- 1Davidai Arrhythmia Center, Sheba Medical Center, Ramat Gan, Israel
- 2Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
- 3Department of Cardiac Surgery, Sheba Medical Center, Ramat Gan, Israel
- 4Population Health Research Institute, McMaster University, Hamilton, ON, Canada
- 5Jesselson Integrated Heart Center, Shaare Zedek Medical Center, Jerusalem, Israel
Background: Postoperative atrial fibrillation (POAF) is the most common complication of cardiac surgery, requiring interventions and prolonging hospital stay. POAF is associated with increased mortality and a higher rate of systemic thrombo-embolism. The rates of recurrent AF, optimal follow-up and management remain unclear. We aimed to evaluate the incidence of recurrent atrial fibrillation (AF) events, during long term follow-up in patients with POAF following cardiac surgery.
Methods: Patients with POAF and a CHA2DS2-VASc score of ≥2 were randomized in a 2:1 ratio to either implantation of a loop recorder (ILR) or ECG monitoring using periodic Holters. Participants were followed prospectively for 2 years. The primary end point was the occurrence of AF longer than 5 min.
Results: The final cohort comprised of 22 patients, of whom 14 received an ILR. Over a median follow up of 25.7 (IQR of 24.7–44.4) months, 8 patients developed AF, representing a cumulative annualized risk of AF recurrence of 35.7%. There was no difference between ILR (6 participants, 40%) and ECG/Holter (2 participants, 25% p = 0.917). All 8 patients with AF recurrence were treated with oral anticoagulation. There were no cases of mortality, stroke or major bleeding. Two patients underwent ILR explantation due to pain at the implantation site.
Conclusions: The rate of recurrent AF in patients with POAF after cardiac surgery and a CHA2DS2-VASc score of ≥2 is approximately 1 in 3 when followed systematically. Further research is need to assess the role of ILRs in this population.
Introduction
Postoperative atrial fibrillation (POAF), defined as new-onset atrial fibrillation (AF) in the immediate period after surgery (1, 2), is the most common complication of cardiac surgery (3, 4). Affecting 10%–65% of patients (3, 5), this arrhythmia is associated with increased mortality, and morbidity including stroke and hemodynamic deterioration (6). POAF prolongs the hospital stay and increases health costs. The incidence of POAF is increasing (7), resulting from an increase in the age and burden of arrhythmic risk factors (3) in patients undergoing cardiac surgery.
Despite the high incidence of POAF, its association with recurrent AF after hospital discharge remains unclear. There is uncertainty with respect to the long term follow up and management of these patients.
The first aim of the current study was to assess the incidence of recurrent AF events, during long term follow-up in patients that presented with POAF following cardiac surgery and were discharged in sinus rhythm. Secondly, we aimed to assess the efficacy of an implanted cardiac monitor, as compared to usual care, to detect recurrent AF events during follow-up.
Methods
The study was conducted at 2 tertiary medical centers in Israel (Sheba medical center and Shaare Zedek Medical Center) from August 2017 through March 2021 (NCT 02522364). We recruited adult patients with documented new-onset AF lasting at least 5 min occurring during the index hospitalization following cardiac surgery. Furthermore, patients were required to have a CHA2DS2-VASc score of 2 or higher. Consenting participants were randomized in a 1:2 ratio to either ECG monitoring using periodic Holters (at 2 and 6 months post discharge) alone (No-ILR) and periodic Holters plus implantation of a loop recorder (ILR). The main exclusion criteria were a contraindication for oral anticoagulation, a dual chamber cardiac implantable device and active systemic infection.
All participants were discharged in sinus rhythm and were followed prospectively for a minimum of 2 years. The follow up included Holter monitoring at 3 and 6 months post discharge and clinic visits biannually. Patients randomized to ILR were implanted with a Biomonitor 2 device (Biotronik, Berlin, Germany) within 2 weeks of surgery. Participants were also connected to a remote monitoring system, allowing for continuous surveillance of arrhythmic events. All patients were treated with apixaban for a minimum of 3 months. Treatment with anticoagulation was at the discretion of the attending physician but would be continued to a maximum duration of 6 weeks after hospital discharge unless AF reoccurred. Any documentation of recurrent AF of ≥5 min would trigger continuation or re-initiation of OAC.
The primary end point was the occurrence of AF lasting at least 5 min. Additional endpoints were all-cause death, stroke, rapid AF requiring hospitalization and initiation of long term anticoagulation. The main safety end points were acute complication of ILR implantation and major bleeding.
Results are presented as median [interquartile-range (IRQ)] or mean ± SD as appropriate. Continues variables were compared using a student-t test or Wilcoxon test and binary variables were compared using χ2 tests. Predictors of AF recurrence were evaluated by a univariable cox proportional hazards model followed by a multivariable model. Statistical analysis was carried out using SPSS v21 (Chicago, Ill., USA). A two-sided p value <0.05 was considered statistically significant.
The study was approved by local institutional ethical boards and complied with the Declaration of Helsinki. All study participants gave written informed consent.
Funding
This investigator-initiated study was funded by an unrestricted grant form Pfizer Inc. via the BMS/Pfizer European Thrombosis Investigator Initiated Research Program (ERISTA) and by an additional unrestricted grant form Biotronik Inc. Neither company was involved in the study design, data collection, analysis or interpretation. The manuscript was written solely by the authors.
Results
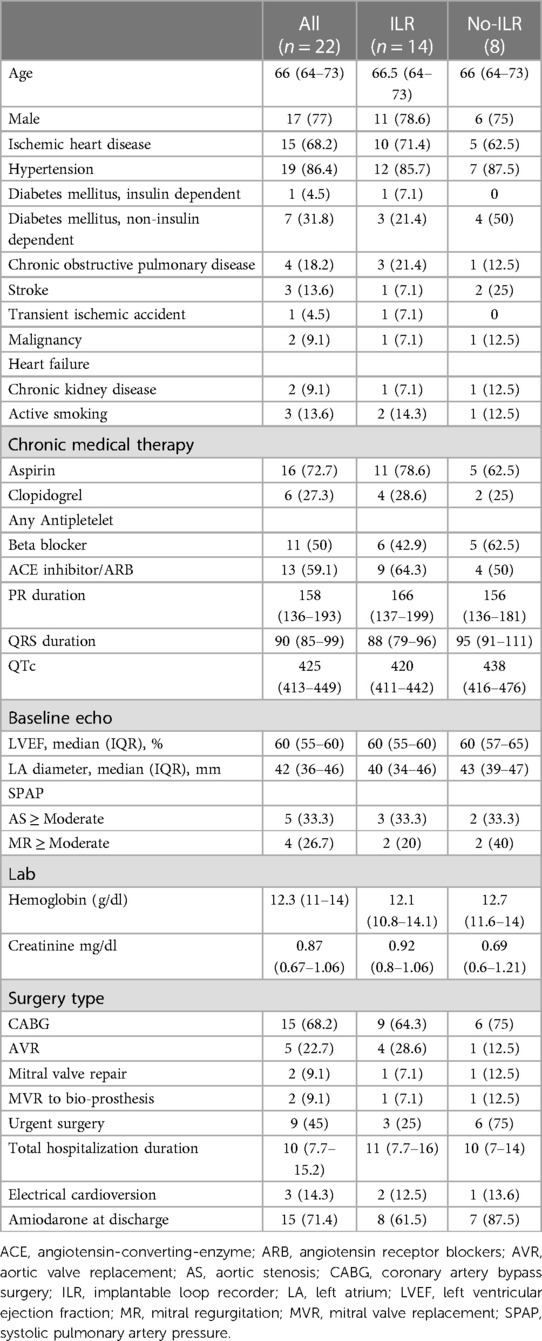
A total of 29 patients were recruited (see Supplementary Table for baseline characteristics), among whom 7 withdrew consent after randomization. One participant that was randomized to ILR crossed over to the no-ILR arm (Figure 1). The final study cohort was comprised of 22 participants {median age 66 [interquartile range (IQR) 64–73], 23% female}, of whom 14 underwent ILR implantation.
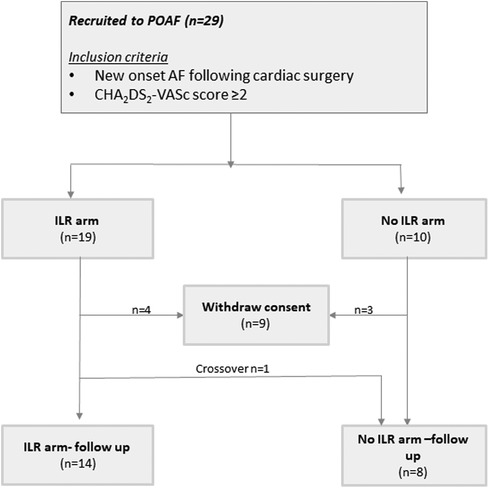
Figure 1. Patient flow. AF, atrial fibrillation; ILR, implantable loop recorder; POAF, post operative atrial fibrillation.
All participants in the study were treated with amiodarone during their hospitalization. This resulted in cardioversion in 20 (90) % the reminder underwent electrical cardioversion. All patients were discharged in sinus rhythm (as required by the study protocol) and 7 were discharged with amiodarone. The baseline characteristics of the as-treated cohort are detailed in Table 1.
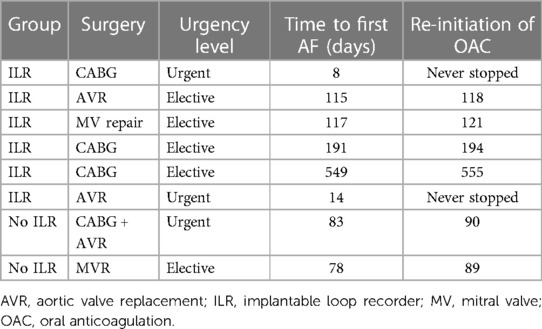
Over a median follow up of 25.7 (IQR 24.7–44.4) months, 8 patients developed AF, representing a cumulative risk of 33.8% (23.3%–44.4%) of AF recurrence at 1 year. Six (40%) were diagnosed by ILR recording while 2 (25%) by ECGs or Holter monitoring (Table 2). The median time to first AF detection was 99 (IQR 30, 172) days and was similar in both groups (log rank p = 0.690). All 8 participants were treated with oral anticoagulation (OAC). The patients that had early AF recurrences continued OAC throughout the study. In the remaining 6, the treatment was re-initiated within a median of 5 (3–11) days from detection of AF recurrence. Treatment with amiodarone was stopped after 6 weeks in all but 2 patients who had early AF recurrence.
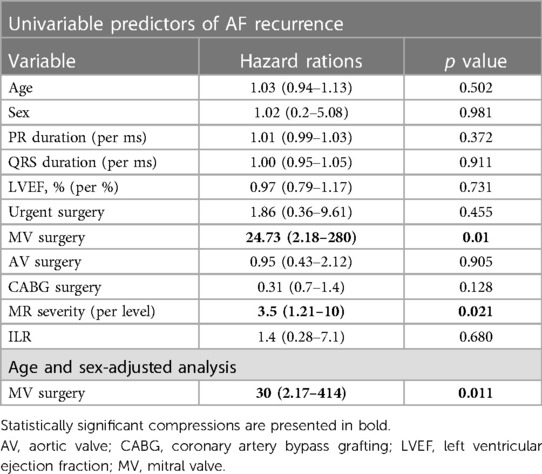
In our cohort, the risk of AF recurrence was not affected by sex, age nor the urgency of the surgery (Table 3). The risk was higher in patients that underwent mitral valve surgery (mitral valve repair or replacement to a biological valve). The association remained statistically significant after adjustment for age and sex [OR 2.18 (2.18–414)]. Similarly the risk of AF recurrence increased in proportion to the severity of MR [OR 2.18 (2.18–414)].
There were no cases of mortality, stroke or systemic embolism during follow up. Two patients underwent ILR explanation; one due to pain at the implant site and the other as part of treatment of deep sternal infection attributed to the index surgery.
Discussion
Our study showed a high rate of AF recurrence in patients that developed new onset AF following cardiac surgery. Although the crude rates of AF detection were higher in patients who received an ILR, this difference was not statistically significant. Most AF recurrences were observed within 6 months of the surgery suggesting that intense arrhythmic monitoring during this period may be effective. As defined by the study protocol, all study participants had a CHA2DS2-VASc score ≥2, therefore each detection of AF recurrence resulted in initiation of OAC therapy.
Our results add to the growing body of evidence that a substantial proportion of patients with POAF develop recurrent AF over time. The recurrence rate in our study (37.5%) is higher than what was observed during long-term follow-up of 3,023 patients in the Arterial Revascularization Trial (18.5% over a median of 6 years) (6). The higher rate observed in our study may be explained by the systematic use of continuous monitoring for recurrent AF.
The long-held notion that POAF is a benign phenomenon is not supported by contemporary data. Multiple studies have shown increased risk of stroke, heart failure or death (6, 8, 9). Yet, the association between POAF and adverse events appears to be stronger early after surgery (9). This, taken with recurrence rates below 50% suggest that identification of POAF alone is not sufficient to identify patients that would benefit from lifelong OAC therapy. This is further supported by a systematic review that failed to show a decrease in the risk of thromboembolic events with the use of OAC following POAF (10) and is reflected by the contemporary European Society of Cardiology Guidelines (1). At this time there are not clear data to guide the risk stratification and selection of patients with POAF that would benefit from OAC. Therefore, systematic follow-up for recurrent AF may be the appropriate strategy. In addition to provision of OAC, such a strategy may identify patients who stand to benefit from rhythm control. The importance of a timely diagnosis of AF is further evident by the results of the recent EAST-AFNET 4 (11) study showing that early implementation of a rhythm control strategy leads to improved outcome, irrespective of AF related symptoms.
Consistent with previous studies (12, 13) the risk of AF recurrence after POAF was high among patients that underwent mitral valve repair or replacement. All of these patients had hemodynamically significant MR that may lead to dilatation and remodeling of the left atrium, creating substrate for AF. Both severe MR and MR surgery may serve as important markers for use in risk stratification.
The results of our study alongside similar other studies emphasize the need for a large prospective randomized study that would be powered not only to more precisely estimate the incidence of AF recurrence and identify its predictors but also to evaluate the potential clinical benefit of early detection of AF recurrence and the impact on clinical outcomes of patients with POAF. Such a study could guide our approach to monitoring these patients, test the incremental value of ILRs over periodic Holters and help select patients that would benefit from life-long OAC. A high-yield population on which to focus may be those undergoing mitral valve surgery.
The main strengths of this study are the randomized design, pre-registration and systematic follow-up. Furthermore, the use of ILR ensures that all AF events were captured, yielding the true incidence of progression from POAF to AF the majority of the study cohort (ILR arm). The study has important limitations. The small sample size, withdrawal of 7 participants, plus the crossover of one study participant to the no-ILR arm may have obscured between group differences. Furthermore, the study is unable to identify specific predictors of AF recurrence.
Conclusions
Among patients with POAF after cardiac surgery and a CHA2DS2-VASc score of 2 or more, the rate of AF recurrence as detected with systematic follow up is approximately 1 in 3. In the present study, ILR monitoring did not result in higher rates of AF detection, however between-group differences were likely obscured by small sample sizes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Sheba medical center, Tel HaShomer, Israel and the Shaare Zedek Medical Center, Jerusalem, Israel. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AS, RB, and MG contributed to conception and original design of the study. The design was revised by RB and AB. AS and MG secured the research grants. RB and ER supervised the project. AB and DV compiled and organized the database. AS and WB performed the statistical analysis. AS and WB wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study is investigator-initiated and funded by Pfizer Inc. via the BMS/Pfizer European Thrombosis Investigator Initiated Research Program (ERISTA) and by an unrestricted grant form Bitronik Inc.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1153275/full#supplementary-material.
References
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2020) 2021(42):373–498. doi: 10.1093/eurheartj/ehaa612
2. McIntyre WF. Post-operative atrial fibrillation after cardiac surgery: challenges throughout the patient journey. Front Cardiovasc Med. (2023) 10:1156626. doi: 10.3389/fcvm.2023.1156626
3. Aranki SF, Shaw DP, Adams DH, Rizzo RJ, Couper GS, VanderVliet M, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. (1996) 94:390–7. doi: 10.1161/01.CIR.94.3.390
4. Mitchell LB, CCS Atrial Fibrillation Guidelines Committee. Canadian cardiovascular society atrial fibrillation guidelines 2010: prevention and treatment of atrial fibrillation following cardiac surgery. Can J Cardiol. (2011) 27:91–7. doi: 10.1016/j.cjca.2010.11.005
5. Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. (2001) 135:1061–73. doi: 10.7326/0003-4819-135-12-200112180-00010
6. Benedetto U, Gaudino MF, Dimagli A, Gerry S, Gray A, Lees B, et al. Postoperative atrial fibrillation and long-term risk of stroke after isolated coronary artery bypass graft surgery. Circulation. (2020) 142:1320–29. doi: 10.1161/CIRCULATIONAHA.120.046940
7. Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. (1993) 56:539–49. doi: 10.1016/0003-4975(93)90894-N
8. Wang MK, Meyre PB, Heo R, Devereaux PJ, Birchenough L, Whitlock R, et al. Short-term and long-term risk of stroke in patients with perioperative atrial fibrillation after cardiac surgery: systematic review and meta-analysis. CJC Open. (2022) 4:85–96. doi: 10.1016/j.cjco.2021.09.011
9. Conen D, Wang MK, Devereaux PJ, Whitlock R, McIntyre WF, Healey JS, et al. New-onset perioperative atrial fibrillation after coronary artery bypass grafting and long-term risk of adverse events: an analysis from the CORONARY trial. J Am Heart Assoc. (2021) 10:e020426. doi: 10.1161/JAHA.120.020426
10. Fragão-Marques M, Teixeira F, Mancio J, Seixas N, Rocha-Neves J, Falcão-Pires I, et al. Impact of oral anticoagulation therapy on postoperative atrial fibrillation outcomes: a systematic review and meta-analysis. Thromb J. (2021) 19:89. doi: 10.1186/s12959-021-00342-2
11. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. (2020) 383:1305–16. doi: 10.1056/NEJMoa2019422
12. Shen J, Lall S, Zheng V, Buckley P, Damiano RJ Jr, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. (2011) 141:559–70. doi: 10.1016/j.jtcvs.2010.03.011
Keywords: post-operative atrial fibrillation, implantable loop recorder, stroke, atrial fibrillation, cardiac surgery
Citation: Sabbag A, Berkovich A, Raanani E, Volvovitch D, McIntyre WF, Kassif Y, Kogan A, Glikson M and Beinart R (2023) Subclinical postoperative atrial fibrillation: a randomized trial. Front. Cardiovasc. Med. 10:1153275. doi: 10.3389/fcvm.2023.1153275
Received: 29 January 2023; Accepted: 2 May 2023;
Published: 25 May 2023.
Edited by:
Rohan Wijesurendra, University of Oxford, United KingdomReviewed by:
Harry Crijns, Maastricht University, NetherlandsMartin Manninger, Medical University of Graz, Austria
Christine Lemeš, Ordensklinikum Linz, Austria
© 2023 Sabbag, Berkovich, Raanani, Volvovitch, McIntyre, Kassif, Kogan, Glikson and Beinart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Avi Sabbag YXZpLnNhYmJhZ0BzaGViYS5oZWFsdGguZ292Lmls
 Avi Sabbag
Avi Sabbag Anat Berkovich1,2
Anat Berkovich1,2 William F. McIntyre
William F. McIntyre Michael Glikson
Michael Glikson Roy Beinart
Roy Beinart