaum SR, Murray B, O'Malley TA, et al. Cardiac risk factors and complications in non-cardiac surgery. Medicine (Baltimore). (1978) 57:357–70. doi: 10.1097/00005792-197807000-00006
46. Chapman GC, Sheyn D, Slopnick EA, Hijaz AK, Mahajan ST, Mangel J, et al. Perioperative safety of surgery for pelvic organ prolapse in elderly and frail patients. Obstet Gynecol. (2020) 135:599–608. doi: 10.1097/aog.0000000000003682
Keywords: Tibet autonomous region, high altitude, perioperative cardiac complications, noncardiac surgery, risk factor
Citation: Zhu Q, Duan H, Liu Z, Li Y, Zhang Y, Shen L and Huang Y (2023) The incidence and risk factors of perioperative cardiac complications in noncardiac major surgery in high-altitude areas: A prospective trial in Tibet autonomous region, China. Front. Cardiovasc. Med. 10:1158711. doi: 10.3389/fcvm.2023.1158711
Received: 4 February 2023; Accepted: 17 March 2023;
Published: 3 April 2023.
Edited by:
Lars Norgren, Örebro University, SwedenReviewed by:
Alben Sigamani, Numen Health, IndiaFilippo Toriello, Università degli studi di Milano, Italy
© 2023 Zhu, Duan, Liu, Li, Zhang, Labaciren, Shen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zijia Liu bGl1LXpqMDJAMTI2LmNvbQ== Labaciren bGJjcjAxMDIwM0AxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Cardiovascular Epidemiology and Prevention, a section of the journal Frontiers in Cardiovascular Medicine
 Qianmei Zhu
Qianmei Zhu Hanyu Duan2,†
Hanyu Duan2,† Yuelun Zhang
Yuelun Zhang