
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 May 2023
Sec. Pediatric Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1158227
This article is part of the Research TopicFrom Interventional Pearls to Pioneering Technologies in Transcatheter Treatment of Congenital Heart DefectsView all 22 articles
 Elchanan Bruckheimer1*†
Elchanan Bruckheimer1*† Kristoffer Steiner1,2,†
Kristoffer Steiner1,2,† Yuval Barak-Corren1
Yuval Barak-Corren1 Leonel Slanovic1
Leonel Slanovic1 Michael Levinzon3
Michael Levinzon3 Alexander Lowenthal1
Alexander Lowenthal1 Gabriel Amir1
Gabriel Amir1 Tamir Dagan1
Tamir Dagan1 Einat Birk1
Einat Birk1
Purpose: Evaluate Piccolo and ADOII devices for transcatheter patent ductus arteriosus (PDA) closure. Piccolo has smaller retention discs reducing risk of flow disturbance but residual leak and embolization risk may increase.
Methods: Retrospective review of all patients undergoing PDA closure with an Amplatzer device between January 2008 and April 2022 in our institution. Data from the procedure and 6 months follow-up were collected.
Results: 762 patients, median age 2.6 years (range 0–46.7) years and median weight 13 kg (range 3.5–92) were referred for PDA closure. Overall, 758 (99.5%) had successful implantation: 296 (38.8%) with ADOII, 418 (54.8%) with Piccolo, and 44 (5.8%) with AVPII. The ADOII patients were smaller than the Piccolo patients (15.8 vs. 20.5 kg, p < 0.001) and with larger PDA diameters (2.3 vs. 1.9 mm, p < 0.001). Mean device diameter was similar for both groups. Closure rate at follow-up was similar for all devices ADOII 295/296 (99.6%), Piccolo 417/418 (99.7%), and AVPII 44/44 (100%). Four intraprocedural embolizations occurred during the study time period: two ADOII and two Piccolo. Following retrieval the PDA was closed with an AVPII in two cases, ADOI in one case and with surgery in the fourth case. Mild stenosis of the left pulmonary artery (LPA) occurred in three patients with ADOII devices (1%) and one patient with Piccolo device (0.2%). Severe LPA stenosis occurred in one patient with ADOII (0.3%) and one with AVPII device (2.2%).
Conclusions: ADOII and Piccolo are safe and effective for PDA closure with a tendency to less LPA stenosis with Piccolo. There were no cases of aortic coarctation related to a PDA device in this study.
Transcatheter closure of a PDA is a common and straight-forward procedure in the paediatric age group with a variety of devices and methods available. Choice of device is usually determined by a combination of patient age and weight, the morphology of the ductus and the physician's personal preference. Coils were popular due to the ability to use low-profile systems via arterial access in small patients but were often complicated by incomplete closure or embolization (1). First generation occluders usually required larger delivery systems and a venous approach and the presence of a disc or an umbrella which could protrude to the aortic side limiting the use in smaller patients (2–4). The newer generations of the Amplatzer duct occluders, ADOII and Piccolo (previously called ADOII additional sizes), require a 4 or 5 French delivery system and can be implanted from the aortic or venous side (5, 6). Although available for clinical use since 2011, the Piccolo received FDA approval for PDA closure in children with a body weight over 700 grams in 2019 and has proved to be a highly useful device for transcatheter PDA closure of extremely preterm born children (7–11).
The major difference between the ADOII and the Piccolo is the smaller size of the retention discs in the latter which although advantageous in avoiding flow disturbances may make it less occlusive and prone to embolisation. We report on a large single institution, retrospective study on the outcome of transcatheter PDA closure with the Piccolo in a non-premature cohort.
Consecutive patients diagnosed with a PDA who underwent an attempt at transcatheter closure between January 2008 and April 2022 at our institution were identified from the cardiac catheterization database. Patient data, procedural characteristics, hemodynamic and angiographic findings, echocardiographic findings, and clinical status were recorded from the patient records. All aortic angiograms were reviewed by the same physician (KS). Data was collected retrospectively. The study was approved by the Institutional Review Board. Informed, written consent was obtained before each procedure.
The ADOII and Piccolo (St Jude Medical, St. Paul, Minnesota) devices have been described previously [reference]. The ADOII Is available in diameters of 3, 4, 5 and 6 mm and lengths of 4 and 6 mm with retention disks 6 mm larger than the device waist. Piccolo is similar but with smaller retention disks which are 4, 5.25 or 6.5 mm when device diameters are 3, 4 or 5 mm, respectively. The Piccolo is also available in a length of 2 mm. The symmetrical design offers the possibility to deploy the Piccolo devices through a 4Fr. delivery sheath either from the aortic or the pulmonary side and the disks, altered from curved to flat, in addition to their occlusive properties, anchor the device on both sides while minimizing risk of protrusion.
Procedures were performed typically under general anesthesia due to patient age. Following percutaneous access, biplane aortography was performed in right anterior oblique (RAO) and lateral planes and the PDA was closed using standard techniques (12) using a 4–5Fr delivery system from the aortic or pulmonary side. If the size or shape of the PDA was not appropriate for either of these devices an AVPII plug was used. Device selection was at the sole discretion of the interventionalist. PDA morphology on biplane aortogram was classified using the Krichenko classification and modification suggested by Philip (13, 14). A device was chosen so that the diameter of the waist was approximately twice the size of the narrowest part of the ductus, usually at the pulmonary end. The length of the Piccolo was chosen to suit the ductal anatomy, so that in a conical-tubular shaped ductus the aortic disk would lie deep in the ductus at the tip of the cone and in a tubular ductus at the ampulla without extending in to the aortic lumen. Stability of the device was assessed by a mild push-pull the delivery cable. Aortography was performed after implantation to confirm position and residual leak after device release. Color-Doppler echocardiography was performed the following day before discharge.
A complete color-Doppler echocardiogram was performed in all patients prior to catheterization, 1 day after PDA occlusion and on all follow up visits. Pulmonary artery stenosis was defined as echocardiographic evidence of turbulent flow and a Doppler velocity >2 m/s that was not demonstrated on echocardiogram before intervention and when greater than 2.5 m/s the stenosis was defined as significant, similar to the national study from UK and Ireland that used 2 m/s as definition for pulmonary artery stenosis (5). Coarctation of the aorta was defined as a pullback peak gradient above 10 mmHg post device deployment if it was not present before device placement. Presence of a residual shunt was defined as echocardiographic evidence of residual flow across the PDA the day following implantation.
Statistical analysis was performed using Kruskal-Wallis nonparametric Anova test and Dunńs Multiple Comparison Test.
Between January 2008 and April 2022, 762 patients were referred for PDA closure. Patients and PDA characteristics are summarized in Table 1. The use of Piccolo increased from 10% in 2011 to around 80% of all device-occluded PDA cases in 2021–2022 (Figure 1).
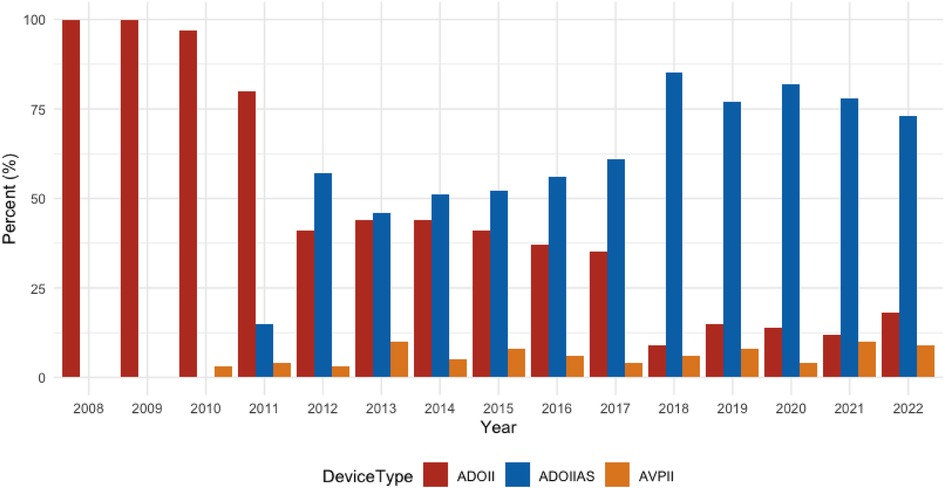
Figure 1. Device use over time 2008–2022. Over this time period, the ADOIIAS became our preferred device, used today in about 80% of all PDA closures.
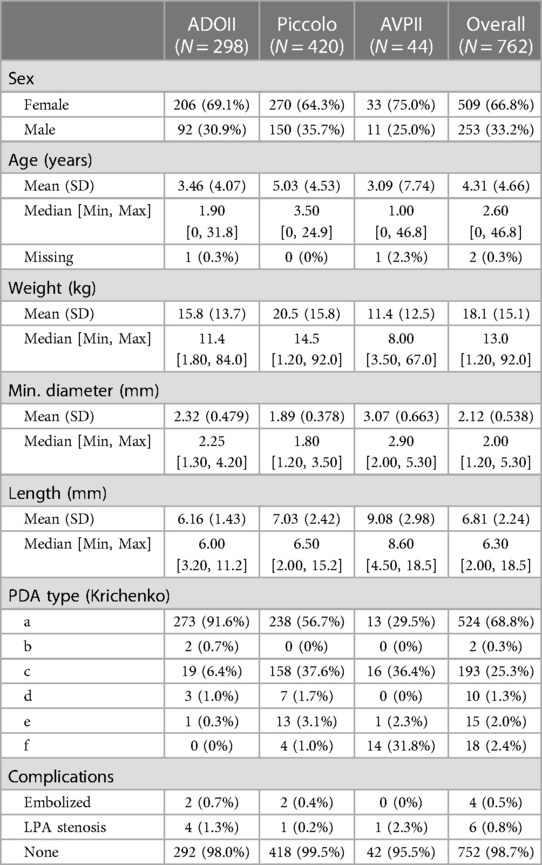
Table 1. Patient, PDA and device characteristics. The ADOII patients were smaller than the Piccolo p < 0.001 with larger PDA diameters p < 0.001.
The patients who underwent closure with ADOII were significantly younger and smaller than the Piccolo patients. The minimal PDA diameter in the ADOII group was significantly larger than that of the Piccolo and since the mean device diameter was similar for both groups the device:PDA ratio was significantly larger for the Piccolo device group. There were significantly more Krichenko type-C PDAs in the Piccolo group compared to ADOII.
The closure rate at follow up was similar for all devices at 99.6%–100%. All patients were discharged the following day with normal distal pulses palpated and with no venous or arterial complications.
There were four implantation failures, two in a type A PDA and two in a type C. In these cases, the Piccolo was demonstrated to be unstable before release. The device was retrieved without releasing in all cases and the PDA was closed successfully with an AVPII 6 mm device. There were two device embolizations, both to the right pulmonary artery, which were successfully retrieved, and the PDA was closed with an AVPII 6 mm with no further complications. On mean follow up 8.4 ± 15.2 months, one patient had mild left pulmonary artery stenosis on echocardiography with a maximal velocity of 2.5 m/s. There were no cases of aortic flow disturbance or significant LPA stenosis.
There were two cases of device embolization (ADOII 4-4, ADOII 5-4) to the right pulmonary artery, one patient was treated with surgical PDA closure the same day. In the other patient the device was successfully retrieved and the PDA was closed using an ADOI 8-6 device. On mean follow up 11.9 ± 20.4 months, there were four cases of LPA stenosis, three mild and one significant stenosis which underwent balloon dilation 4 years later with a good result. There were no cases of aortic flow disturbance. One patient suffered from endocarditis and a residual shunt after PDA closure with an ADOII 5-4 mm device. The shunt eventually disappeared on echocardiographic follow up 14 months after implantation. Summary of all complications by weight of patient is found in Table 2.
AVPII was used for PDA closure in 44 patients who were smaller and younger than the Piccolo and ADOII patients. On a mean follow up of 7.4 ± 13.4 months there was one complication. A 7-month-old patient (6 kg), who underwent PDA closure with an 8 mm AVPII device, had significant LPA stenosis and underwent balloon angioplasty of the left pulmonary artery with a 6 mm Savvy balloon (SAVVY® Cordis, Miami Lakes, United States). On follow up, the patient demonstrated a perfusion distribution of 30% to the left lung and 70% to the right lung.
This retrospective study demonstrates that the Amplatzer ductal occluders are safe and effective devices for the transcatheter closure of a patent ductus arteriosus. The lower profile Piccolo device was used in small and larger patients with smaller PDA diameters with an excellent closure rate and very few complications. The major concerns of embolization and residual leak due to its smaller retention disks were not supported when a device:PDA diameter of approximately 2:1 was maintained. In the one case of embolization a review of the angiogram demonstrated the narrowing of the PDA to be dynamic in the RAO plane with a diameter of 3.5 mm which was overlooked at the time of choice of device. A relatively high rate of PDA device embolizations of 2.6% has been reported in a meta- analysis (15). This was not our experience in our cohort (n = 4, 0.5%). The reasons for this may relate to our routine use of biplane angiography which enhances the identification and sizing of the narrowest ductal diameter. Since the PDA is often not necessarily viewed best in the lateral plane and can be foreshortened, the 40 degree RAO plane is a good angle for allowing a supplemental view of the PDA morphology. Using both angled views simultaneously can demonstrate fixed or dynamic narrowest ductal diameters more easily.
Once the diameter is established, when using the Piccolo device the delivery is from the aortic side as we have described in our previous report. In this approach the pulmonary disk is extruded completely in the main pulmonary artery (MPA) and approximated to the end of the delivery sheath so that the central body of the device is restricted in the sheath. When pulling the sheath back no further part of the device is released until the pulmonary disk becomes concave, indicating that it is up against the pulmonary arterial wall. While maintaining tension, withdrawal of the sheath releases the body of the Piccolo device in the ductus and aortic ampulla without any part of the body in the narrowing. If the body of the device is in the narrowed part of the ductus it can “stent” the ductus open and possibly make embolization more likely. In a conical ductus [Krichenko A] we use the largest diameter and shortest length Piccolo device so that it fills up the peak of the cone with the device's aortic disk deep in the ampulla providing both excellent closure, even in a moderate ductus, with no peri-device leak and preventing embolization (Figures 2, 3). This approach also prevents any significant pulmonary or aortic flow disturbance. When the ductal diameter is greater than 3 mm we generally used an AVPII device since this is a more robust device with larger diameters available with smaller disks than the ADOII.
When using the ADOII we rarely used the 6 mm long device for PDA closure since the disks are more likely to stand proud of the aortic or pulmonary wall which could cause flow disturbance and inferior closure. In type C ductal anatomy, elongation deformity of the aortic disk causes it to have less traction on the wall and it can prolapse into the ductus and embolize. In this type of ductal anatomy, we recommend the use of a Piccolo or an AVPII.
LPA stenosis of some degree has been reported after transcatheter PDA closure especially in patients below 4 kg body weight and a larger PDA minimal diameter (16). However, in premature and small infants the stenosis is usually transient since the Piccolo device is implanted within the ductus. In larger and older patients the pulmonary disk is placed in the pulmonary lumen and often, in the case of the ADOII, the edge of the disk lies partially across the take-off of the LPA. While a very mild degree of stenosis/flow disturbance may be unavoidable, in our series a significant stenosis was quite unusual, occurring in only six cases (6/762, 0.8%, 4 mild and 2 severe stenoses). This is probably related to the extensive use of the Piccolo device and a higher procedural age. Many authors report on the obvious advantages of this device in extremely premature infants (7–10), however, we and others (17–21) believe that the Piccolo is also very useful for closing small and moderate ductus in all age groups and has become our first choice for transcatheter PDA closure of these ducts. This observation can be seen in the bar chart documenting our choice of device selection over the study period (Figure 1).
The limitations of this report are it being retrospective and a non-randomized comparison of devices, however the relatively large numbers of consecutive patients referred for transcatheter PDA occlusion lends support to our observations.
ADOII and Piccolo are safe and effective for PDA closure with a tendency to less LPA stenosis with ADOIIAS devices.
Transcatheter closure of a PDA is a common procedure that should result in effective closure and minimal complications. In our opinion, the Piccolo device is a safe and effective device for transcatheter PDA closure and is our preferred choice for transcatheter closure of small to moderate PDAs in all age groups. This technique has replaced coil closure of PDA in our institution. We prefer a retrograde approach through a 4Fr system with device waist-to-PDA diameter ratio of greater than 2:1 with a length that places the aortic disc inside the diverticulum.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethic Committee, Schneider Children's Medical Center, Petah Tikva. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
KS has received fellowship grants from Gåvåfonden Karolinska Sjukhuset, Ränkdonation Stockholm, Sällskapet Barnavård, Stiftelsen Barnforskning Astrid Lindgrens Barnsjukhus and Hjärtebarnsfonden.
EB is a proctor for Abbott Vascular.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ADOI, Amplatzer duct occluder I; ADOII, Amplatzer duct occluder II; ADOIIAS, Amplatzer duct occluder II additional sizes; AVPII, Amplatzer vascular plug II; Fr, French; LAT, lateral; LPA, left pulmonary artery; PDA, patent ductus arteriosus; RAO, right anterior oblique; Vmax, velocity maximal.
1. Patel HT, Cao QL, Rhodes J, Hijazi ZM. Long-term outcome of transcatheter coil closure of small to large patent ductus arteriosus. Catheter Cardiovasc Interv. (1999) 47(4):457–61. doi: 10.1002/(SICI)1522-726X(199908)47:4%3C457::AID-CCD15%3E3.0.CO;2-A
2. Janorkar S, Goh T, Wilkinson J. Transcatheter closure of patent ductus arteriosus with the use of rashkind occluders and/or gianturco coils: long-term follow-up in 123 patients and special reference to comparison, residual shunts, complications, and technique. Am Heart J. (1999) 138(6 Pt 1):1176–83. doi: 10.1016/S0002-8703(99)70085-2
3. Beck A, Birk E, Blieden LC, Bruckheimer E. Stent implantation for coarctation of aorta caused by a rashkind patent ductus arteriosus umbrella. Catheter Cardiovasc Interv. (2004) 63(1):80–2. doi: 10.1002/ccd.20128
4. Moore JW, Levi DS, Moore SD, Schneider DJ, Berdjis F. Interventional treatment of patent ductus arteriosus in 2004. Catheter Cardiovasc Interv. (2005) 64(1):91–101. doi: 10.1002/ccd.20243
5. Kang SL, Jivanji S, Mehta C, Tometzki AJ, Derrick G, Yates R, et al. Outcome after transcatheter occlusion of patent ductus arteriosus in infants less than 6 kg: a national study from United Kingdom and Ireland. Catheter Cardiovasc Interv. (2017) 90(7):1135–44. doi: 10.1002/ccd.27212
6. Bruckheimer E, Godfrey M, Dagan T, Levinzon M, Amir G, Birk E. The Amplatzer duct occluder II additional sizes device for transcatheter PDA closure: initial experience. Catheter Cardiovasc Interv. (2014) 83(7):1097–101. doi: 10.1002/ccd.25445
7. Fraisse A, Bautista-Rodriguez C, Burmester M, Lane M, Singh Y. Transcatheter closure of patent ductus arteriosus in infants with weight under 1,500 grams. Front Pediatr. (2020) 8:558256. doi: 10.3389/fped.2020.558256
8. Georgiev S, Tanase D, Eicken A, Peters J, Hörer J, Transvenous EP. Echocardiographically guided closure of persistent ductus arteriosus in 11 premature infants: a pilot study. JACC Cardiovasc Interv. (2021) 14(7):814–6. doi: 10.1016/j.jcin.2021.01.009
9. Sathanandam S, Balduf K, Chilakala S, Washington K, Allen K, Knott-Craig C, et al. Role of transcatheter patent ductus arteriosus closure in extremely low birth weight infants. Catheter Cardiovasc Interv. (2019) 93(1):89–96. doi: 10.1002/ccd.27808
10. Sathanandam SK, Gutfinger D, O'Brien L, Forbes TJ, Gillespie MJ, Berman DP, et al. Amplatzer piccolo occluder clinical trial for percutaneous closure of the patent ductus arteriosus in patients ≥700 grams. Catheter Cardiovasc Interv. (2020) 96(6):1266–76. doi: 10.1002/ccd.28973
11. Zahn EM, Nevin P, Simmons C, Garg R. A novel technique for transcatheter patent ductus arteriosus closure in extremely preterm infants using commercially available technology. Catheter Cardiovasc Interv. (2015) 85(2):240–8. doi: 10.1002/ccd.25534
12. Thanopoulos B, Eleftherakis N, Tzannos K, Stefanadis C. Transcatheter closure of the patent ductus arteriosus using the new Amplatzer duct occluder: initial clinical applications in children. Am Heart J. (2008) 156(5):917.e1–.e6. doi: 10.1016/j.ahj.2008.08.001
13. Krichenko A, Benson LN, Burrows P, Möes CA, McLaughlin P, Freedom RM. Angiographic classification of the isolated, persistently patent ductus arteriosus and implications for percutaneous catheter occlusion. Am J Cardiol. (1989) 63(12):877–80. doi: 10.1016/0002-9149(89)90064-7
14. Philip R, Waller BR 3rd, Agrawal V, Wright D, Arevalo A, Zurakowski D, et al. Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv. (2016) 87(2):310–7. doi: 10.1002/ccd.26287
15. Backes CH, Rivera BK, Bridge JA, Armstrong AK, Boe BA, Berman DP, et al. Percutaneous patent ductus arteriosus (PDA) closure during infancy: a meta-analysis. Pediatrics. (2017) 139(2):e20162927. doi: 10.1542/peds.2016-2927
16. Tomasulo CE, Gillespie MJ, Munson D, Demkin T, O'Byrne ML, Dori Y, et al. Incidence and fate of device-related left pulmonary artery stenosis and aortic coarctation in small infants undergoing transcatheter patent ductus arteriosus closure. Catheter Cardiovasc Interv. (2020) 96(4):889–97. doi: 10.1002/ccd.28942
17. VanLoozen D, Sandoval JP, Delaney JW, Pedra C, Calamita P, Dalvi B, et al. Use of Amplatzer vascular plugs and Amplatzer duct occluder II additional sizes for occlusion of patent ductus arteriosus: a multi-institutional study. Catheter Cardiovasc Interv. (2018) 92(7):1323–8. doi: 10.1002/ccd.27824
18. Agnoletti G, Marini D, Villar AM, Bordese R, Gabbarini F. Closure of the patent ductus arteriosus with the new duct occluder II additional sizes device. Catheter Cardiovasc Interv. (2012) 79(7):1169–74. doi: 10.1002/ccd.23477
19. Mahmoud HT, Santoro G, Gaio G, D'Aiello FA, Capogrosso C, Palladino MT, et al. Single-center experience in percutaneous closure of arterial duct with Amplatzer duct occluder II additional sizes. Catheter Cardiovasc Interv. (2017) 89(6):1045–50. doi: 10.1002/ccd.26860
20. Fiszer R, Chojnicki M, Szkutnik M, Haponiuk I, Chodór B, Białkowski J. Are the AMPLATZER duct occluder II additional sizes devices dedicated only for smaller children? EuroIntervention. (2017) 12(17):2100–3. doi: 10.4244/EIJ-D-15-00238
Keywords: PDA closure, interventional pediatric cardiology, Piccolo, ADOII, AVPII
Citation: Bruckheimer E, Steiner K, Barak-Corren Y, Slanovic L, Levinzon M, Lowenthal A, Amir G, Dagan T and Birk E (2023) The Amplatzer duct occluder (ADOII) and Piccolo devices for patent ductus arteriosus closure: a large single institution series. Front. Cardiovasc. Med. 10:1158227. doi: 10.3389/fcvm.2023.1158227
Received: 3 February 2023; Accepted: 10 April 2023;
Published: 4 May 2023.
Edited by:
Petru Liuba, Lund University, SwedenReviewed by:
Nazmi Narin, Izmir Katip Celebi University, Türkiye© 2023 Bruckheimer, Steiner, Barak-Corren, Slanovic, Levinzon, Lowenthal, Amir, Dagan and Birk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elchanan Bruckheimer ZWxjaGFuYW5iQGJlemVxaW50Lm5ldA==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.