- 1Department of Orthopedics, People’s Hospital of Deyang City, Deyang, China
- 2Department of Orthopedics, The First Affiliated Hospital of Dalian Medical University, Dalian, China
Aims: To investigate the potential relationship between anatomic distribution of lower extremity deep venous thrombosis (LEDVT) and pulmonary embolism (PE).
Methods: A retrospective case-control study was performed in patients diagnosed with LEDVT, which were confirmed by bilateral lower extremity compression ultrasonography (CUS) examination. According to the ultrasound reports, thrombus sidedness was categorized as unilateral and bilateral lower extremity, thrombus location was classified into distal and proximal LEDVT. Anatomic distributions of LEDVT were further subdivided depending on the combination of thrombus sidedness and location. Patients with PE were identified using the International Classification of Diseases-10 (ICD-10) codes (I26.0 and I26.9), and divided into PE group and Non-PE group. Univariate and multivariate logistic regression analyses were used to assess the association between anatomic distribution of LEDVT and PE. Sensitivity analyses were also conducted.
Results: A total of 2,363 consecutive patients with LEDVT were included, of whom 66.10% and 33.90% were unilateral and bilateral LEDVT, as well as 71.39% and 28.61% were isolated distal and proximal LEDVT, respectively. After the diagnosis of LEDVT, 185 patients (7.83%) developed PE. The proportions of PE ranged between the lowest (4.07%) in unilateral-distal LEDVT and highest (14.55%) in bilateral-proximal LEDVT. Multivariate logistic regression analysis showed that bilateral LEDVT (odds ratios [OR] = 2.455, 95% confidence interval [CI]: 1.803–3.344, P < 0.001) and proximal LEDVT (OR = 1.530, 95% CI: 1.105–2.118, P = 0.010) were risk factors for developing PE. Moreover, unilateral-proximal (OR = 2.129, 95% CI: 1.365–3.320, P = 0.00), bilateral-distal (OR = 3.193, 95% CI: 2.146–4.752, P < 0.001) and bilateral-proximal LEDVT(OR = 3.425, 95% CI: 2.093–5.603, P < 0.001) were significantly associated with an increased risk of PE. Sensitivity analyses also confirmed the robustness of these associations.
Conclusion: Patients with unilateral-proximal, bilateral-distal or bilateral-proximal are more likely to suffer from PE than those with unilateral-distal LEDVT.
Introduction
Deep venous thrombosis (DVT), the formation of blood thrombus in the deep veins, remains a serious and growing public health problem (1). In several large population-based studies, the overall incidence rates of DVT per 100,000 person-years were as high as 147 in USA (2), 123 in Taiwan (3), 108 in Norway (4), and 80.9 in Canada (5). As is well known, thrombus originating in the lower extremity into the pulmonary arteries is considered to be the most common mechanism for pulmonary embolism (PE) (6). With the increasing number of hospitalized DVT patients, the incidence rate of PE was also markedly elevated (7–9). Notably, PE has become the third leading cause of cardiovascular death globally, accounting for 8–13 per 1,000 deaths in women and 2–7 per 1,000 deaths in men (10). On the other hand, global public awareness for DVT and PE was significantly lower than other thromboembolic disease (11). To date, venous thromboembolism (VTE), including DVT and PE, continues to impose a substantial social and economic burden worldwide (11).
With regard to DVT treatment, one of the most important goals is to detect and prevent the occurrence of PE (12). Due to the lack of specific clinical symptoms and signs, diagnosing PE remains a clinical challenge (13). Computed tomography pulmonary angiography (CTPA), which requires the injection of iodinated contrast material, has been widely used as a gold-standard diagnostic modality in patients with suspected PE (14). However, CTPA exposes patients to risks of false-positive result, allergic reaction, renal failure and cumulative radiation-induced cancer (13, 14). Moreover, CTPA was found to be overused, leading to ineffective utilization of hospital resources (15). Therefore, there is an urgent need to identify DVT patients at high risk of PE, and to implement effective preventive measures (12).
Recently, the occurrence of PE seems to be closely related to the anatomic distribution of lower extremity DVT (LEDVT) (16, 17). In general, isolated distal LEDVT is presumed to be more benign than proximal LEDVT, presenting a lower risk of PE, VTE recurrence, post-thrombotic syndrome and mortality (18–20). On the other hand, thrombus sidedness, especially bilateral LEDVT, was found to be associated with an increased risk of PE (12, 17). However, limited studies have focused on the single relationship between thrombus sidedness or thrombus location and PE, and yielded inconsistent results (12, 16, 17, 21–24).
Hospital information system (HIS) is one of the most widely used information systems in the health care. In this study, we used our hospital HIS database over a 10-year period (2012–2022) to identify all LEDVT patients, and then explored the association between thrombus sidedness, thrombus location and risk of PE. Subsequently, anatomic distributions of LEDVT were subdivided depending on the combination of thrombus sidedness and location, and further analyzed this relationship in different anatomic distribution of LEDVT.
Materials and methods
Study subjects and design
This retrospective case-control study was conducted at People's Hospital of Deyang City, a 1838-bed tertiary hospital located in Southwest China. Between January 1, 2012 and July 31, 2022, all hospitalized patients were screened by searching the electronic HIS database (n = 671,456). Initially, 20,730 patients who underwent lower extremity compression ultrasonography (CUS) were identified. After that, patients were excluded if they met any of the following criteria: (1) no thrombus; (2) unilateral CUS examination; (3) arterial thrombosis; (4) lower extremity superficial vein thrombosis; (5) PE on admission or before LEDVT diagnosis; (6) age <18 years; (7) incomplete data. The study flowchart is illustrated in Figure 1. The study protocol was approved by the Institutional Ethics Committee of our hospital (Number: 2021-04-019-K01), and performed in accordance with the Declaration of Helsinki. Patient informed consent was waived owing to the use of anonymous retrospective data.
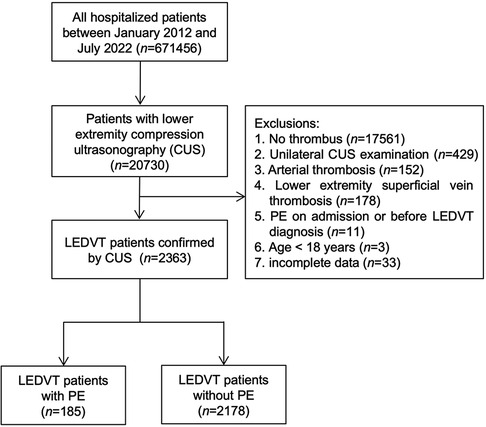
Figure 1. Flowchart of patient inclusion and exclusion. CUS, compression ultrasonography; PE, pulmonary embolism; LEDVT, lower extremity deep venous thrombosis.
Data collection and definition
For diagnosis of LEDVT, CUS examinations were generally performed by an experienced radiologist, and the results were reviewed and verified by another radiologist. Briefly, the following deep veins of the thigh and calf were sequentially scanned: common femoral vein, superficial femoral vein, deep femoral vein, popliteal vein, anterior tibial vein, posterior tibial vein, peroneal vein and calf muscle vein. According to the results of ultrasound reports, thrombus sidedness included left, right and bilateral lower extremity, and thrombus location included proximal (thrombus occurring in the popliteal vein and/or above), distal (thrombus below the popliteal vein), and mixed LEDVT (both proximal and distal thrombus) (23). Previous studies have found a similar PE risk between left and right LEDVT (12, 16, 24), and this study also did not reach statistical significance (Supplementary Table S1 and Figure S1). For this reason, thrombus sidedness was categorized as unilateral or bilateral lower extremity. Since LEDVT is thought to progress from distal to proximal location, and the number of isolated proximal LEDVT patients was relatively small in the study, subjects with both distal and proximal LEDVT were regarded as proximal LEDVT (25). Thereafter, anatomic distributions of LEDVT were further subdivided into 4 groups: unilateral-distal, unilateral-proximal, bilateral-distal and bilateral-proximal LEDVT.
At our institute, the diagnosis of PE was based on the presence of an intraluminal filling defect in the pulmonary artery tree on CTPA. In accordance with a previous study (26), the International Classification of Diseases-10 (ICD-10) codes were used to identify patients with PE (I26.0 and I26.9). ICD-10 I26.0 represents PE patients with acute cor pulmonale, while I26.9 represents PE patients without acute cor pulmonale. Meanwhile, the diagnosis of PE was confirmed using discharge diagnoses as “pulmonary embolism, pulmonary thrombosis, pulmonary artery embolism or pulmonary infarction”. Thereby, patients with LEDVT were divided into PE group (case subjects with PE) and Non-PE group (control subjects without PE).
Moreover, other clinical characteristics were collected, including age at admission, sex, body mass index (BMI), as well as history of tobacco and alcohol use which were recorded in the electronic admission note. Based on the Working Group on Obesity in China (27), obesity was defined as BMI ≥ 28.0 kg/m2. Smoking and drinking status were classified as never, former, current or unknown. Also, comorbidities associated with PE were extracted using ICD-10 codes of discharge diagnoses (28): hypertension (I10–I13, I15), diabetes mellitus (E11–E14), chronic obstructive pulmonary disease (COPD, J42–J44), atrial fibrillation (I48), heart failure (I50), varicose vein (I83.9), hepatic insufficiency (K72.0–K72.1, K72.9), renal insufficiency (N17–N19), and cancer (C00–C97, D00–D09). In this study, all data were cleaned independently by two authors (JZ and YC), and cross-checked for accuracy.
Statistical analysis
Prior to analysis, all variables were checked for missing values, and 19.85% of obesity data were found to be missing (n = 469). Considering the large number of missing value, we created a missing obesity category using missing indicator method rather than multiple imputation (29).
For continuous variable (age), normality was first checked using the Shapiro-Wilk test, and described as mean ± standard deviation (SD), whereas other categorical variables were reported as numbers (percentages). Differences between the two groups were compared by Student's t-test for continuous variable and Pearson's χ2 test for categorical variables. The proportions of PE with 95% confidence intervals (CI) were calculated using the Wilson/Brown method. Differences in proportions were evaluated by χ2 test, and P values were adjusted for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR) correction, with the FDR set at 5% (30).
Univariate logistic regression analyses were performed to identify factors associated with PE. All variables with P value ≤0.1 in univariate analyses were included in the multivariate logistic regression analyses. The assumption of linearity in the logit for the continuous variable was checked by the Box-Tidwell test, it was found to be violated for age. For this, age was transformed into categorical variable based on the cut-off value determined by the receiver operating characteristic curve (ROC) analysis. Multicollinearity was evaluated by variance inflation factor (VIF), with a VIF >10 indicating excessive multicollinearity (31). In this study, two multivariate analysis models were constructed. Model 1 was adjusted for thrombus sidedness (unilateral/bilateral), thrombus location (distal/proximal), and statistically significant variables (age, sex, obesity, hypertension, renal insufficiency, cancer), while Model 2 was adjusted for anatomic distribution of LEDVT (unilateral-distal/unilateral-proximal/bilateral-distal/bilateral-proximal) and statistically significant variables. Moreover, sensitivity analyses excluding those patients with missing data were conducted to test the robustness of the results. Using logistic regression analyses, crude and adjusted odds ratios (OR) with 95% CI were calculated. Existing studies have shown that patients with unilateral or distal LEDVT were less likely to suffer from PE (12, 23). Thus, unilateral, distal and unilateral-distal LEDVT was used as a reference.
All reported P values are two-sided, and P < 0.05 was considered statistically significant. All analyses were conducted using JMP Pro software (version 16.0.0; SAS Institute Inc., Cary, NC, United States) and GraphPad Prism (version 9.1.1; GraphPad Software, San Diego, California, United States).
Results
Patient characteristics
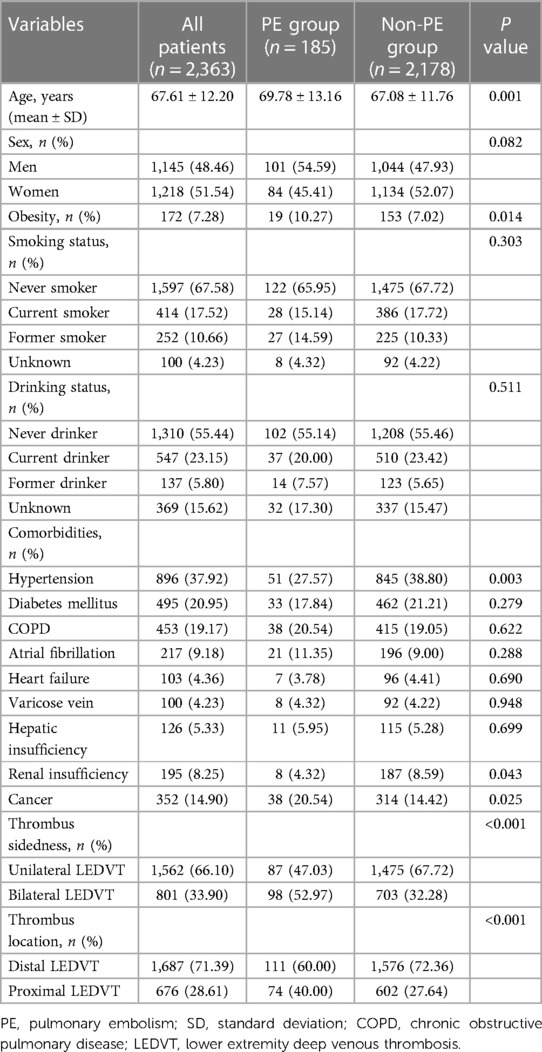
As confirmed by ultrasound, a total of 2,363 consecutive hospitalized patients with LEDVT were included in the final analysis. Patient characteristics are summarized in Table 1. The mean age was 67.61 years (ranging from 20 to 98 years), and 51.54% were women. Among these patients, 801 (33.90%) were bilateral LEDVT, and 1,562 (66.10%) were unilateral LEDVT (852 in the left and 710 in the right lower extremity). For the thrombus location, 1,687 (71.39%) had isolated distal LEDVT, 676 (28.61%) had proximal LEDVT (417 with distal LEDVT and 259 without distal LEDVT). During hospitalization, 185 patients developed PE after the diagnosis of LEDVT. Overall, the proportion of PE among LEDVT patients was 7.83% (95% CI: 6.81%–8.98%). More specifically, the proportions of PE were 5.57% (95% CI: 4.54%–6.82%) for unilateral LEDVT, and 12.24% (95% CI: 10.14%–14.69%) for bilateral LEDVT, the difference was statistically significant (P < 0.001). Regarding the thrombus location, patients with proximal LEDVT (10.95%, 95% CI: 8.81%–13.52%) had a higher proportion of PE than those with distal LEDVT (6.58%, 95% CI: 5.49%–7.86%, P < 0.001). When compared with patients without PE, patients with PE were older and obesity (P < 0.05). The other characteristics were comparable between the two groups, except for hypertension, renal insufficiency and cancer (P < 0.05).
Anatomic distribution of LEDVT
As shown in Table 2, unilateral-distal LEDVT (46.08%) was the most common type of thrombosis, whereas PE occurred most frequently in bilateral-distal LEDVT (35.68%). Based on the anatomic distribution of LEDVT, the proportions of PE ranged between the lowest (4.07%, 95% CI: 3.05%–5.40%) in unilateral-distal LEDVT and highest (14.55%, 95% CI: 10.50%–19.81%) in bilateral-proximal LEDVT. When compared with unilateral-distal LEDVT, patients with unilateral-proximal, bilateral-distal and bilateral-proximal LEDVT had higher proportions of PE (adjusted P < 0.001).
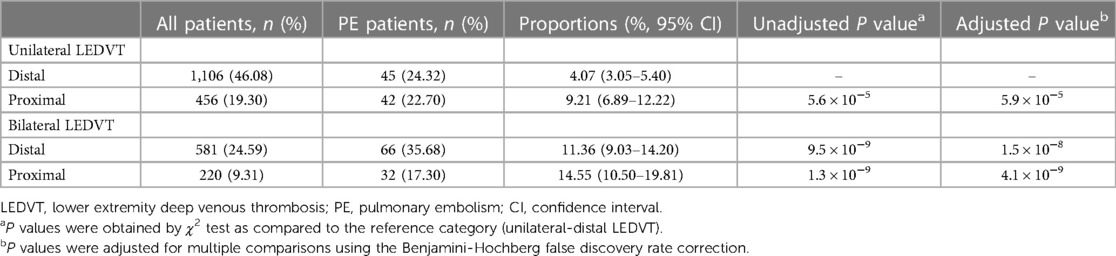
Table 2. Proportions of pulmonary embolism stratified by the anatomic distribution of lower extremity deep venous thrombosis.
Thrombus sidedness and location associated with PE
Using ROC analysis for PE risk (Supplementary Figure S2), the optimal cut-off point for age was 75.0 years [area under curve (AUC): 0.575, 95% CI: 0.536–0.614; sensitivity: 77.30%, specificity: 38.02%]. As shown in Table 3, univariate analyses found that age, obesity, hypertension, renal insufficiency, cancer, thrombus sidedness and thrombus location were significantly associated with PE (P < 0.05). Sex was close to achieving statistical significance (P = 0.083). The above factors were entered into the multivariate logistic analysis (Model 1), and multicollinearity results showed that VIF ranged from 1.014 to 1.762 (Supplementary Table S2), indicating that there was no multicollinearity between these variables. In the multivariate analysis, obesity, hypertension, thrombus sidedness and thrombus location were independently associated with PE (P < 0.05). In other words, bilateral LEDVT (OR = 2.455, 95% CI: 1.803–3.344, P < 0.001) and proximal LEDVT (OR = 1.530, 95% CI: 1.105–2.118, P = 0.010) were risk factors for developing PE.
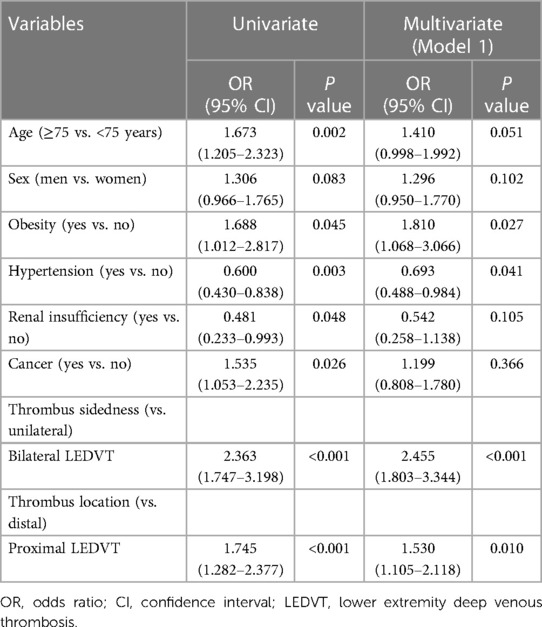
Table 3. Univariate and multivariate logistic regression analyses of factors associated with pulmonary embolism (Model 1).
Anatomic distribution of LEDVT associated with PE
Anatomic distribution of LEDVT, instead of thrombus sidedness and thrombus location, was included into the multivariate logistic analysis (Model 2, Table 4), and no significant multicollinearity was detected between variables (Supplementary Table S2). When compared with unilateral-distal LEDVT, the adjusted OR for PE was highest in bilateral-proximal LEDVT (OR = 3.425, P < 0.001), followed by bilateral-distal LEDVT (OR = 3.193, P < 0.001) and unilateral-proximal LEDVT (OR = 2.129, P = 0.001). In addition, obesity and hypertension were also found to be independently associated with PE (P < 0.05).
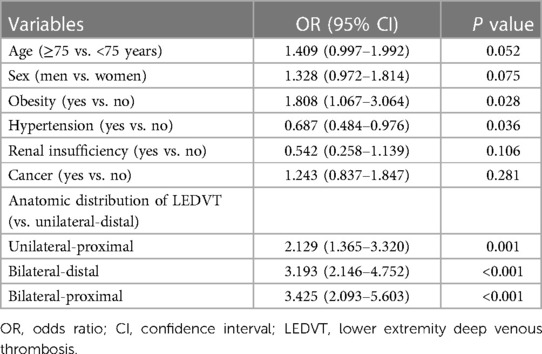
Table 4. Multivariate logistic regression analyses of factors associated with pulmonary embolism (Model 2).
Sensitivity analysis
After excluding 469 patients with missing data, 1,894 patients were assessed for sensitivity analyses. The results are graphically illustrated as forest plots in Figure 2. Consistent with the main analyses, bilateral and proximal LEDVT remained independently associated with an increased risk of PE (Model 1, P < 0.01, Figure 2A), whereas unilateral-proximal, bilateral-distal and bilateral-proximal LEDVT exhibited higher risk of PE (Model 2, P < 0.01, Figure 2B).
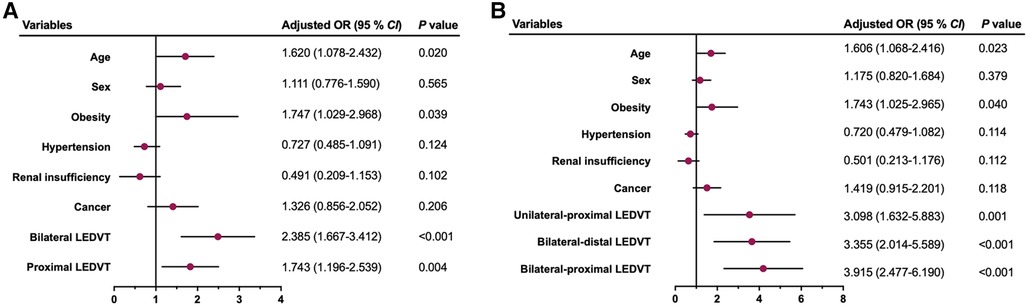
Figure 2. Multivariate sensitivity analysis by excluding patients with missing data for the risk of pulmonary embolism. (A) Model 1. (B) Model 2. OR, odds ratio; CI, confidence interval; LEDVT, lower extremity deep venous thrombosis.
Discussion
Although DVT and PE often differ substantially in terms of risk factors, disease presentation and clinical outcomes, DVT is believed to contribute to the occurrence of PE (32). Recently, data from the RE-COVERY DVT/PE global observational study showed that 35.21% of PE patients had LEDVT, while 20.63% of LEDVT patients had PE (33). In another international, prospective, observational WHITE study, 10.21% of LEDVT patients had confirmed concomitant PE (34). After the diagnosis of LEDVT, we found the proportion of PE among LEDVT patients was 7.83%, which was lower than the above-mentioned (33, 34), but slightly higher than that reported in another study (6.15%) (16).
CUS is the first-line tool for imaging LEDVT, and recommended to undergo bilateral examinations because of a high incidence of clinically silent contralateral thrombosis (35). Using the ultrasound, anatomic distribution of LEDVT can be observed clearly, including thrombus sidedness (left, right and bilateral) and thrombus location (proximal, distal and mixed). In the present study, patients with bilateral LEDVT had a higher proportion of PE than those with unilateral LEDVT (12.24% vs. 5.57%). This association remained significant even after multivariable adjustments and sensitivity analysis (Model 1). This finding indicated that bilateral LEDVT was independent risk factor for developing PE, leading to a 2.455-fold increase in PE risk. Consistent with our study, Bikdeli et al. (17) included 30,445 patients with LEDVT, and patients with bilateral DVT had markedly higher rates of PE than left or right LEDVT (46.62% vs. 22.13% vs. 24.83%), as well as a significantly higher rate of subsequent 90-day new PE (1.73% vs. 0.72% vs. 0.91%). This relationship was also confirmed by other studies (16, 24).
In addition, several prior studies have shown that patients with right LEDVT were more likely to suffer from PE than those with left LEDVT (12, 23, 36). Iliac vein compression syndrome, also known as May-Thurner syndrome, is an uncommon anatomical variant characterized by compression of left common iliac vein by the overlying right iliac artery. For this, left iliac vein compression may potentially limit the migration of the thrombus from this stenotic segment to the pulmonary arteries, leading to a relatively low incidence of PE (36). This speculation was supported by the results of Chen et al. (36), who confirmed that left iliac vein thrombosis (IVT) was associated with a lower incidence of symptomatic PE than right LEDVT (5.4% vs. 13.8%). In this study, we observed a similar trend, but this was not statistically significant in multivariate analysis (Supplementary Table S1, P = 0.053) and sensitivity analysis (Supplementary Figure S1, P = 0.113). Also, the literature mentioned above reported no differences in PE incidence between left non-IVT, right IVT and right non-IVT (12.8% vs. 10.1% vs. 16.6%, P = 0.38) (36). Due to a relatively small number of proximal LEDVT patients included in this study, this may be the reason that right LEDVT did not reach a statistically significant level.
Commonly, patients with proximal LEDVT had a higher likelihood for development of PE than those with distal LEDVT (18, 23). For this, the CHEST guideline emphasizes the importance of thrombus location in treatment choices between serial imaging and anticoagulation therapy (37). As recommended by the 2019 European Society of Cardiology (ESC) guideline, thrombus location can also be used to confirm PE (38). Except for the different anatomic location, proximal and distal LEDVT differ substantially in many aspects, including age, sex, comorbidity burden and VTE risk factors (e.g., recent surgery) (18, 19, 33). After adjusting for confounding factors, we also found that proximal LEDVT was risk factor for developing PE in multivariate analysis and sensitivity analysis (Model 1).
Based on the results of thrombus sidedness and thrombus location, we further subdivided the anatomic distributions of LEDVT into 4 categories (unilateral-distal, unilateral-proximal, bilateral-distal and bilateral-proximal LEDVT). In this study, the proportions of PE ranged between the lowest (4.07%) in unilateral-distal LEDVT and highest (14.55%) in bilateral-proximal LEDVT. The results from multivariate analysis also found that unilateral-proximal, bilateral-distal and bilateral-proximal LEDVT exhibited a 2.129-fold, 3.193-fold and 3.425-fold increase risk for PE, respectively (Model 2). In line with this, Qiu et al. (16) reported that the incidence of PE was highest in patients with bilateral-proximal LEDVT (15.4%), followed by bilateral-distal LEDVT (11.1%), left-proximal LEDVT (7.2%) and right-proximal LEDVT (5.5%). In fact, the clinicians have paid more attention to patients with proximal LEDVT, as evidenced by the treating decision of extension of secondary prophylaxis (34). Notably, the PE risk in isolated distal LEDVT was still high, even without leg edema and/or pain (39). As bilateral-distal LEDVT (24.59%) was the second most prevalent thrombosis after unilateral-distal LEDVT in the study, anatomic distributions of LEDVT deserved special attention, especially bilateral-distal LEDVT.
Moreover, obesity and hypertension were found to be independently associated with PE, while age (Model 2, P = 0.052) and sex (Model 2, P = 0.075) were close to achieving statistical significance. Indeed, age, sex and obesity have already clearly been identified as risk factors for PE (40). Interestingly, the adjusted ORs of hypertension were less than 1.0, implying that hypertension appeared to be a protective factor for PE, which seems controversial with clinical practice. Consistent with this finding, Hu et al. (41) performed a summary-level Mendelian randomization analysis by extracting data from public and large-scale genome-wide association studies, and found that per SD increase of systolic blood pressure (SBP) could decrease the risk of PE by 1% (95% CI: 0.98–1.00, P = 0.003), and the presence of hypertension was associated with a lower risk of PE, although statistical significance was not reached (OR = 0.69, 95% CI: 0.42–1.15, P = 0.16). Another meta-analysis including 9 prospective studies also supported an inverse association between SBP and PE, and patients with hypertension had a lower risk of PE (hazard ratio = 0.93, 95% CI: 0.85–1.03) (42). The potential mechanisms behind the protective effect of hypertension are not well understood. It was likely that the increased medical attention after hypertension diagnosis may be a protective factor against PE (41). Further studies are needed to validate this finding.
Cancer has long been recognized as an important risk factor for PE (28, 40). In this study, patients with PE had a higher proportion of cancer than those without (20.54% vs. 14.42%), and univariate analysis showed that patients with cancer had a 1.535-fold increase in PE risk (P = 0.026). However, this relationship lost its independent significance in the multivariate analysis and sensitivity analysis (Model 1 and Model 2). When compared with patients without cancer, those with cancer were younger (65.26 vs. 70.32 years, P < 0.001), and less obesity (5.40% vs. 7.61%, P = 0.038). This may have contributed to the lack of statistical significance found in cancer.
Some potential limitations should be noted. First, this was a retrospective study, some important variables related to PE could not be accessed, including other risk factors (e.g., prior VTE, immobility, trauma), anticoagulation and antiplatelet use before hospital admission, and location of PE (central [main or lobar pulmonary artery branch] vs. peripheral [segmental or subsegmental branch]). Meanwhile, anticoagulants after LEDVT diagnosis were not obtained due to lack of detailed medication regimen in the database. Second, all patients were identified from our hospital, hence selection bias inevitably existed. Also, excluding a large number of patients with unilateral CUS examination from the analysis may have led to a selection bias. Despite the strengths of our study, the findings should be interpreted with some caution. Third, although the overall sample size was large, the sample size for PE were relatively small. For this reason, a further subdivision of anatomic distribution of LEDVT (left-distal, left-proximal, right-distal, right-proximal) could not be analyzed. Therefore, more studies are necessary to validate these associations.
In conclusion, patients with unilateral-proximal, bilateral-distal or bilateral-proximal are more likely to suffer from PE than those with unilateral-distal LEDVT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of People's Hospital of Deyang City (2021-04-019-K01). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZW and XC conceived and designed the study. JZ, YC, YL and ML collected the data. ZW and XC performed the statistical analysis. JZ and YC wrote the manuscript. ZW and XC supervised the study and approved the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Sichuan Science and Technology Program (2021JDR0337, 2020YFS0520), and Health and Family Planning Commission of Sichuan Province (20PJ249, 20PJ250).
Acknowledgments
We thank Xiaohong Qin from the Shanghai ClinBrain Information Technology Co., Ltd, for assistance with data extraction.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1154875/full#supplementary-material.
Supplementary Figure S1
Association between left, right LEDVT and pulmonary embolism risk using a multivariate sensitivity analysis by excluding patients with missing data.
Supplementary Figure S2
Receiver operating characteristic (ROC) curve of age for the detection of pulmonary embolism.
References
1. Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA. (2020) 324(17):1765–76. doi: 10.1001/jama.2020.17272
2. Wendelboe AM, Campbell J, Ding K, Bratzler DW, Beckman MG, Reyes NL, et al. Incidence of venous thromboembolism in a racially diverse population of Oklahoma county, Oklahoma. Thromb Haemost. (2021) 121(6):816–25. doi: 10.1055/s-0040-1722189
3. Chang SL, Huang YL, Lee MC, Hu S, Hsiao YC, Chang SW, et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. (2018) 319(8):807–17. doi: 10.1001/jama.2018.0246
4. Arshad N, Isaksen T, Hansen JB, Brækkan SK. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur J Epidemiol. (2017) 32(4):299–305. doi: 10.1007/s10654-017-0238-y
5. Payne JG, Tagalakis V, Wu C, Lazo-Langner A. Current estimates of the incidence of acute venous thromboembolic disease in Canada: a meta-analysis. Thromb Res. (2021) 197:8–12. doi: 10.1016/j.thromres.2020.10.030
6. Duffett L, Castellucci LA, Forgie AM. Pulmonary embolism: update on management and controversies. Br Med J. (2020) 370:m2177. doi: 10.1136/bmj.m2177
7. Sonne-Holm E, Kjærgaard J, Bang LE, Fosbøl E, Carlsen J, Winther-Jensen M. Pulmonary embolism: age specific temporal trends in incidence and mortality in Denmark 1999–2018. Thromb Res. (2022) 210:12–9. doi: 10.1016/j.thromres.2021.12.011
8. Zhang Z, Lei J, Shao X, Dong F, Wang J, Wang D, et al. Trends in hospitalization and in-hospital mortality from VTE, 2007 to 2016, in China. Chest. (2019) 155(2):342–53. doi: 10.1016/j.chest.2018.10.040
9. Martin KA, Molsberry R, Cuttica MJ, Desai KR, Schimmel DR, Khan SS. Time trends in pulmonary embolism mortality rates in the United States, 1999 to 2018. J Am Heart Assoc. (2020) 9(17):e016784. doi: 10.1161/jaha.120.016784
10. Barco S, Mahmoudpour SH, Valerio L, Klok FA, Münzel T, Middeldorp S, et al. Trends in mortality related to pulmonary embolism in the European region, 2000-15: analysis of vital registration data from the WHO mortality database. Lancet Respir Med. (2020) 8(3):277–87. doi: 10.1016/s2213-2600(19)30354-6
11. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118(9):1340–7. doi: 10.1161/circresaha.115.306841
12. Hou J, Wang W, Cai H, Chen J, Chen B, Shen Z, et al. Patients with right lower extremity deep vein thrombosis have a higher risk of symptomatic pulmonary embolism: a retrospective study of 1585 patients. Ann Vasc Surg. (2022) 81:240–8. doi: 10.1016/j.avsg.2021.08.049
13. Roy PM, Friou E, Germeau B, Douillet D, Kline JA, Righini M, et al. Derivation and validation of a 4-level clinical pretest probability score for suspected pulmonary embolism to safely decrease imaging testing. JAMA Cardiol. (2021) 6(6):669–77. doi: 10.1001/jamacardio.2021.0064
14. Guan X, Lan Q, Liang Y, Ke H, Chen S, Long L. Comparative study of diagnostic efficacy of single phase-computed tomography pulmonary angiography and dual phase-computed tomography pulmonary angiography in the diagnosis of pulmonary embolism. Front Cardiovasc Med. (2022) 9:846805. doi: 10.3389/fcvm.2022.846805
15. van der Hulle T, Cheung WY, Kooij S, Beenen LFM, van Bemmel T, van Es J, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. (2017) 390(10091):289–97. doi: 10.1016/s0140-6736(17)30885-1
16. Qiu T, Zhang T, Liu L, Li W, Li Q, Zhang X, et al. The anatomic distribution and pulmonary embolism complications of hospital-acquired lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. (2021) 9(6):1391–1398.e3. doi: 10.1016/j.jvsv.2021.03.004
17. Bikdeli B, Sharif-Kashani B, Bikdeli B, Valle R, Falga C, Riera-Mestre A, et al. Impact of thrombus sidedness on presentation and outcomes of patients with proximal lower extremity deep vein thrombosis. Semin Thromb Hemost. (2018) 44(4):341–7. doi: 10.1055/s-0037-1621716
18. Bikdeli B, Caraballo C, Trujillo-Santos J, Galanaud JP, di Micco P, Rosa V, et al. Clinical presentation and short- and long-term outcomes in patients with isolated distal deep vein thrombosis vs proximal deep vein thrombosis in the RIETE registry. JAMA Cardiol. (2022) 7(8):857–65. doi: 10.1001/jamacardio.2022.1988
19. Schellong SM, Goldhaber SZ, Weitz JI, Ageno W, Bounameaux H, Turpie AGG, et al. Isolated distal deep vein thrombosis: perspectives from the GARFIELD-VTE registry. Thromb Haemost. (2019) 119(10):1675–85. doi: 10.1055/s-0039-1693461
20. Lidstrom SC, Wiggins KL, Harrington LB, McKnight B, Blondon M, Smith LN. Incident thrombus location and predicting risk of recurrent venous thromboembolism. Res Pract Thromb Haemost. (2022) 6(5):e12762. doi: 10.1002/rth2.12762
21. Wang XH, Cui LB, Liu Y, Han X, Chi J, Yang B, et al. Association between risk stratification for pulmonary embolism and deep vein thrombosis of lower extremities. Clin Respir J. (2020) 14(7):631–7. doi: 10.1111/crj.13177
22. Chen X, Liu X, Liu J, Zhang D. Pulmonary embolism secondary to deep venous thrombosis: a retrospective and observational study for clinical characteristics and risk stratification. Phlebology. (2021) 36(8):627–35. doi: 10.1177/0268355521990964
23. Zhang C, Li Q, Yu H, Wang F, Lin Z, Yin W, et al. Relationship between the site of thrombosis and the prevalence of pulmonary embolism in acute lower extremity deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. (2020) 8(5):725–33. doi: 10.1016/j.jvsv.2019.11.010
24. El-Menyar A, Asim M, Jabbour G, Al-Thani H. Clinical implications of the anatomical variation of deep venous thrombosis. Phlebology. (2018) 33(2):97–106. doi: 10.1177/0268355516687863
25. Ma J, Du P, Qin J, Zhou Y, Liang N, Hu J, et al. Incidence and risk factors predicting deep venous thrombosis of lower extremity following spinal fractures. Sci Rep. (2021) 11(1):2441. doi: 10.1038/s41598-021-82147-x
26. Johnson SA, Signor EA, Lappe KL, Shi J, Jenkins SL, Wikstrom SW, et al. A comparison of natural language processing to ICD-10 codes for identification and characterization of pulmonary embolism. Thromb Res. (2021) 203:190–5. doi: 10.1016/j.thromres.2021.04.020
27. Zhou B. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi. (2002) 23(1):5–10. doi: 10.3760/j.issn:0254-6450.2002.01.003
28. Glise Sandblad K, Rosengren A, Sörbo J, Jern S, Hansson PO. Pulmonary embolism and deep vein thrombosis-comorbidities and temporary provoking factors in a register-based study of 1.48 million people. Res Pract Thromb Haemost. (2022) 6(4):e12714. doi: 10.1002/rth2.12714
29. Choi J, Dekkers OM, le Cessie S. A comparison of different methods to handle missing data in the context of propensity score analysis. Eur J Epidemiol. (2019) 34(1):23–36. doi: 10.1007/s10654-018-0447-z
30. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. (2014) 67(8):850–7. doi: 10.1016/j.jclinepi.2014.03.012
31. Cheng J, Sun J, Yao K, Xu M, Cao Y. A variable selection method based on mutual information and variance inflation factor. Spectrochim Acta A Mol Biomol Spectrosc. (2022) 268:120652. doi: 10.1016/j.saa.2021.120652
32. Wenger N, Sebastian T, Engelberger RP, Kucher N, Spirk D. Pulmonary embolism and deep vein thrombosis: similar but different. Thromb Res. (2021) 206:88–98. doi: 10.1016/j.thromres.2021.08.015
33. Schellong S, Ageno W, Casella IB, Chee KH, Schulman S, Singer DE, et al. Profile of patients with isolated distal deep vein thrombosis versus proximal deep vein thrombosis or pulmonary embolism: RE-COVERY DVT/PE study. Semin Thromb Hemost. (2022) 48(4):446–58. doi: 10.1055/s-0041-1729169
34. Palareti G, Bignamini AA, Urbanek T, Cini M, Li YJ, Madaric J, et al. Influence of clinical presentation, site, and extent of venous thrombosis on decision about duration of anticoagulation: data from the international, prospective, observational WHITE study. Thromb Res. (2022) 211:140–6. doi: 10.1016/j.thromres.2022.01.025
35. Pennell RC, Mantese VA, Westfall SG. Duplex scan for deep vein thrombosis–defining who needs an examination of the contralateral asymptomatic leg. J Vasc Surg. (2008) 48(2):413–6. doi: 10.1016/j.jvs.2008.03.046
36. Chen F, Huang JG, Liu X, Zhou W. Left iliac vein involvement is a protective factor against symptomatic pulmonary embolism in lower left extremity deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. (2022) 10(6):1272–8. doi: 10.1016/j.jvsv.2022.05.015
37. Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. (2021) 160(6):e545–608. doi: 10.1016/j.chest.2021.07.055
38. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. (2020) 41(4):543–603. doi: 10.1093/eurheartj/ehz405
39. Kim SM. Clinical presentation of isolated calf deep vein thrombosis in inpatients and prevalence of associated pulmonary embolism. J Vasc Surg Venous Lymphat Disord. (2022) 10(5):1037–43. doi: 10.1016/j.jvsv.2022.02.011
40. Fujieda K, Nozue A, Watanabe A, Shi K, Itagaki H, Hosokawa Y, et al. Malignant tumor is the greatest risk factor for pulmonary embolism in hospitalized patients: a single-center study. Thromb J. (2021) 19(1):77. doi: 10.1186/s12959-021-00334-2
41. Hu M, Li X, Yang Y. Causal associations between cardiovascular risk factors and venous thromboembolism. Semin Thromb Hemost. (2023). doi: 10.1055/s-0042-1760335
42. Mahmoodi BK, Cushman M, Anne Næss I, Allison MA, Bos WJ, Brækkan SK, et al. Association of traditional cardiovascular risk factors with venous thromboembolism: an individual participant data meta-analysis of prospective studies. Circulation. (2017) 135(1):7–16. doi: 10.1161/circulationaha.116.024507
Keywords: pulmonary embolism, lower extremity deep venous thrombosis, anatomic distribution, risk factor, compression ultrasonography
Citation: Zhang J, Chen Y, Wang Z, Chen X, Liu Y and Liu M (2023) Anatomic distribution of lower extremity deep venous thrombosis is associated with an increased risk of pulmonary embolism: A 10-year retrospective analysis. Front. Cardiovasc. Med. 10:1154875. doi: 10.3389/fcvm.2023.1154875
Received: 31 January 2023; Accepted: 7 March 2023;
Published: 22 March 2023.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Fulvio Pomero, Michele e Pietro Ferrero Hospital, ItalyDoris Barcellona, University of Cagliari, Italy
© 2023 Zhang, Chen, Wang, Chen, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhicong Wang d2FuZ3pjb25nQGhvdG1haWwuY29t Xi Chen Y2hlbnhpZG9jdG9yQDE2My5jb20=
†These authors have contributed equally to this work.
Specialty Section: This article was submitted to Thrombosis, a section of the journal Frontiers in Cardiovascular Medicine
 Jianjun Zhang1,†
Jianjun Zhang1,† Zhicong Wang
Zhicong Wang