- 1Department of Cardiology, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, China
- 2Department of Gastroenterology Centre, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou, China
- 3Department of Cardiology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, China
Background and aims: The roles of sodium-glucose cotransporter 2 inhibitor (SGLT2i) in acute heart failure (AHF) risk after acute myocardial infarction (AMI) remain unclear. In this study, we explored the correlation between SGLT2i administration and short-term in-hospital AHF risk in AMI patients.
Methods: This single-center, retrospective, and observational study included 990 AMI patients comprising 386 non-ST-segment elevation myocardial infarction (NSTEMI) and 604 segment elevation myocardial infarction (STEMI) patients enrolled from January 2019 to March 2022. Demographic information, clinical characteristics, medical treatment, and laboratory examination results during hospitalization were extracted from an electronic medical record system. The primary outcome was defined as all-cause AHF during hospitalization.
Results: In NSTEMI patients, a significantly lower proportion received SGLT2i treatment in the AHF group compared with the non-AHF group. During hospitalization, SGLT2i significantly reduced brain natriuretic peptide levels both in STEMI and NSTEMI patients. Multivariate logistic regression and stratification analyses suggested that SGLT2i is associated with reduced in-hospital AHF risk, and has a strong protective effect against AHF in NSTEMI patients with hypertension. Furthermore, SGLT2i significantly reduced the risk of in-hospital AHF for both patients with diabetes and non-diabetes.
Conclusions: SGLT2i can reduce the risk of AHF in AMI patients during hospitalization.
Introduction
Acute myocardial infarction (AMI) is a serious and fatal cardiovascular emergency. Rupture of vulnerable coronary plaques can result in thrombosis, leading to complete or partial coronary artery occlusion, and eventually causing myocardial ischemia or necrosis. With advances in coronary intervention technology and the standardization of admission process for patients with chest pain, the mortality rate of AMI patients has been considerably reduced, and the complications caused by necrotic myocardial tissue have been greatly decreased. However, the adverse cardiovascular events such as acute heart failure (AHF) and arrhythmia that occur among in-hospital AMI patients still pose a serious burden on postoperative management and the rational allocation of medical resources (1, 2).
Studies have confirmed that sodium-glucose cotransporter 2 inhibitor (SGLT2i) can significantly improve cardiovascular and renal outcomes (3). A meta-analysis of randomized trials reveals that SGLT2i reduce mortality and morbidity in patients with heart failure (4). Although current guidelines generally recommend that SGLT2i should be discontinued during AMI, a recent JACC report highlighted the potential for improved patient outcomes through early application of SGLT2i in AMI (5). The safety issue of SGLT2i for type 2 diabetes mellitus (T2DM) patients combined with AMI deserves our attention. SGLT2i treatment may result in an asymptomatic increase in blood ketone body levels, but the vast majority of patients can compensate for this slight increase in ketone body levels. From the perspective of mechanism, SGLT2i related diabetes ketoacidosis can be predicted, prevented and controlled. The increase in ketone body levels is a metabolic adaptation of the body to glucose loss. In the presence of metabolic stress, especially in patients with diabetes and heart failure, the ketone body energy supply is more efficient and plays a protective role in the heart (6). Due to differences in the number and extent of lesions and emergency treatment strategies between ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI) patients, the mechanism and incidence of AHF during hospitalization are also different. At present, the discrepancies in the protection provided by SGLT2i against AHF in STEMI or NSTEMI patients during hospitalization are unclear.
In this retrospective study, we aimed to investigate the effect of SGLT2i intervention on HF indicators in hospitalized STEMI and NSTEMI patients, and to explore the correlation between SGLT2i administration and short-term risk of AHF during hospitalization in AMI patients.
Patients and methods
Participants
This study was performed in compliance with the Declaration of Helsinki, and was approved by the Committee of Clinical Investigation of The Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University (KY314-01).
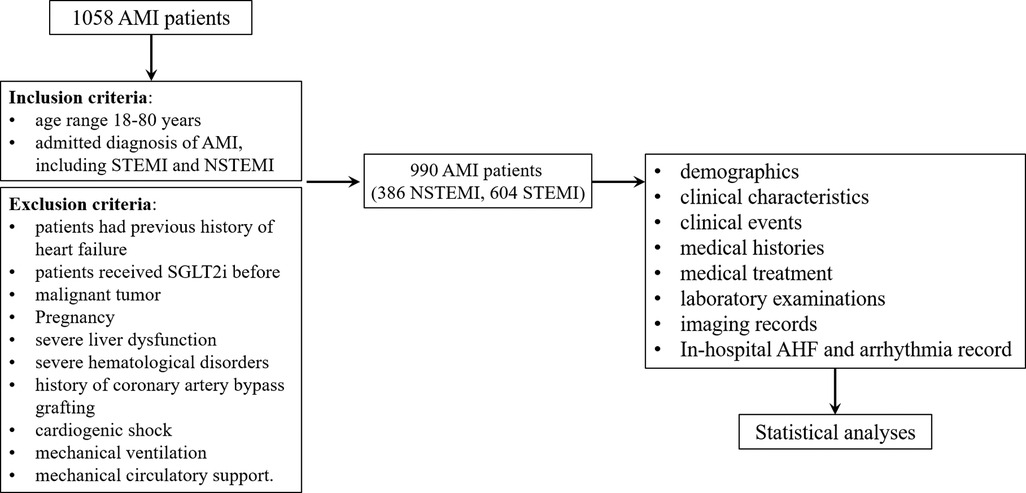
This single-center, retrospective, and observational study was registered in the China Clinical Trial Registration Center (ChiCTR2300067892). In total, 990 patients comprising 386 NSTEMI and 604 STEMI patients admitted to the Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University from January 2019 to March 2022 were enrolled in this study. The research protocol was shown in Figure 1.
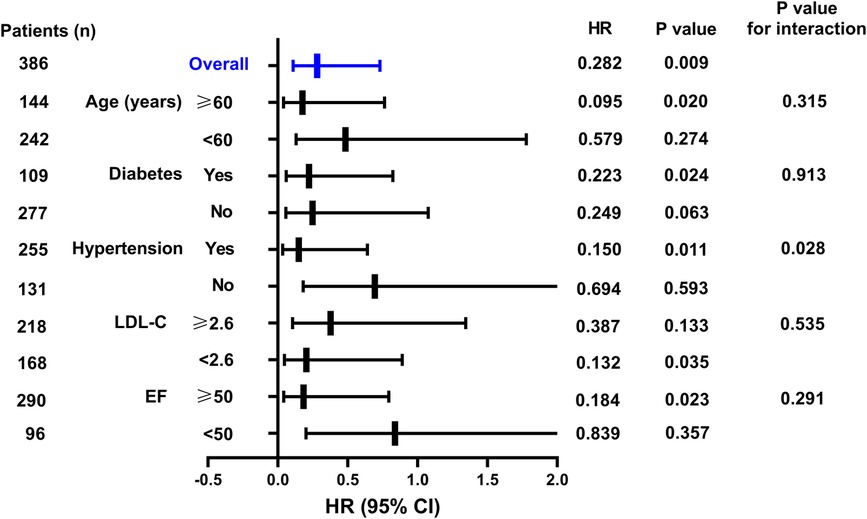
Figure 1. Hazard ratios of the SGLT2i for predicting in-hospital AHF in NSTEMI group in the subgroup analysis..
The inclusion criteria were as follows: (1) age range 18–80 years, (2) admitted diagnosis of AMI, including STEMI and NSTEMI. Diagnostic criteria of STEMI: (1) a history of chest pain/discomfort; (2) at admission, continuous elevation of ST segments in two or more adjacent ECG leads ≥0.1 mV (>30 min, V2, V3 ≥ 0.2 mV) or new onset of left bundle branch block; (3) myocardial injury markers (troponin, CK-MB) increased beyond the 99th percentile of the laboratory reference limit. Diagnostic criteria of NSTEMI: (1) a history of chest pain/discomfort; (2) in leads with R wave dominant or R/S > 1, new horizontal or downwardly inclined ST segment depression ≥0.05 mV or T wave inversion ≥0.1 mV appears in two adjacent leads; (3) myocardial injury markers (troponin, CK-MB) increased beyond the 99th percentile of the laboratory reference limit.
The patient exclusion criteria were as follows: (1) patients had previous history of heart failure, (2) patients received SGLT2i before, (3) malignant tumor, (4) pregnancy, (5) severe liver dysfunction, (6) severe hematological disorders, (7) history of coronary artery bypass grafting, (8) cardiogenic shock, (9) mechanical ventilation, and (10) mechanical circulatory support. Severe liver dysfunction was diagnosed as the elevated serum transaminases, severely elevated serum bilirubin, decreased albumin concentration, and coagulation disorders. Severe hematological disorders were considered as multiple myeloma, lymphoma, myelodysplastic syndrome, or leukemia.
Data collection and definition
Information relating to demographics, clinical characteristics, clinical events, medical histories, medical treatment, laboratory examinations, and imaging records collected during hospitalization were extracted from an electronic medical record system.
The primary end-point was in-hospital AHF, and the second end-points were BNP levels and in-hospital arrhythmia. AHF was diagnosed based on typical symptoms, signs, and laboratory tests of factors, such as orthopnea, acute pulmonary edema, and BNP levels. Considering that during the acute phase, AHF events and subsequent respiratory distress may result in discontinuation of feeding and avoidance of SGLT2i, only AHF events occurring 48 h after admission were recorded. Arrhythmia was defined as at least one episode of atrial fibrillation, atrial flutter, ventricular fibrillation, or ventricular flutter. Only arrhythmia events occurring 48 h after admission were recorded. The Gensini score was used to assess the severity of coronary artery disease and was calculated according to a previously described protocol (7).
Statistical analysis
All data were tested for normal distribution. Approximately normally distributed data were expressed as the mean ± standard deviation and skewed continuous variables were expressed as the median (interquartile range). Continuous variables between two groups were compared using Student's t-test or the Mann–Whitney U-test. The χ2 test was used for comparisons of categorical variables between groups. Univariate and multivariate logistic regression analyses were performed to identify the predictive value of SGLT2i intervention for AHF or arrhythmia risk during hospitalization. All tests were two-sided. P-values <0.05 were considered statistically significant. Statistical analyses were performed using SPSS software 22.0 (SPSS Inc., Chicago, IL, USA).
Results
Table 1 shows the clinical and biochemical characteristics of the 990 patients enrolled in this retrospective study. All-cause AHF was recorded for 38 of 604 STEMI and 51of 386 NSTEMI patients during hospitalization. Among the STEMI patients, the mean age of patients with AHF was significantly higher than that of patients without AHF. In addition, the proportion of concurrent arrhythmia in the AHF group was significantly higher than that in the non-AHF group for both STEMI and NSTEMI patients. The Gensini score was used to reflect the severity of coronary lesions. In NSTEMI patients, the Gensini score in the AHF group was significantly higher than that in the non-AHF group, while there was no significant difference among the STEMI patients. Comparison of biochemical test data revealed that in NSTEMI patients, the AHF group had lower HDL-C levels compared with the non-AHF group, and higher levels of myocardial injury and heart failure markers (creatine phosphokinase, creatine kinase-MB, hydroxybutyrate dehydrogenase, and BNP), while there were no significant differences among the STEMI patients. Furthermore, patients with NSTEMI and AHF had lower EF values than those without AHF. Finally, we analyzed the patients' drug interventions during hospitalization. Among NSTEMI patients, the proportions of patients receiving angiotensin receptor-neprilysin inhibitor (ARNI) and SGLT2i therapies in the AHF group were significantly lower than those in the non-AHF group, while the opposite trend was observed for mineralocorticoid receptor antagonist (MRA) treatment.
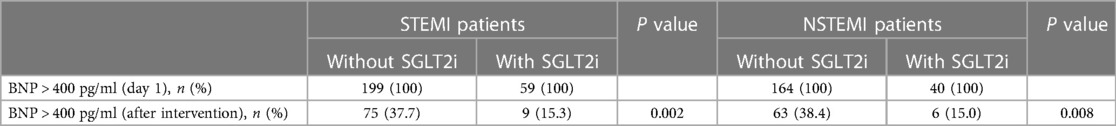
Since BNP tests are performed to evaluate cardiac function and prognosis for in-hospital AMI patients, we grouped STEMI and NSTEMI patients according to whether SGLT2i was used. In total, 462 patients (BNP >400 ng/ml, day 1) were enrolled, comprising 258 patients in the STEMI group (59 in the SGLT2i group and 199 in the non-SGLT2i group) and 204 patients in the NSTEMI group (40 in the SGLT2i group and 164 in the non-SGLT2i group). Table 2 showed that SGLT2i administration markedly decreased the proportion of patients whose BNP levels were over 400 ng/ml, compared with effects observed in the SGLT2i-free groups.
Multivariate logistic regression analyses showed that SGLT2i therapy was associated with reduced in-hospital AHF risk in STEMI patients (P < 0.05 for models 3–5) (Table 3). In both univariate and multivariate logistic regression analyses, SGLT2i intervention was associated with a reduction in the risk of AHF occurrence during hospitalization of NSTEMI patients (P < 0.05 for models 1–4) (Table 3). In both univariate and multivariate logistic regression analyses, SGLT2i was not associated with reduced in-hospital arhythmia risk in STEMI and NSTEMI patients (P > 0.05 for model 1–5) (Table 4).
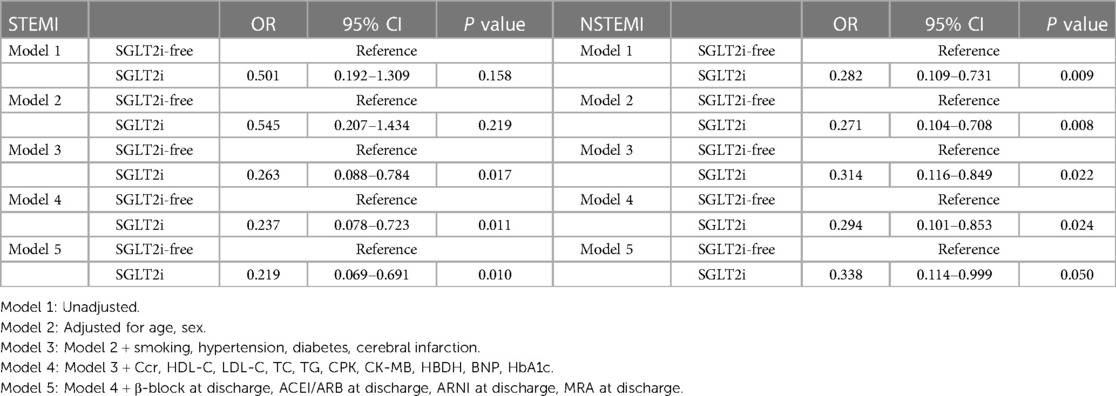
Table 3. Univariate and multivariate logistic analyses for the in-hospital AHF risk according to the SGLT2i administration.
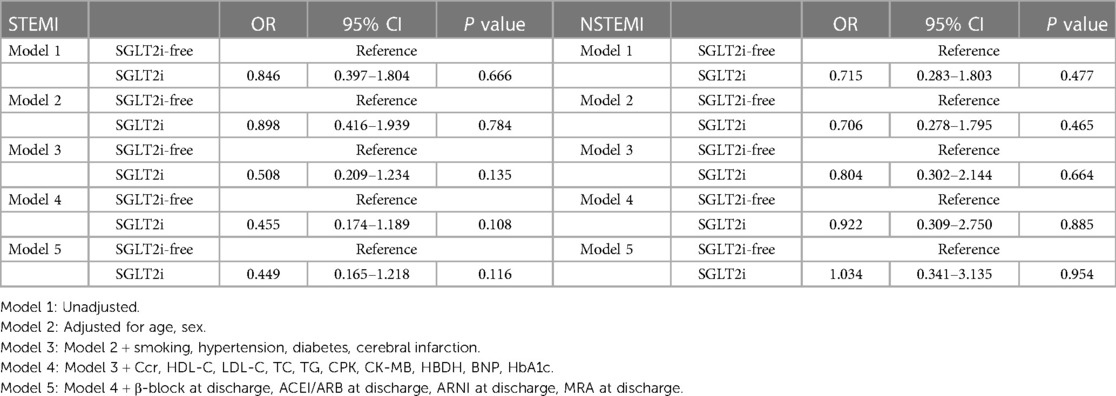
Table 4. Univariate and multivariate logistic analyses for the in-hospital arrhythmia risk according to the SGLT2i administration.
As shown in Figure 2, when stratified by age, for patients aged ≥60 years, the in-hospital AHF occurrence rate in the SGLT2i group was increased by 0.177-fold (95% CI: 0.041–0.764, P = 0.020) compared to that in the SGLT2i-free group. For diabetic patients, the in-hospital AHF risk in the SGLT2i group was 0.223-fold lower than that in the SGLT2i-free group (95% CI: 0.060–0.824, P = 0.024), although the prediction values in non-diabetic patients were not statistically significant. For patients with hypertension, the in-hospital AHF risk in the SGLT2i group was 0.150-fold lower than that in the SGLT2i-free group (95% CI: 0.035–0.641, P = 0.011). The prediction values in patients without hypertension were not statistically significant, and the P value for interaction was 0.028. For patients with LDL-C < 2.6 mmol/L, the in-hospital AHF risk in the SGLT2i group was 0.204-fold lower than that in the SGLT2i-free group (95% CI: 0.047–0.891, P = 0.035), while the prediction values in patients (LDL-C ≥ 2.6 mmol/L) were not statistically significant. For patients with EF ≥50%, the in-hospital AHF risk in the SGLT2i group was 0.184-fold lower than that in the SGLT2i-free group (95% CI: 0.043–0.794, P = 0.023), However, in EF <50% subgroup, there was no significant difference between the SGLT2i and SGLT2i-free groups in terms of in-hospital AHF risk prediction.
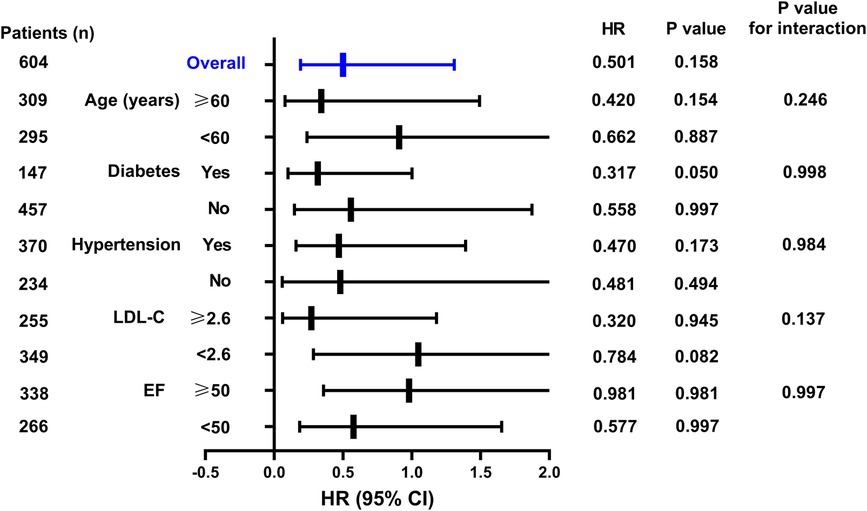
Figure 2. Hazard ratios of the SGLT2i for predicting in-hospital AHF in NSTEMI group in the subgroup analysis.
As shown in Figure 3, after stratifying STEMI patients by age (<60 or ≥60 years), diabetes (yes or no), hypertension (yes or no), LDL-C (<2.6 or ≥2.6) and EF (<50% or ≥50%), the AHF risk prediction values for SGLT2i treatment remained statistically insignificant.
Furthermore, we explored the effect of SGLT2i on the occurrence of in-hospital AHF and in-hospital arrhythmia in diabetics or non-diabetics. Table 5 demonstrated that SGLT2i could reduce the occurrence of in-hospital AHF both in diabetics and non-diabetics. However, for the occurrence of in-hospital arrhythmia, there was no significant difference between diabetics and non-diabetics.
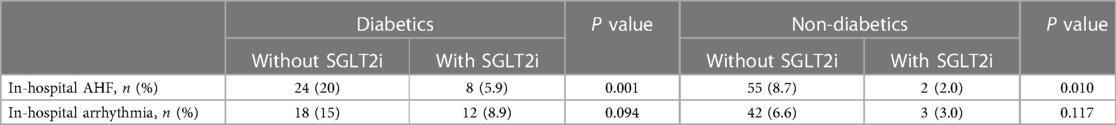
Table 5. SGLT2i significantly reduced the risk of in-hospital AHF for both diabetics and non-diabetics.
Considering that part of the included patients used ARNI, which may have a synergistic effect with SGLT2i, we finally analyzed the impact of ARNI use on the SGLT2i effect. Table 6 suggested that ARNI use has no significant effect on SGLT2i reducing the risk of AHF in both STEMI and NSTEMI patients.

Table 6. ARNI had no significant effect on SGLT2i reducing the risk of AHF in both STEMI and NSTEMI patients.
Discussion
In this retrospective study of 990 patients, we found in NSTEMI patients, the proportion of SGLT2i intervention in the AHF group was significantly lower than that in the non-AHF group. In addition, we found that SGLT2i significantly reduced the BNP levels of STEMI and NSTEMI patients. SGLT2i was also found to be associated to improve the outcome for in-hospital AHF risk both in STEMI and NSTEMI patients, and had strong protective effects against in-hospital AHF risk in NSTEMI patients with hypertension. Furthermore, SGLT2i significantly reduced the risk of in-hospital AHF for both diabetics and non-diabetics.
Patients with AMI are at high risk of HF, severe arrhythmia, and cardiovascular death. Despite significant advances in early treatment strategies for myocardial infarction, patients are still exposed to residual cardiovascular risks associated with current drug treatments, especially in the critical early stage after AMI, when it is necessary to prevent adverse cardiac remodeling, AHF, and cardiovascular death (8). According to domestic and foreign guidelines and consensuses, SGLT2i can be used for patients with chronic HF with and without diabetes as well as those with chronic kidney disease (9, 10). SGLT2i can also be used in adult patients with reduced ejection fraction of heart failure (HFrEF) (NYHA II–IV) to decrease the risk of cardiovascular death and hospitalization for HF, regardless of whether the patient has diabetes (11). However, very few studies have been conducted on the effect of SGLT2i on the risk of in-hospital AHF in AMI patients.
In the initial analyses of basic clinical characteristics, we unexpectedly found that, among STEMI patients, there were no significant differences in basic clinical characteristics, biochemical analysis, echocardiographic results, and drug intervention between the AHF and non-AHF groups, with the exception of age and arrhythmia records. However, in NSTEMI patients, the AHF group had higher coronary severity, more significant traditional cardiovascular risk factors and indicators, and lower usage rate of ARNI and SGLT2i compared to the non-AHF group. It can be speculated that this difference of caused by most STEMI patients having relatively few coronary lesions, and therefore, emergency PCI is largely effective in preventing myocardial necrosis and subsequent complications, while in NSTEMI patients, coronary lesions are more diffuse, although the lesions are caused by non-transmural necrosis. Patients admitted to hospital are usually treated conservatively and with optional coronary interventions for some lesions. Therefore, patients with more significant traditional cardiovascular factors are prone to AHF. Thus, these results also suggested that the absence of ARNI or SGLT2i is, to some extent, related to the occurrence of AHF. Therefore, we then analyzed the effect of SGLT2i on BNP levels, and the results were as expected in that SGLT2i reduced BNP levels both in STEMI and non-STEMI patients. Although the previous study has shown that the protective effect of SGLT2i on HF does not depend on ARNI (12), our results suggested that ARNI has no significant effect on SGLT2i reducing the risk of AHF in both STEMI and NSTEMI patients, further indicating the protective effect of SGLT2i on AHF.
Logistic regression analyses suggested that SGLT2i treatment independently predicts the occurrence of AHF both in STEMI and NSTEMI patients. Logistic regression analyses for in-hospital AHF prediction showed that the HR of the SGLT2i group was lower than that of the SGLT2i-free group. Since AHF and arrhythmia were not consistent, the absence of statistical significance in the arrhythmia analysis was considered to be related to the small sample size. Furthermore, the stratified analysis suggested that, in NSTEMI patients, SGLT2i is more likely to reduce the risk of AHF in patients with hypertension, which reflected the specific cardiovascular protective mechanisms of SGLT2i. SGLT2i may not directly inhibit coronary thrombosis, but instead may inhibit neurohumoral activation (13), myocardial cell necrosis (14), and reperfusion injury (15). It may also enhance endothelial cell function and vasodilation (16), promote myocardial energy metabolism, maintain myocardial contractility (17), inhibit oxidative stress (18), improve coronary blood flow, and reduce ventricular load (19). These mechanisms of action may further prevent myocardial hypertrophy, myocardial fibrosis, and heart failure (20). SGLT2i may also have additional cardiometabolic benefits in high-risk groups following myocardial infarction, including reduced ventricular afterload and preload (21), improved glycemic control (22), and weight loss (23). Specifically, SGLT2i benefits the heart through mechanisms such as diuresis, natriuresis, reduction of inflammation and oxidative stress, promotion of red blood cell generation, inhibition of sympathetic nervous activity, improvement of cardiac energy metabolism and cardiac remodeling, and ultimately improvement of vascular function (24, 25). Therefore, in the face of extremely complex cardiac and vascular homeostasis disorders, SGLT2i can exert its unique cardiovascular protective effect to prevent the occurrence of AHF. Finally, we investigated the effect of SGLT2i on in-hospital AHF and the arrhythmia in diabetics or non-diabetics, which was consistent with previous reports, SGLT2i has an inhibitory effect on the occurrence of AHF in the non-diabetic patients. However, SGLT2i has no significant improvement effect on the occurrence of arrhythmia. From the data analysis, it can be seen that the use of SGLT2i can reduce the risk of the occurrence of arrhythmia. Further expanding the sample size may have different results.
There were some limitations in this study. As a single-center and retrospective study, bias caused by confounding factors was a prominent problem, although methods such as stratified analysis and both univariate and multivariate logistic regression analysis were used to eliminate the interference of confounding factors.
Conclusions
In conclusion, SGLT2i can reduce the risk of AHF in AMI patients during hospitalization and is associated with a strong protective effect against AHF in NSTEMI patients with hypertension. SGLT2i significantly reduced the risk of in-hospital AHF for both diabetics and non-diabetics, which further suggests a protective role of SGLT2i in AMI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Committee of Clinical Investigation of The Affiliated Changzhou No.2 People's Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YZ drafted the manuscript. JZ contributed to the case collection and database organization. HJ was responsible for statistical analysis of the data. FW and YJ contributed to the conception and design of this article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Changzhou Health and Wellness Committee Research Project (CQ20210122) and the Changzhou Health and Wellness Committee Research Project (ZD202112).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lecour S, Andreadou I, Bøtker HE, Davidson SM, Heusch G, Ruiz-Meana M, et al. IMproving preclinical assessment of cardioprotective therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST action. Basic Res Cardiol. (2021) 116(1):52. doi: 10.1007/s00395-021-00893-5
2. Ko DT, Khera R, Lau G, Qiu F, Wang Y, Austin PC, et al. Readmission and mortality after hospitalization for myocardial infarction and heart failure. J Am Coll Cardiol. (2020) 75(7):736–46. doi: 10.1016/j.jacc.2019.12.026
3. Gulsin GS, Graham-Brown MPM, Squire IB, Davies MJ, McCann GP. Benefits of sodium glucose cotransporter 2 inhibitors across the spectrum of cardiovascular diseases. Heart. (2022) 108(1):16–21. doi: 10.1136/heartjnl-2021-319185
4. Camilli M, Lombardi M, Chiabrando JG, Zito A, Del Buono MG, Vergallo R, et al. Sodium-Glucose cotransporter inhibitors reduce mortality and morbidity in patients with heart failure: evidence from a meta-analysis of randomized trials. Am J Ther. (2021) 29(2):e199–204. doi: 10.1097/MJT.0000000000001452
5. Omar M, Jensen J, Frederiksen PH, Kistorp C, Videbæk L, Poulsen MK, et al. Effect of empagliflozin on hemodynamics in patients with heart failure and reduced ejection fraction. J Am Coll Cardiol. (2020) 76(23):2740–51. doi: 10.1016/j.jacc.2020.10.005
6. He Z, Lam K, Zhao W, Yang S, Li Y, Mo J, et al. SGLT-2 inhibitors and euglycemic diabetic ketoacidosis/diabetic ketoacidosis in FAERS: a pharmacovigilance assessment. Acta Diabetol. (2023) 60(3):401–11. doi: 10.1007/s00592-022-02015-6
7. Liu HH, Cao YX, Li S, Guo YL, Zhu CG, Wu NQ, et al. Impacts of prediabetes Mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes.. Hypertension. (2018) 71(6):1039–46. doi: 10.1161/HYPERTENSIONAHA.118.11063
8. Song L, Zhao X, Chen R, Li J, Zhou J, Liu C, et al. Association of PCSK9 with inflammation and platelet activation markers and recurrent cardiovascular risks in STEMI patients undergoing primary PCI with or without diabetes. Cardiovasc Diabetol. (2022) 21(1):80. doi: 10.1186/s12933-022-01519-3
9. Wong CKH, Lau KTK, Tang EHM, Lee CH, Lee CYY, Woo YC, et al. Cardiovascular benefits of SGLT2 inhibitors in type 2 diabetes, interaction with metformin and role of erythrocytosis: a self-controlled case series study. Cardiovasc Diabetol. (2022) 21(1):92. doi: 10.1186/s12933-022-01520-w
10. Kaze AD, Zhuo M, Kim SC, Patorno E, Paik JM. Association of SGLT2 inhibitors with cardiovascular, kidney, and safety outcomes among patients with diabetic kidney disease: a meta-analysis. Cardiovasc Diabetol. (2022) 21(1):47. doi: 10.1186/s12933-022-01476-x
11. Al Rifai M, Newby LK, Nair AP, Misra A, Rogers JG, Fedson S, et al. SGLT-2 Inhibitors for patients with heart failure: what have we learned recently? Curr Atheroscler Rep. (2022) 24(8):627–34. doi: 10.1007/s11883-022-01038-2
12. Camilli M, Lombardi M, Chiabrando JG, Del Buono MG, Montone RA, Biondi-Zoccai G, et al. Efficacy of sodium-glucose cotransporter-2 inhibitors in heart failure patients treated with dual angiotensin receptor blocker-neprilysin inhibitor: an updated meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2021) 7(4):e74–6. doi: 10.1093/ehjcvp/pvab034
13. Liu Y, Xu J, Wu M, Xu B, Kang L. Empagliflozin protects against atherosclerosis progression by modulating lipid profiles and sympathetic activity. Lipids Health Dis. (2021) 20(1):5. doi: 10.1186/s12944-021-01430-y
14. Paolisso P, Bergamaschi L, Santulli G, Gallinoro E, Cesaro A, Gragnano F, et al. Infarct size, inflammatory burden, and admission hyperglycemia in diabetic patients with acute myocardial infarction treated with SGLT2-inhibitors: a multicenter international registry. Cardiovasc Diabetol. (2022) 21(1):77. doi: 10.1186/s12933-022-01506-8
15. Nikolaou PE, Mylonas N, Makridakis M, Makrecka-Kuka M, Iliou A, Zerikiotis S, et al. Cardioprotection by selective SGLT-2 inhibitors in a non-diabetic mouse model of myocardial ischemia/reperfusion injury: a class or a drug effect? Basic Res Cardiol. (2022) 117(1):27. doi: 10.1007/s00395-022-00934-7
16. Li X, Preckel B, Hermanides J, Hollmann MW, Zuurbier CJ, Weber NC. Amelioration of endothelial dysfunction by sodium glucose co-transporter 2 inhibitors: pieces of the puzzle explaining their cardiovascular protection. Br J Pharmacol. (2022) 179(16):4047–62. doi: 10.1111/bph.15850
17. Salah HM, Verma S, Santos-Gallego CG, Bhatt AS, Vaduganathan M, Khan MS, et al. Sodium-Glucose cotransporter 2 inhibitors and cardiac remodeling. J Cardiovasc Transl Res. (2022) 15(5):944–56. doi: 10.1007/s12265-022-10220-5
18. Dyck JRB, Sossalla S, Hamdani N, Coronel R, Weber NC, Light PE, et al. Cardiac mechanisms of the beneficial effects of SGLT2 inhibitors in heart failure: evidence for potential off-target effects. J Mol Cell Cardiol. (2022) 167:17–31. doi: 10.1016/j.yjmcc.2022.03.005
19. Kim HK, Ishizawa R, Fukazawa A, Wang Z, Bezan Petric U, Chang Hu M, et al. Dapagliflozin attenuates sympathetic and pressor responses to stress in young prehypertensive spontaneously hypertensive rats. Hypertension. (2022) 79(8):1824–34. doi: 10.1161/hypertensionaha.122.19177
20. Withaar C, Meems LMG, Markousis-Mavrogenis G, Boogerd CJ, Silljé HHW, Schouten EM, et al. The effects of liraglutide and dapagliflozin on cardiac function and structure in a multi-hit mouse model of heart failure with preserved ejection fraction. Cardiovasc Res. (2021) 117(9):2108–24. doi: 10.1093/cvr/cvaa256
21. Shen X, Shen X. Promise of sodium-glucose co-transporter-2 inhibitors in heart failure with mildly reduced ejection fraction. ESC Heart Failure. (2022) 9(4):2239–48. doi: 10.1002/ehf2.14005
22. Galderisi A, Tamborlane W, Taylor SI, Attia N, Moretti C, Barbetti F. SGLT2i Improves glycemic control in patients with congenital severe insulin resistance. Pediatrics. (2022) 150(1):e2021055671. doi: 10.1542/peds.2021-055671
23. Chan YH, Chen SW, Chao TF, Kao YW, Huang CY, Chu PH. The risk of consequent nephropathy following initial weight loss in diabetic patients treated with sodium glucose cotransporter 2 inhibitors. Cardiovasc Diabetol. (2021) 20(1):167. doi: 10.1186/s12933-021-01361-z
24. Chen Y, Peng D. New insights into the molecular mechanisms of SGLT2 inhibitors on ventricular remodeling. Int Immunopharmacol. (2023) 118:110072. doi: 10.1016/j.intimp.2023.110072
Keywords: SGLT2I, AHF, AMI, NSTEMI, hospitalization
Citation: Zhu Y, Zhang J-l, Jin H, Ji Y and Wang F-f (2023) The effect of SGLT2i on in-hospital acute heart failure risk in acute myocardial infarction patients—a retrospective study. Front. Cardiovasc. Med. 10:1158507. doi: 10.3389/fcvm.2023.1158507
Received: 15 February 2023; Accepted: 3 May 2023;
Published: 16 May 2023.
Edited by:
Vittorio Palmieri, San Sebastiano & Sant'Anna National Hospital, ItalyReviewed by:
Mohammad Ahmad Zaki Al-Ani, University of Florida, United StatesMassimiliano Camilli, Agostino Gemelli University Polyclinic (IRCCS), Italy
© 2023 Zhu, Zhang, Jin, Ji and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-fang Wang bGlnaHR5ZWFyd2ZmQDE2My5jb20=
 Yi Zhu
Yi Zhu Jia-li Zhang2
Jia-li Zhang2