- 1Yanqing Hospital of Beijing Chinese Medicine Hospital, Beijing, China
- 2Clinical Department of Integrative Traditional Chinese and Western Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 4Sun Yat-sen University Cancer Center, Guangzhou, China
Introduction: Preventing ischemia-reperfusion injury is the main direction of myocardial infarction treatment in the convalescent stage. Some studies have suggested that saponins in Traditional Chinese medicine (TCM) preparations can protect the myocardium by various mechanisms. Our meta-analysis aims to evaluate the efficacy of TCM saponins in treating myocardial ischemia-reperfusion injury (MIRI) and to summarize the potential molecular mechanisms further.
Methods: We conducted a literature search in six electronic databases [Web of Science, PubMed, Embase, Cochrane Library, Sinomed, China National Knowledge Infrastructure (CNKI)] until October 2022.
Results: Seventeen eligible studies included 386 animals (254 received saponins and 132 received vehicles). The random effect model is used to calculate the combined effect. The effect size is expressed as the weighted average difference (WMD) and 95% confidence interval (CI). Compared with placebo, saponins preconditioning reduced infarct size after MIRI significantly (WMD: −3.60,95% CI: −4.45 to −2.74, P < 0.01, I2: 84.7%, P < 0.001), and significantly increased EF (WMD: 3.119, 95% CI: 2.165 to 4.082, P < 0.01, I2: 82.9%, P < 0.0 L) and FS (WMD: 3.157, 95% CI: 2.218 to 4.097, P < 0.001, I2: 81.3%, P < 0.001).
Discussion: The results show that the pre-administration of saponins from TCM has a significant protective effect on MIRI in preclinical studies, which provides an application prospect for developing anti-MIRI drugs with high efficiency and low toxicity.
1. Introduction
Timely percutaneous coronary intervention (PCI) remains by far the most effective treatment for the salvage of acute myocardial infarction (1). However, subsequent ischemia/reperfusion (I/R) injury can still lead to massive loss of cardiomyocytes, thereby exacerbating cardiac dysfunction (2). Now, MIRI has become a vital issue in clinical practice, attracting extensive attention from researchers. MIRI involves multiple mechanisms, such as inflammation, oxidative stress, mitochondrial damage, and calcium overload (3). Recently, researchers have begun to focus on ferroptosis, apoptosis, and autophagy during MIRI. Several drug and non-drug therapies have emerged to treat I/R injury (4). However, given that treatments targeting I/R injury would be used only once in most patients, their development, compared to drugs that require longer-term use, is at an obvious disadvantage.
TCM emphasizes the holistic concept. The herbal prescriptions are usually complex, targeting multiple pathways (5, 6). In TCM, the causes of MIRI include Qi deficiency, blood stasis, and phlegm resistance (7–9). According to TCM theories, doctors mainly use herbs that nourish qi, promote blood circulation, remove blood stasis, and expel phlegm. Recently, multiple studies have found that saponins from traditional Chinese herbs have sound anti-MIRI effects (10–12). The mechanisms include regulating calcium homeostasis and energy metabolism and inhibiting inflammation and oxidative stress (13–16). Saponins mainly contain steroid saponins and triterpenoid saponins. Due to their structural specificity, saponins have different MIRI protection mechanisms (17). Therefore, we conducted a comprehensive and systematic meta-analysis of all relevant articles to evaluate the effectiveness and summarize the possible means of TCM saponin extract intervention in alleviating I/R injury.
2. Methods
2.1. Data sources and search strategies
We searched Web of Science, PubMed, Embase, Cochrane Library, Sinomed, and China National Knowledge Infrastructure (CNKI) for articles on the cardioprotective effects of saponins in myocardial ischemia-reperfusion injury. The search terms were “myocardial ischemia-reperfusion injury,” “myocardial I/R injury,” “myocardial ischemia-reperfusion injury,” “myocardial ischemia-reperfusion injury,” “saponins,” “ginsenosides,” “Holothurin,” and “Quillaja Saponins,”. The publication time was limited to before October 2022. Two reviewers (Fan Yang and Xin Su) independently assessed articles for eligibility, and disagreements were resolved by discussion with the corresponding author.
2.2. Inclusion and exclusion criteria
Inclusion criteria: (a) Type of study: In vivo animal studies. (b) Interventions and controls: Saponin extracts derived from traditional Chinese herbs compared with placebo treatment. (c) Outcomes: At least one of the following outcome measures should be reported: Infarct size [infarct size expressed as a percentage of infarct area over the area at risk (AAR)], left ventricle ejection fraction percentage (LVEF), left ventricle fractional shortening percentage (LVFS).
Exclusion criteria: (a) Duplicate reports. (b) Studies with incomplete data and unclear outcome indicators. (c) Studies where the outcome indicators could not be transformed into mergeable data. (d) Studies using incorrect statistical methods that could not be corrected.
2.3. Data extraction
Two researchers (Jiahao Sun and Jiarong Fan) independently extracted baseline information (i.e., sample size, country, author, and year), animal characteristics (e.g., age, weight, strain, or species), detailed treatments of included studies Strategy (such as the type of saponin, source plant, dose, method and time of administration) and methods for measuring myocardial infarction size after myocardial I/R injury.
2.4. Quality assessment
The quality of included studies was assessed and scored by two researchers (Yang Fan and Su Xin) according to the CAMARADES(Collaborative Approach to Meta Analysis and Review of Animal Data from Experimental Stroke) list (18). For each article, we scored 1 point for each of the following terms: statement of a potential conflict of interest, sample size calculation, random allocation to groups, a peer-reviewed publication, compliance with animal welfare regulations, and blinded outcomes assessment. We define 1–2 points as high risk, 3–4 points as medium risk, and 5–6 points as low risk. Disagreements were resolved through discussions with the corresponding author.
2.5. Data statistical and analysis
This meta-analysis used continuous variables as mean and standard deviation. We calculated pooled effect sizes using random effects models. Effect sizes were expressed as weighted mean differences (WMD) with 95% confidence intervals (CI). To assess heterogeneity, we used the Q statistic and the I2 statistic for quantifying. Publication bias was evaluated initially by funnel plots and further detected by Egger's test. If there were significant heterogeneity among studies (p < 0.10), the analysis would be performed by deleting each study, post hoc subgroup analysis (i.e., duration of reperfusion, study type, species, and schedule of preconditioning), and trim-and-fill method to achieve sensitivity analysis. Univariate regression (i.e., study type, species, states, sample size, route of administration and duration of reperfusion, and temporal regimen of preconditioning) was proposed to explore potential heterogeneity sources. For all results except heterogeneity, P < 0.05 was considered statistically significant. We used STATA version 15.1 (STATA Corporation, CollegeStation, TX, USA) for statistical analyses and graphs.
3. Results
3.1. Characteristics of included studies
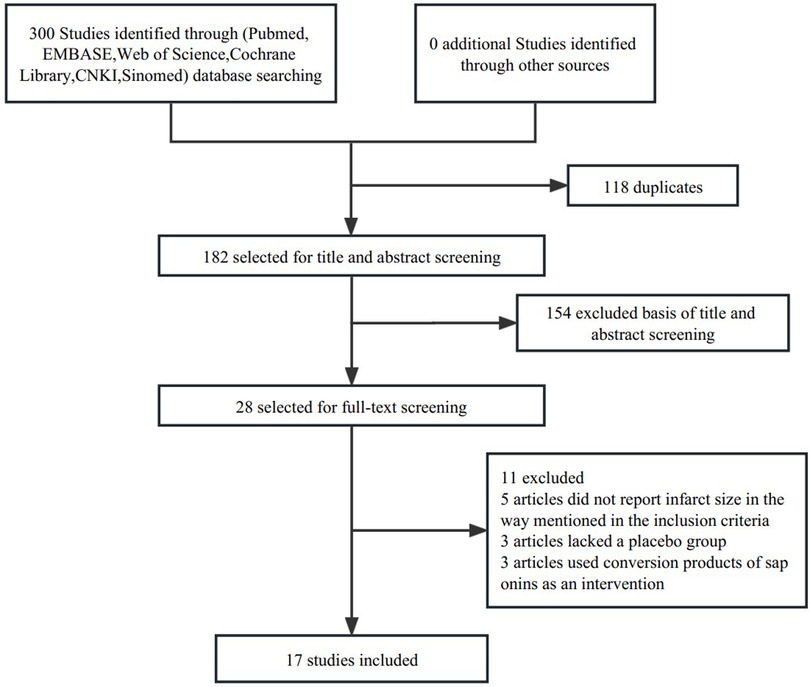
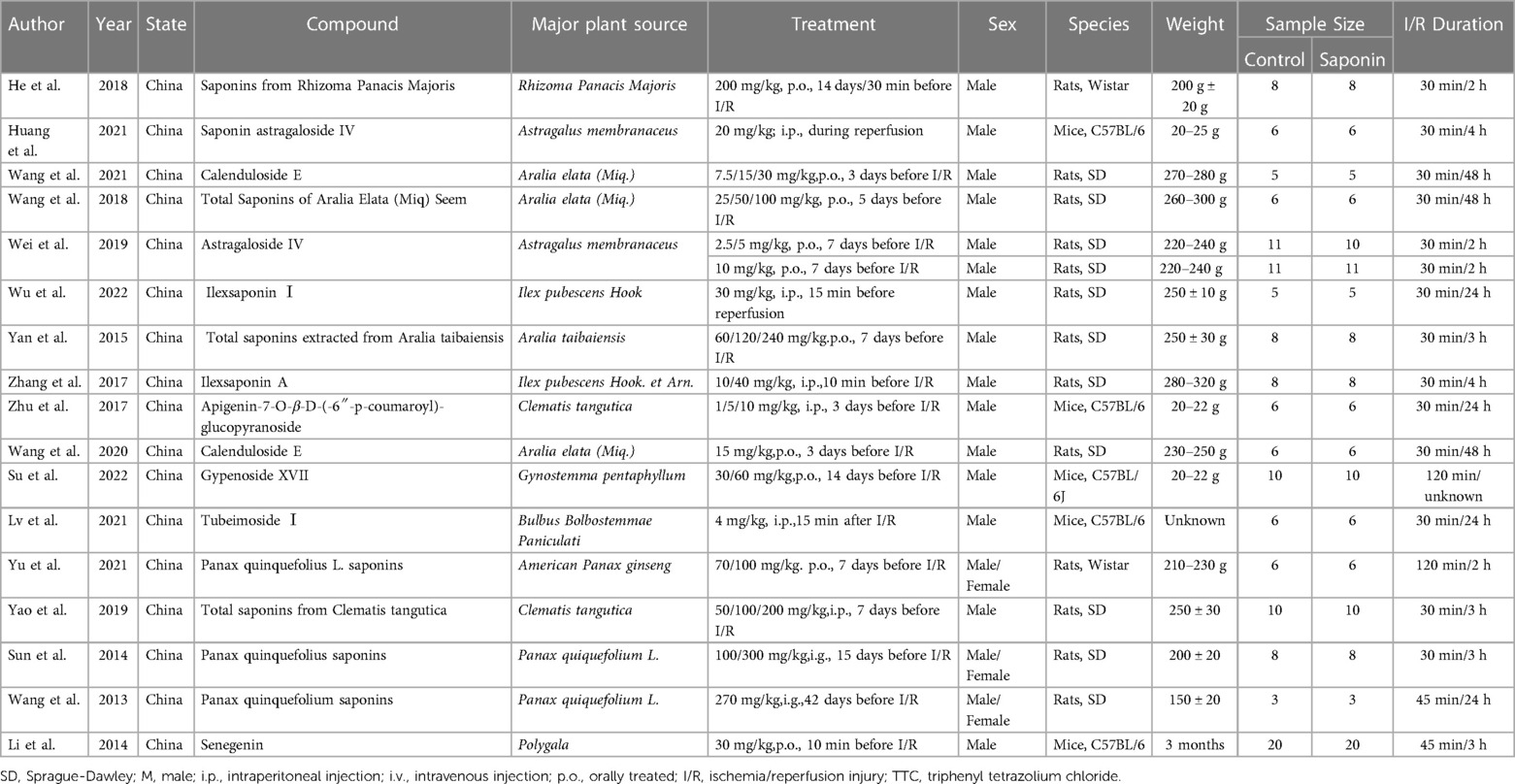
We obtained 300 related articles (PubMed: 64, EMBASE: 47, Web of Science: 55, Cochrane Library: 1, CNKI: 91, Sinomed: 42) by searching in databases, and 182 articles were retained after removing duplicate articles, among which 154 were excluded after the title and abstract screening, and 28 were eligible for full-text review. Five articles did not report infarct size as mentioned in the inclusion criteria, three lacked a placebo group, and three used saponin conversion products as an intervention. Finally, 17 studies of 386 animals (254 in the saponin treatment group and 132 in the control group) met the predetermined inclusion criteria and were eventually included in our meta-analysis (Figure 1) (4, 5, 10, 13, 17, 19–30). Table 1 shows comprehensive information for each study. All studies used mice (C57BL/6) and rats (Sprague-Dawley or Wistar) to investigate the cardioprotective effects of saponins. All studies measured infarct size by Evans blue/TTC double staining. Saponins were administered orally or intravenously, or intraperitoneally. In most studies, the administration began a few days before myocardial ischemia, and some studies were administered within half an hour before ischemia or during reperfusion. All 17 studies were conducted in China and were published between 2013 and 2022.
3.2. Quality assessment of studies
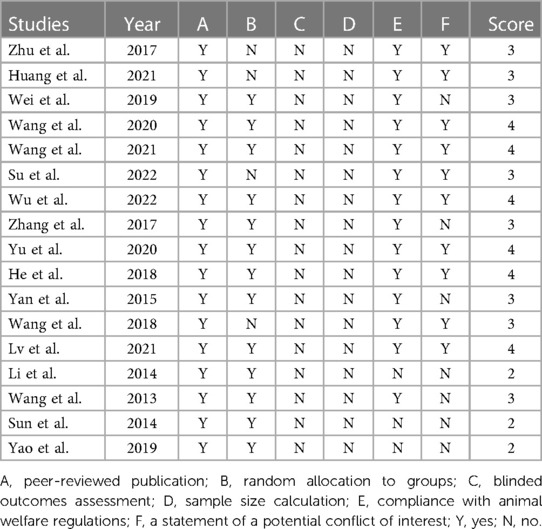
The quality of the included studies was evaluated in Table 2. Most studies (82.4%) were scored 3 to 4, indicating that the data were generally reliable and the risk of bias was acceptable. Three studies were judged as having a high risk of bias.
3.3. Infarct size
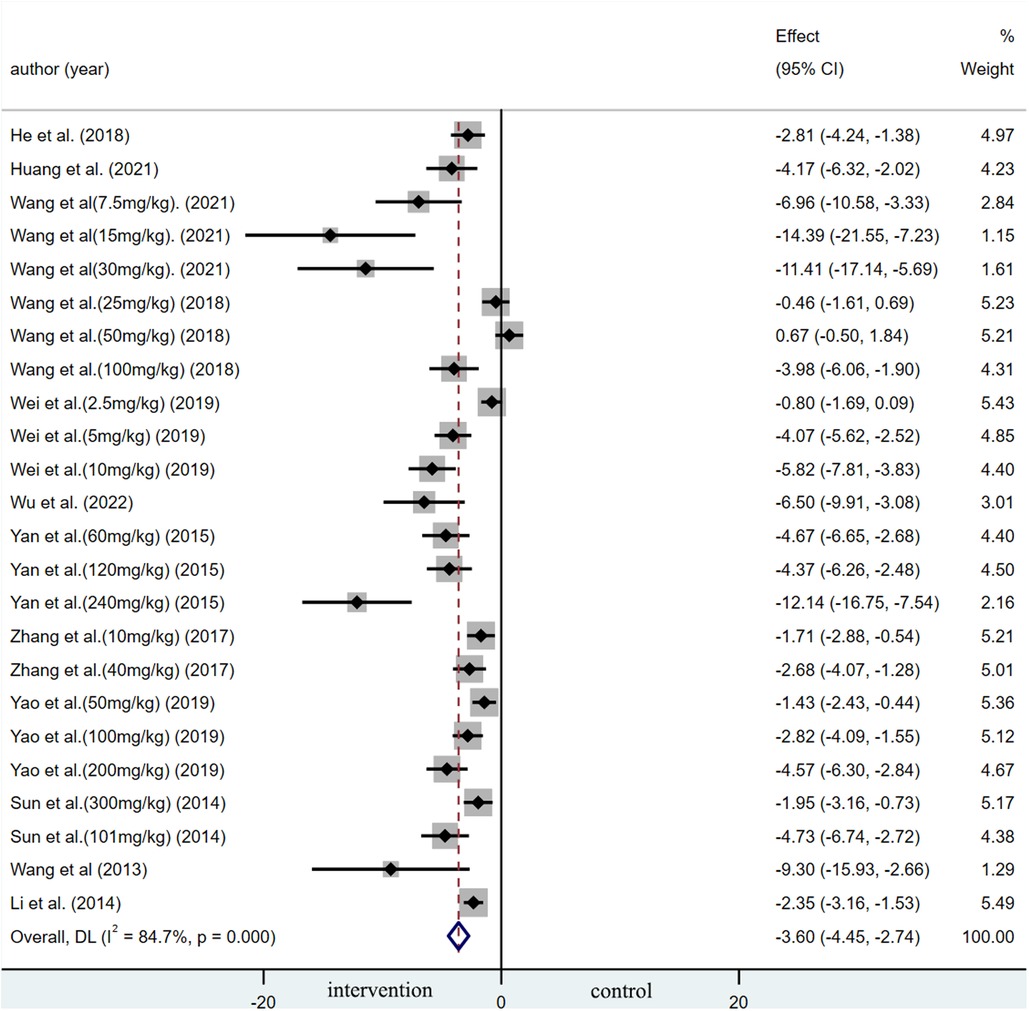
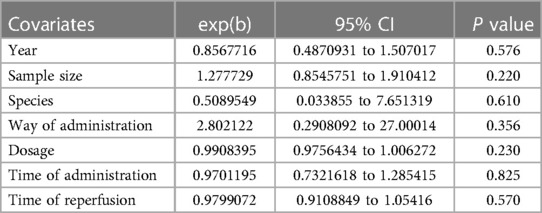
A summary analysis of 24 arms with 290 animals (intervention group = 192, control group = 98) showed the consistent effect of saponins on myocardial infarction size (Figure 2). Random effect model analysis showed that compared with placebo treatment, saponins significantly reduced infarct size (WMD: −3.60, 95% CI: −4.45 to−2.74, P < 0.01). There is a large amount of heterogeneity between studies (I2: 84.7%, P < 0.001). The funnel plot and Egger test (P < 0.001) showed the existence of publication bias (Figure 3). After introducing possibly unpublished studies through the trim and fill method, the results were steady (WMD: −2.81, 95% Cl: −3.69 to−1.93, P < 0.001), and the systematic deletion of each study did not affect the summary WMD and corresponding P values (Figure 4), which means that the beneficial effect of saponins in myocardial protection is stable. Subgroup analysis to explore the sources of heterogeneity between studies showed that sample size was a possible source of heterogeneity (Figure 5). Univariate regression failed to reveal any significant correlation between research covariables and effect sizes (Table 3).
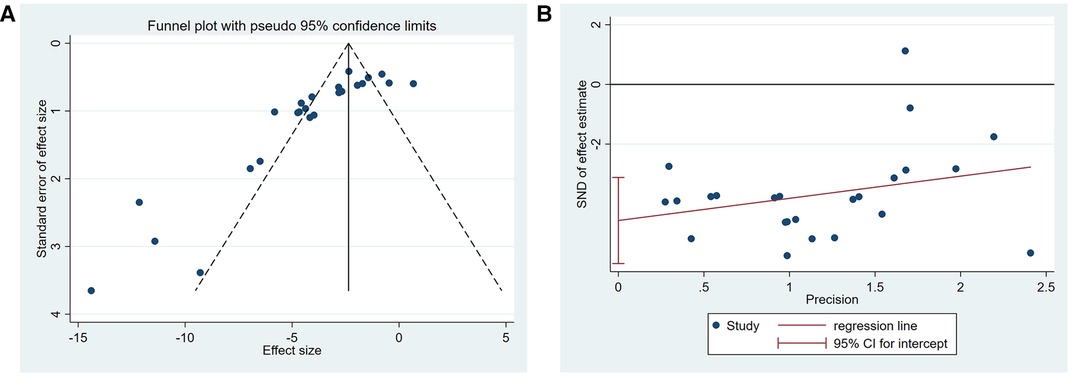
Figure 3. Publication bias among the studies reporting the infarct size. (A) Funnel plot, (B) Egger's test.
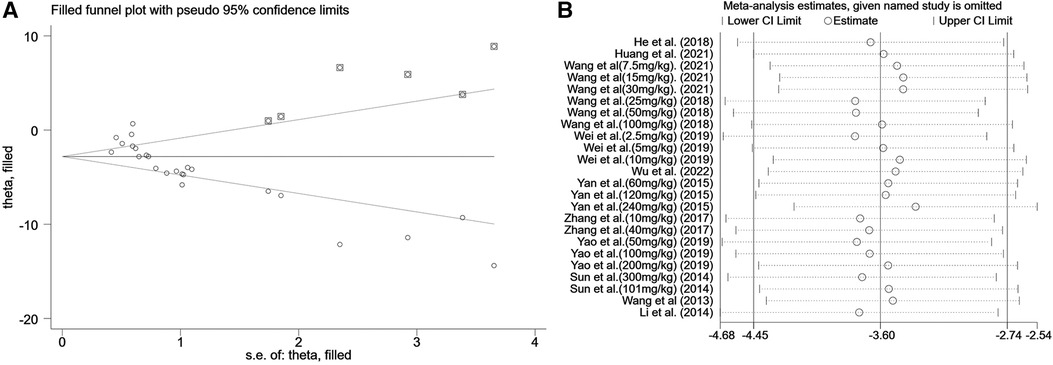
Figure 4. Sensitivity analysis of summary WMD of infarct size for saponins treatment vs. vehicle in myocardial I/R injury. (A) Filled funnel plot with pseudo 95% confidence limits. (B) Pooled effect sizes and 95% confidence limits after each study were omitted.
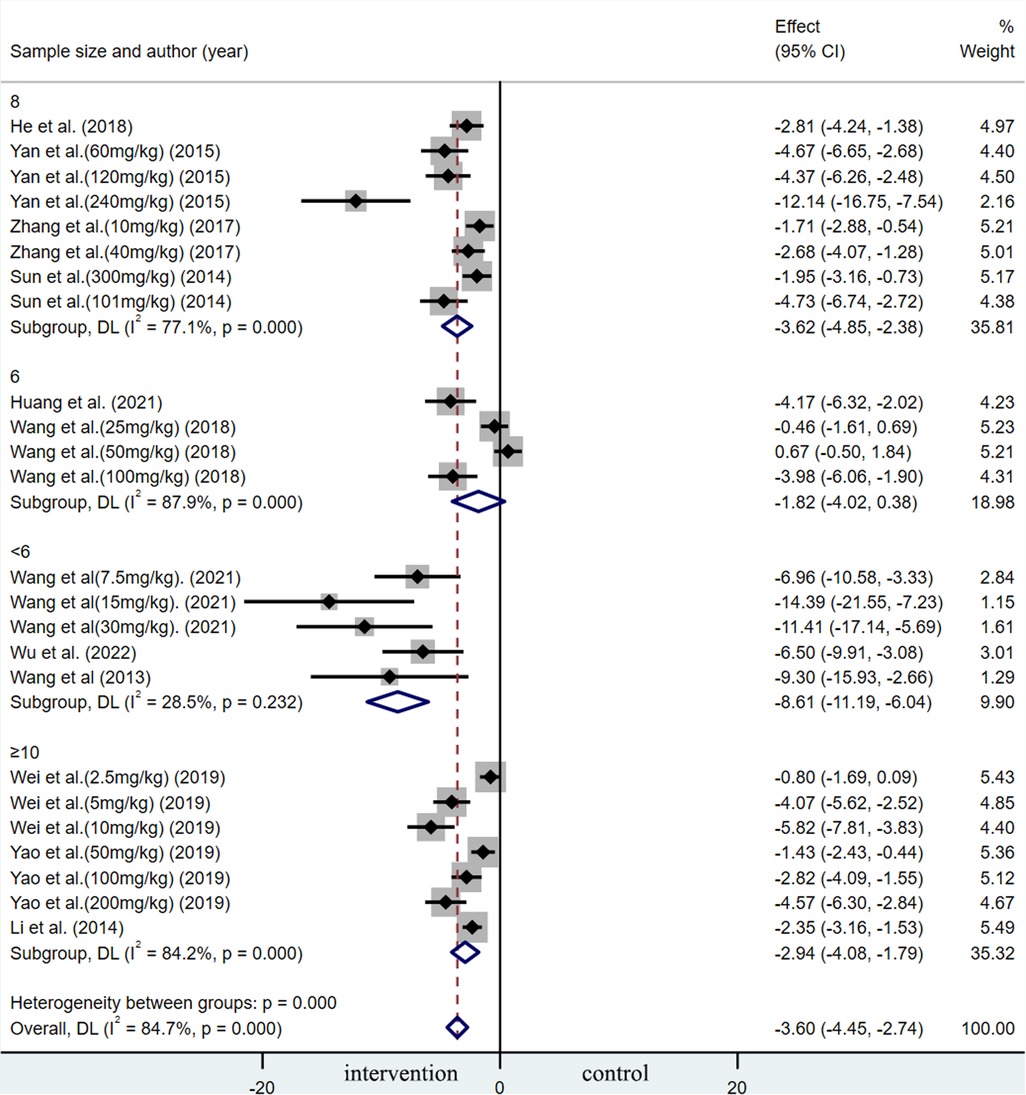
Figure 5. Subgroup analysis of summary WMD of infarct size for saponins treatment vs. vehicle in MIRI.
3.4. Cardiac function
3.4.1. Left ventricular ejection fraction percentage
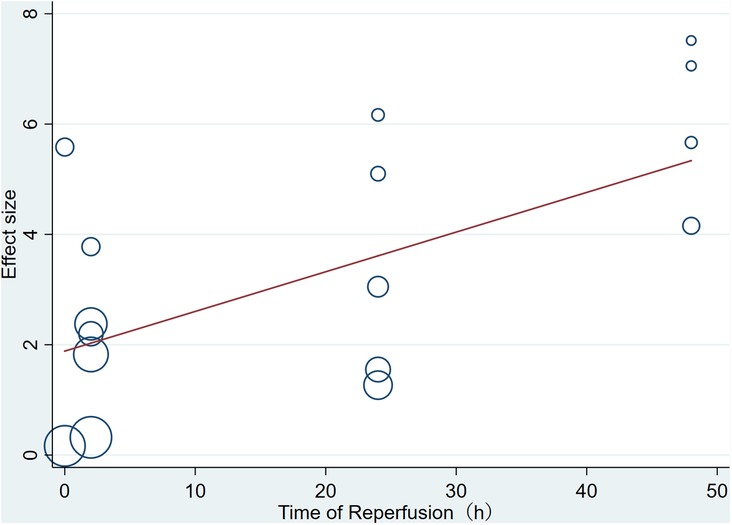
Pooled data from 16 arms involving 168 animals showed that saponin treatment significantly increased LVEF after myocardial I/R injury compared with placebo treatment (WMD: 3.119, 95% CI: 2.165 to 4.082, P < 0.01) (Figure 6). Heterogeneity was high (I2 = 82.9%, P < 0.01). The funnel plot and Egger (P < 0.001) test showed publication bias (Figure 7). Sensitivity analysis by trim and fill method showed that WMD: 1.548, 95% CI: 0.529 to 2.568, P = 0.003. The difference was statistically significant. Systematically deleting each study did not significantly affect WMD and P values, indicating that the beneficial effect of saponin treatment on LVEF is stable (Figure 8). Univariate regression showed that the effect size was significantly correlated with reperfusion time (Coefficient: 1.074572, 95% CI: 1.014757 to 1.137913, P = 0.017) (Figure 9), suggesting that the inter-study heterogeneity may be mainly due to the differences in the reperfusion time (Table 4).
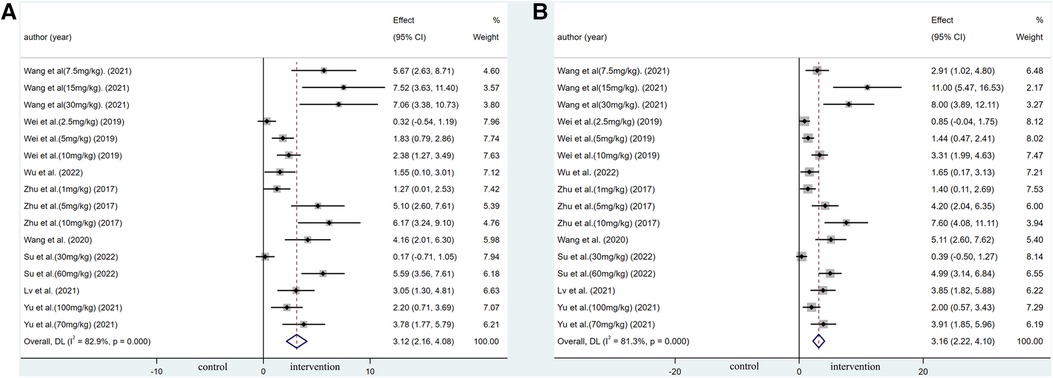
Figure 6. Forest plot of studies investigating the effects of saponins treatment on cardiac function vs. vehicle in myocardial I/R injury. (A) Summary WMD of LVEF. (B) Summary WMD of LVFS.

Figure 7. Assessment of publication bias among the studies reporting EF and FS. (A) Funnel plot of studies reporting EF. (B) Funnel plot of studies reporting FS.
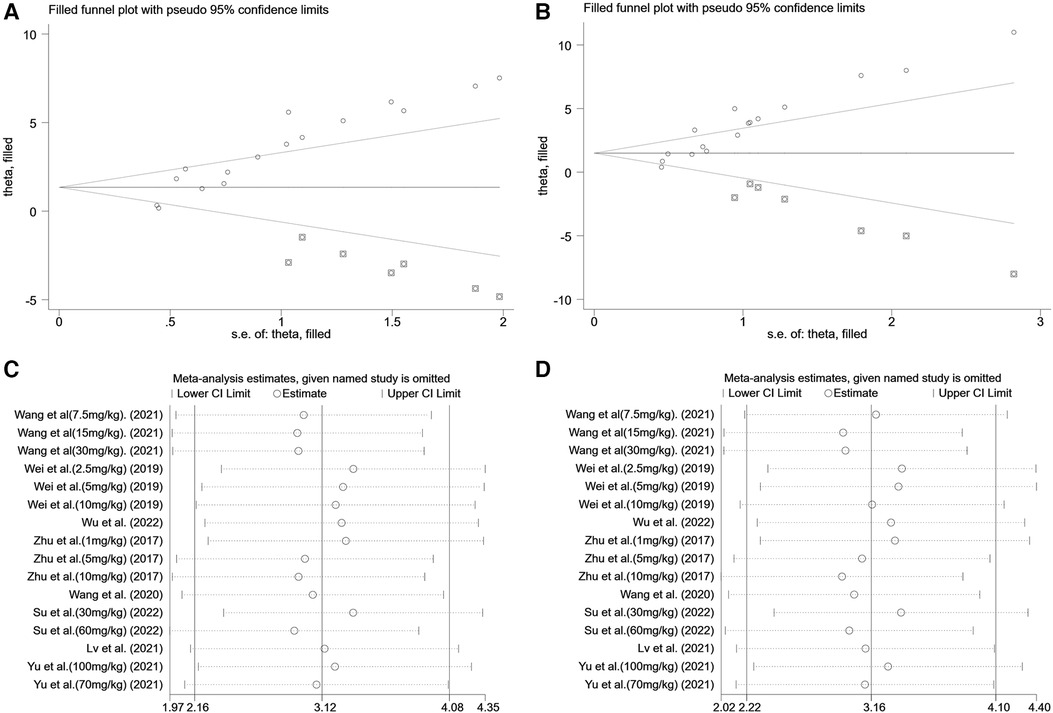
Figure 8. Sensitivity analysis of summary WMD of EF&FS for saponins treatment vs. vehicle in myocardial I/R injury. (A) Filled funnel plot with pseudo 95% confidence limits of EF. (B) Filled funnel plot with pseudo 95% confidence limits of FS. (C) Pooled effect sizes and 95% confidence limits of studies reporting EF after each study were omitted. (D) Pooled effect sizes and 95% confidence limits of studies reporting FS after each study were omitted.
3.4.2. Left ventricle fractional shortening percentage
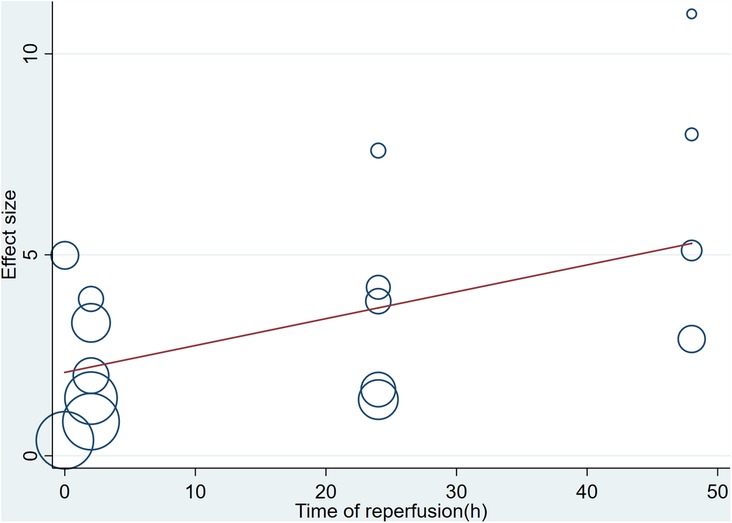
A meta-analysis of 16 arms involving 168 experimental animals showed that saponin administration significantly increased LVFS after myocardial I/R injury compared with placebo treatment (WMD: 3.157, 95% CI: 2.218 to 4.097, P < 0.001) (Figure 6) with high heterogeneity (I2 = 81.3%, P < 0.001). The funnel plot and Egger test (P < 0.001) showed publication bias (Figure 7). Sensitivity analysis by trim and fill method obtained no significant influence of publication bias on pooled effect size (WMD: 1.697, 95% CI: 0.698 to 2.695, P = 0.01). Systematically deleting the WMD and corresponding P values did not significantly affect the summary of each study. The results showed that the beneficial effect of saponin administration on FS was stable (Figure 8). Univariate regression showed that the effect size was significantly correlated with the reperfusion time (Coefficient: 1.069146, 95% CI: 1.002232 to 1.140527, P = 0.044) (Figure 10), suggesting differences in the reperfusion time may be the primary source of heterogeneity.
3.5. Myocardial enzyme
3.5.1. LDH (lactate dehydrogenase)
Pooled data from 20 arms involving 204 animals showed that saponin treatment significantly decreased LDH after myocardial I/R injury compared with placebo treatment (WMD: −4.035, 95% CI: −4.943 to −3.127, P < 0.01). Heterogeneity was high (I2 = 78.5%, P < 0.01). The funnel plot and Egger test (P < 0.001) showed publication bias. Sensitivity analysis by trim and fill method showed that WMD: −3.110, 95% CI: −4.029 to −2.190, P < 0.001. The result was steady. Systematically deleting each study did not significantly affect WMD and P values, indicating that the beneficial effect of saponin treatment on LDH is stable.
3.5.2. CK-MB (creatine kinase MB isoenzyme)
A meta-analysis of 13 arms involving 126 experimental animals showed that saponin administration significantly decreased CK-MB after myocardial I/R injury compared with placebo treatment (WMD: −4.261, 95% CI: −5.520 to −3.002, P < 0.001) with high heterogeneity (I2 = 79.5%, P < 0.001). The funnel plot and Egger test (P < 0.001) showed publication bias. Sensitivity analysis by trim and fill method obtained no significant influence of publication bias on pooled effect size (WMD: −3.495, 95% CI: −4.825 to −2.165, P < 0.001). Systematically deleting the WMD and corresponding P values did not significantly affect the summary of each study. The results showed that the beneficial effect of saponin administration on CK-MB was stable.
4. Discussion
So far, this is the first study to systematically collect and quantitatively analyze the protective effects of saponins extract from TCM on myocardial I/R injury. In this study, we systematically reviewed 17 articles involving 26 arms. The results showed that saponins significantly decreased the myocardial infarction size and increased the LVEF and LVFS after myocardial infarction compared with the control group, which proves the positive role of saponins in alleviating MIRI, protecting the myocardium, and promoting the recovery of cardiac function.
PCI and thrombolysis are still the most effective methods for treating ischemia in patients with acute myocardial infarction (31). However, evidence shows that I/R injury after blood flow recovery accounts for nearly half of the infarct area, ultimately aggravating ventricular dysfunction after myocardial infarction (19). Therefore, MIRI is an urgent clinical concern. In recent years, some treatments for myocardial ischemia-reperfusion injury have been partially proven effective, but these methods often have drawbacks that cannot be ignored. For example, one way to reduce reperfusion injury is to inflate/deflate the angioplasty balloon within 1 min after reperfusion. Small-scale trials have shown that infarct size is reduced in patients who receive this treatment. However, large-sample randomized clinical trials for STEMI (ST-segment elevation myocardial infarction) post-processing showed neutral results. And repeatedly inflating the balloon at the lesion site may lead to excessive production of thromboembolism. In recent years, there are also some adjuvant drug interventions for myocardial ischemia-reperfusion, most of which have been proved to be ineffective, but some drugs have shown certain efficacy. For example, cyclosporine-a, metoprolol, glucose modulators, and abciximab, among which cyclosporine and abciximab have well-known side effects, glucose modulators lack prospective clinical trial evidence, and β-blockers are also questionable in terms of safety in patients with low systolic blood pressure (<120 mm Hg) and Killip class III patients, limiting their application scenarios. In contrast, as an extract of traditional Chinese medicine, saponins have been widely used by clinicians in China since ancient times. So far, there are no obvious safety problems. This study also confirmed the effectiveness of saponins in reducing ischemia-reperfusion injury in animals. MIRI involves plenty of mechanisms (32). TCM has been used in China for thousands of years. Many of these drugs and therapies have been tested today and have proven superior efficacy (33). Recently, some studies found that saponins from TCM can protect against myocardial ischemia-reperfusion injury through various mechanisms (4, 34–36).
4.1. Necrosis
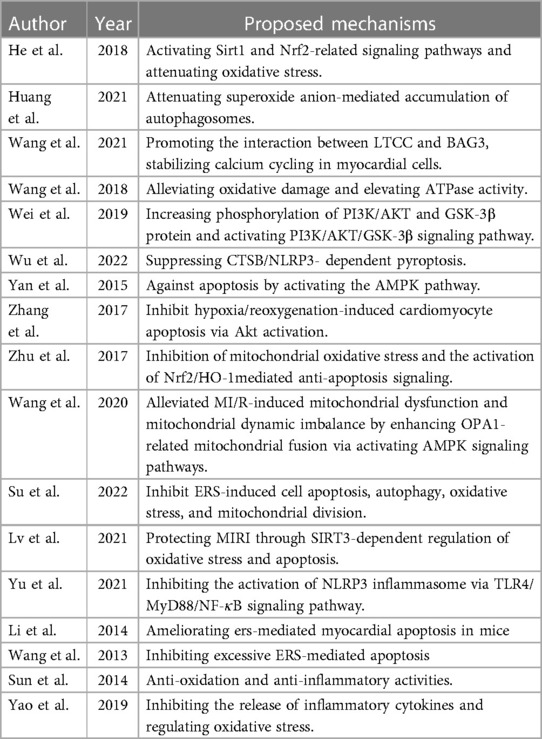
Necrosis is thought to be caused by chemical or physical damage. A decrease in ATP production can cause intracellular calcium overload during myocardial ischemia. During reperfusion, the restoration of energy supply under the premise of calcium overload-induced the oscillatory release and uptake of calcium by the sarcoplasmic reticulum, resulting in uncontrolled excessive muscle fiber contraction and damage to cellular structures. The study of Wang et al. showed that Calenduloside E(CE) could repress L-type calcium current induced by L-type calcium channels(LTCC) agonist and regulate calcium homeostasis (37).
4.2. Oxidative stress
After reperfusion, excessive reactive oxygen species (ROS) are produced, which may lead to the oxidation of nucleic acids, lipids, and proteins, further leading to changes in membrane damage, protein function, metabolic disorders, gene mutation, and resulting in oxidative stress (38–41). Studies have shown that Sirt1, Sirt3, and Nrf2 have the function of regulating oxidative stress. He et al. found that Saponins from Rhizoma Panacis Majoris (SPRM) protect against MIRI by activating Sirt1 and Nrf2-related signaling pathways and attenuating oxidative stress (10). Tubeimoside I (TBM) reduces the production of ROS by inhibiting NOX2. On the other hand, TBM increased antioxidant factors such as Nrf2 and NQO1 (26). Zhu et al. found that a new flavonoid glycoside (APG) increased SOD activity, decreased H2O2 and MDA, inhibited mitochondrial oxidative stress, and finally attenuated MIRI via activating PKCε signaling (19).
4.3. Inflammatory response
After MIRI, neutrophil adhesion and infiltration occur in the coronary artery (5, 42–44). The expression of adhesion molecules increases on the surface of microvascular endothelial cells and leukocytes (9, 45, 46), which promotes adhesion, aggregation, and chemotaxis of neutrophils and resists microcirculation, accelerating the process of MIRI (47). In recent years, the role of NLRP3 inflammatory agents in MIRI has become a research hotspot. NLRP3 can combine with Caspase-1 and Asc to form NLRP3 inflammasome, which requires the activation of NF-κB. Yu et al. found that the pretreatment of Panax notoginseng saponins (PQS) significantly down-regulated the expression of NLRP3, ASC, and caspase-1. This effect may be achieved by inhibiting the TLR4/MyD88/NF- κ B signal pathway (24).
4.4. Intracellular calcium overload
Na+-K+-ATPase is an essential system of cellular energy metabolism. Na+-K+-ATPase activity decreased significantly after MIRI (48). This situation increases intracellular Na+, and more Ca2+ enters the cell through Na+-Ca2+ exchange, resulting in calcium overload (49–51). Total saponins of A. Elata (aralosides, AS) can significantly increase the activity of Na+-K+-ATPase and Ca2+-Mg2+-ATPase (17). The study of Wang et al. showed that Calenduloside E(CE) could promote the interaction between LTCC and bcl2-related athanogene 3 (BAG3), reduce MIR-induced calcium overload and play a protective role in the heart (22).
4.5. Autophagy
Autophagy is a phenomenon in which cells use lysosomes to digest their organelles or some proteins, and it is the key to maintaining normal metabolism and renewal of some organelles. During myocardial ischemia, autophagy can provide critical nutrients for cell survival and inhibit cell apoptosis and necrosis by digesting non-functional proteins. O2•− is a potential mechanism of autophagy. Huang et al. found that Saponin astragaloside IV(ASIV) could increase SOD2 levels and reduce the accumulation of O2•− and autophagosome, alleviating I/R-induced cell death and protecting cardiomyocytes (13).
4.6. Programmed cell death
4.6.1. Apoptosis
Apoptosis is a form of cell death regulated by genes. Once apoptosis is initiated, the volume of apoptotic cells will shrink, the cytoplasm will condense, and finally, apoptotic bodies will be formed. Activation of caspases plays a crucial role in apoptosis. Total saponins extracted from Aralia taibaiensis(sAT) pretreatment significantly inhibited the release of Cyto-c from mitochondria in I/R injured cardiomyocytes, thereby inhibiting the activity and expression of caspase-3 (25). Apigenin-7-O-β-D-(-6″-p-coumaroyl)-glucopyranoside (APG) is a new flavonoid glycoside isolated from Clematis tangutica. Zhu et al. found that APG preconditioning activated Nrf2/HO-1 signaling, down-regulated Bax and cleaved caspase 3, upregulated Bcl2, and reduced the apoptosis rate of IR-damaged cardiomyocytes (19).
4.6.2. Pyroptosis
Pyroptosis is a new form of programmed cell death with an inflammatory response characterized by forming inflammatory bodies and activating caspase and gasdermin (52). Pyrocytosis mainly depends on NLRP3 or caspase-1 (53). Ilexsaponin I(ISI) is a triterpenoid saponin obtained from Ilex pubescens Hook. et Arn. Study by Wu et al. has shown that ISI can promote the formation of the CTSB/HSP70 complex to disturb CTSB/NLRP3 complex, thus inhibiting NLRP3-mediated cell pyrogenesis (23).
4.7. Saponins on multiple signalling pathways
In short, the pathogenesis of MIRI begins with calcium overload and oxidative stress caused by sudden changes in the environment (54). Calcium overload and oxidative stress interact with each other (55, 56), resulting in the destruction of cell structure and function (57, 58), leading to necrosis or apoptosis (1). The necrotic cells cause a different inflammatory response, stimulating other cells to start pyroptosis. Saponins can regulate calcium balance by improving membrane ion channel activity, reducing calcium overload, and preventing calcium overload-induced cell death and oxidative stress. At the same time, saponins reduce the production of reactive oxygen species, promote the removal of reactive oxygen species, reduce autophagy (59–61), apoptosis and inflammatory responses caused by oxidative stress, and reduce pyroptosis caused by inflammatory responses (6, 62–64). Therefore, traditional Chinese medicine saponins can act on multiple targets in the process of MIRI and protect MIRI through the interaction of many pathways.
5. Limitation
There are some limitations in our meta-analysis. First, the studies we included have differences in selecting experimental materials (65–68). The species, strain, age, body weight, and other characteristics of experimental animals may result in heterogeneity in absorption rate. Second, there is no standard scheme for the duration of ischemia/reperfusion in animal models and the administration regimen of saponins (68–72). Significant differences exist in the dosage and treatment scheme of saponins among the studies, which may result in a high heterogeneity and further affect the interpretation of the results. Through subgroup analysis, we found that sample size may be sources of heterogeneity among studies reporting infarct size. It is worth noting that among the study reporting LVEF and LVFS, we found a linear correlation between reperfusion time and the effect of meta-regression. That is, longer reperfusion time is more beneficial to the recovery of cardiac function after myocardial infarction, and that reperfusion time was the primary source of heterogeneity among studies reporting LVEF and LVFS. Funnel plots and Egger tests showed publication bias in this study. However, after adding potential unpublished studies through the trim and fill method, the results were still stable, indicating that publication bias does not affect the conclusions of this study. The sensitivity analysis data also confirmed the effectiveness and reliability of saponins in improving infarct size after reperfusion injury. The included studies reported the immediate efficacy of saponins in improving infarct size and inhibiting secondary cardiac dysfunction. However, one of the disadvantages of traditional Chinese medicine and its extracts is that their components are complex, and their metabolic kinetics in various species is not fully understood. Whether saponins extracted from TCM can maintain their cardioprotective effect for longer is unclear and needs further exploration. Finally, more extensive animal studies have not confirmed the beneficial effects of saponins extracted from TCM. Therefore, there is an urgent need for further research on large animals before human clinical trials.
6. Conclusion and perspectives
This systematic review demonstrated that saponins extracted from TCM protect MIRI by significantly reducing infarct size and improving cardiac function. It provides a theoretical basis for the clinical application of saponins extracted from TCM combined with immediate coronary artery revascularization in treating acute myocardial infarction. The study of saponins against MIRI is still in the initial stage. A systematic summary of the anti-MIRI mechanism of saponins could lay the foundation for studying anti-MIRI effects and structure-activity relationships of saponins, thus contributing to developing anti-MIRI drugs with new mechanisms or new targets. Therefore, studying saponins will play an essential role in developing anti-MIRI drugs. Further research on the intervention of traditional Chinese medicine saponins should consider the use of large animals and more standardized and strict experimental procedures to draw more reliable conclusions to promote its application.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JS, FY, and XS designed the manuscript. JS wrote the manuscript. JS, JF, FY, and XS searched databases and performed the selection of studies. XL and LT revised the manuscript. XL, LT, and CL critically evaluated and commented on the review. FY and XS contributed to the revised version. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81725024 and 81430098), the CACMS Innovation Fund (Grant No. CI2021A00919), and the National Key R&D Program of China (Grant Nos. 2018YFC1704901 and 2018YFC1704900).
Acknowledgments
We express our appreciation to all participants of this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fcvm.2023.1147740/full#supplementary-material
References
1. Wang R, Wang M, Zhou J, Wu D, Ye J, Sun G, et al. Saponins in Chinese herbal medicine exerts protection in myocardial ischemia-reperfusion injury: possible mechanism and target analysis. Front Pharmacol. (2020) 11:570867. doi: 10.3389/fphar.2020.570867
2. Jiang Y, Yu Q, Sui D, Yu X, Xu H, Li M. 20(S)-Protopanaxadiol Alleviates myocardial ischemia/reperfusion injury in rats through suppression of oxidative stress and apoptosis. Nat Prod Commun. (2021) 16:6. doi: 10.1177/1934578X211029528
3. Li D, Liu M, Tao T-Q, Song D-D, Liu X-H, Shi D-Z. Panax quinquefolium saponin attenuates cardiomyocyte apoptosis and opening of the mitochondrial permeability transition pore in a rat model of ischemia/reperfusion. Cell Physiol Biochem. (2014) 34:1413–26. doi: 10.1159/000366347
4. Su S, Wang J, Wang J, Yu R, Sun L, Zhang Y, et al. Cardioprotective effects of gypenoside XVII against ischemia/reperfusion injury: role of endoplasmic reticulum stress, autophagy, and mitochondrial fusion fission balance. Phytother Res. (2022) 36:2982–98. doi: 10.1002/ptr.7493
5. Zhang S, Liu Y, Wang F, Qiang J, Liu P, Zhang J, et al. Ilexsaponin A attenuates ischemia-reperfusion-induced myocardial injury through anti-apoptotic pathway. Plos One. (2017) 12(2):e0170984. doi: 10.1371/journal.pone.0170984
6. Zhan Y, Xu XH, Jiang YP. Protective effects of ginsenoside on myocardiac ischemic and reperfusion injuries. Zhonghua Yi Xue Za Zhi. (1994) 74:626–8. 648.7842343
7. Ning K, Jiang L, Hu T, Wang X, Liu A, Bao Y. ATP-Sensitive Potassium channels mediate the cardioprotective effect of Panax notoginseng saponins against myocardial ischaemia-reperfusion injury and inflammatory reaction. Biomed Res Int. (2020) 2020:3039184. doi: 10.1155/2020/3039184
8. Li M, Li X, Zhou L, Jin Y. Effects of total saponins from panacis majoris rhizoma and its degradation products on myocardial ischemia-reperfusion injury in rats. Biomed Pharmacother. (2020) 130:110538. doi: 10.1016/j.biopha.2020.110538
9. Wang L, Chen X, Wang Y, Zhao L, Zhao X, Wang Y. MiR-30c-5p mediates the effects of panax notoginseng saponins in myocardial ischemia reperfusion injury by inhibiting oxidative stress-induced cell damage. Biomed Pharmacother. (2020) 125:109963. doi: 10.1016/j.biopha.2020.109963
10. He H, Li X, Li D, Ding Y, Zhang Y, Xu H, et al. Saponins from rhizoma panacis majoris attenuate myocardial ischemia/reperfusion injury via the activation of the Sirt1/FoxO1/Pgc-1 alpha and Nrf2/antioxidant defense pathways in rats. Pharmacogn Mag. (2018) 14:297–307. doi: 10.4103/pm.pm_467_17
11. Kim T, Lee S. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem Toxicol. (2010) 48:1516–20. doi: 10.1016/j.fct.2010.03.018
12. Peng Y, Li S, Pei X, Hao K. The multivariate regression statistics strategy to investigate content-effect correlation of multiple components in traditional Chinese medicine based on a partial least squares method. Molecules. (2018) 23(3):545. doi: 10.3390/molecules23030545
13. Huang K, Yu Y, Liu S, Zhou Y, Wang J, Peng Y, et al. A single, acute astragaloside IV therapy protects cardiomyocyte through attenuating superoxide anion-mediated accumulation of autophagosomes in myocardial ischemia-reperfusion injury. Front Pharmacol. (2021) 12. doi: 10.3389/fphar.2021.642925
14. Yin B, Hou X, Lu M. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. (2019) 40:599–607. doi: 10.1038/s41401-018-0082-y
15. Korshunova AY, Blagonravov ML, Neborak EV, Syatkin SP, Sklifasovskaya AP, Semyatov SM, et al. BCL2-regulated Apoptotic process in myocardial ischemia-reperfusion injury (review). Int J Mol Med. (2021) 47. doi: 10.3892/ijmm.2020.4781
16. Li J, Bu Y, Li B, Zhang H, Guo J, Hu J, et al. Calenduloside E alleviates cerebral ischemia/reperfusion injury by preserving mitochondrial function. J Mol Histol. (2022) 53:713–27. doi: 10.1007/s10735-022-10087-5
17. Wang R, Yang M, Wang M, Liu X, Xu H, Xu X, et al. Total saponins of aralia elata (miq) seem alleviate calcium homeostasis imbalance and endoplasmic reticulum stress-related apoptosis induced by myocardial ischemia/reperfusion injury. Cell Physiol Biochem. (2018) 50:28–40. doi: 10.1159/000493954
18. Zeng X, Zhang Y, Kwong JSW, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
19. Zhu Y, Di S, Hu W, Feng Y, Zhou Q, Gong B, et al. A new flavonoid glycoside (APG) isolated from Clematis tangutica attenuates myocardial ischemia/reperfusion injury via activating PKCE signaling. Biochim Biophys Acta-Mol Basis Dis. (2017) 1863:701–11. doi: 10.1016/j.bbadis.2016.12.013
20. Wei D, Xu H, Gai X, Jiang Y. Astragaloside IV alleviates myocardial ischemia-reperfusion injury in rats through regulating PI3K/AKT/GSK-3β signaling pathways. Acta Cir Bras. (2019) 34:e201900708. doi: 10.1590/s0102-865020190070000008
21. Wang M, Wang R, Zhou J, Xie X, Sun G, Sun X. Calenduloside E ameliorates myocardial ischemia-reperfusion injury through regulation of AMPK and mitochondrial OPA1. Oxid Med Cell Longevity. (2020) 2020. doi: 10.1155/2020/2415269
22. Wang R, Wang M, Zhou J, Dai Z, Sun G, Sun X. Calenduloside E suppresses calcium overload by promoting the interaction between L-type calcium channels and Bcl2-associated athanogene 3 to alleviate myocardial ischemia/reperfusion injury. J Adv Res. (2021) 34:173–86. doi: 10.1016/j.jare.2020.10.005
23. Wu J, Chen S, Wu P, Wang Y, Qi X, Zhang R, et al. Cathepsin B/HSP70 complex induced by ilexsaponin I suppresses NLRP3 inflammasome activation in myocardial ischemia/reperfusion injury. Phytomedicine. (2022) 105. doi: 10.1016/j.phymed.2022.154358
24. Yu P, Li Y, Fu W, Li X, Liu Y, Wang Y, et al. Saponins protect myocardial ischemia reperfusion No-reflow through inhibiting the activation of NLRP3 inflammasome via TLR4/MyD88/NF-κB signaling pathway. Front Pharmacol. (2020) 11. doi: 10.3389/fphar.2020.607813
25. Yan J, Duan J, Wu X, Guo C, Yin Y, Zhu Y, et al. Total saponins from aralia taibaiensis protect against myocardial ischemia/reperfusion injury through AMPK pathway. Int J Mol Med. (2015) 36:1538–46. doi: 10.3892/ijmm.2015.2391
26. Lv D, Luo M, Cheng Z, Wang R, Yang X, Guo Y, et al. Tubeimoside I ameliorates myocardial ischemia-reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. Oxid Med Cell Longevity. (2021) 2021. doi: 10.1155/2021/5577019
27. Yao M, Mou F, Zhang W, Li W, Yin Y, Yang Z, et al. Protective effects of the total saponins extracted from Clematis Tangutica on myocardial ischemia/reperfusion injury. China Pharmacist. (2019) 22:2173–6.
28. Sun L, Xun P. Protective effects and mechanism of Panax quinquefolius saponins on myocardial ischemia of rats. Chin J Exp Tradit Med Formulae. (2014) 20:176–9. doi: 10.13422/j.cnki.syfjx.2014240176
29. Wang C, Liu M, Sun S, Song D, Liu X, Shi D. Panax quinquefolium saponins protect rat myocardium from ischemia/reperfusion injury through attenuating excessive endoplasmic reticulum stress. Chin J Pathophysiol. (2013) 29:20–7.
30. Li Z, Chen J, Lv J. Senegenin protects against ischemia-reperfusion injury by ameliorating endoplasmic reticulum stress in mice. Acta Med Univ Sci Technol Huazhong. (2014) 43:409–12.
31. Fu W, Xu H, Yu X, Lyu C, Tian Y, Guo M, et al. 20(S)-Ginsenoside Rg2 attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation: role of SIRT1. RSC Adv. (2018) 8:23947–62. doi: 10.1039/c8ra02316f
32. Xu X, Chen X, Ji H, Li P, Bian Y, Yang D, et al. Astragaloside IV improved intracellular calcium handling in hypoxia-reoxygenated cardiomyocytes via the sarcoplasmic reticulum Ca2+-ATPase. Pharmacology. (2008) 81:325–32. doi: 10.1159/000121335
33. Wang R, Wang M, Liu B, Xu H, Ye J, Sun X, et al. Calenduloside E protects against myocardial ischemia-reperfusion injury induced calcium overload by enhancing autophagy and inhibiting L-type Ca2 + channels through BAG3. Biomed Pharmacother. (2022) 145:112432. doi: 10.1016/j.biopha.2021.112432
34. Ge Z, Xu M, Huang Y, Zhang C, Lin J, Ruan C. Cardioprotective effect of notoginsenoside R1 in a rabbit lung remote ischemic postconditioning model via activation of the TGF-beta 1/TAK1 signaling pathway. Exp Ther Med. (2016) 11:2341–8. doi: 10.3892/etm.2016.3222
35. Cao Y, Li Q, Yang Y, Ke Z, Chen S, Li M, et al. Cardioprotective effect of stem-leaf saponins from panax notoginseng on mice with sleep derivation by inhibiting abnormal autophagy through PI3K/Akt/mTOR pathway. Front Cardiovasc Med. (2021) 8:694219. doi: 10.3389/fcvm.2021.694219
36. Yu Y, Sun G, Luo Y, Wang M, Chen R, Zhang J, et al. Cardioprotective effects of notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep. (2016) 6:21730. doi: 10.1038/srep21730
37. Yang Y, Yao Z, Wang G, Hou J, Liu X. Panax notoginseng saponins protects H9c2 cardiomyocytes against hypoxia/reoxygenation injury via activating the JAK2/STAT3 pathway. Int J Clin Exp Med. (2019) 12:8461–71.
38. Veselova O, Studneva I, Dobrokhotov I, Pal’keeva M, Molokoedov A, Sidorova M, et al. Exogenous galanin reduces hyperglycemia and myocardial metabolic disorders induced by streptozotocin in rats. Int J Pept Res Ther. (2022) 28:103. doi: 10.1007/s10989-022-10412-2
39. Aravinthan A, Kim J, Antonisamy P, Kang C, Choi J, Kim N, et al. Ginseng total saponin attenuates myocardial injury via anti-oxidative and anti-inflammatory properties. J Ginseng Res. (2015) 39:206–12. doi: 10.1016/j.jgr.2014.12.001
40. Liu X, Jiang Y, Yu X, Fu W, Zhang H, Sui D. Ginsenoside-Rb3 protects the myocardium from ischemia-reperfusion injury via the inhibition of apoptosis in rats. Exp Ther Med. (2014) 8:1751–6. doi: 10.3892/etm.2014.2007
41. Si J, Wang N, Wang H, Xie J, Yang J, Yi H, et al. HIF-1α signaling activation by post-ischemia treatment with astragaloside IV attenuates myocardial ischemia-reperfusion injury. PLoS One. (2014) 9:e107832. doi: 10.1371/journal.pone.0107832
42. He K, Yan L, Lin S-Q, Liu Y-Y, Hu B-H, Chang X, et al. Implication of IGF1R signaling in the protective effect of astragaloside IV on ischemia and reperfusion-induced cardiac microvascular endothelial hyperpermeability. Phytomedicine. (2022) 100:154045. doi: 10.1016/j.phymed.2022.154045
43. Wu Y, Cui H, Zhang Y, Yu P, Li Y, Wu D, et al. Inonotus obliquus extract alleviates myocardial ischemia/reperfusion injury by suppressing endoplasmic reticulum stress. Mol Med Rep. (2020) 23. doi: 10.3892/mmr.2020.11716
44. Yuan S, Jing H. Insights into the monomers and single drugs of Chinese herbal medicine on myocardial preservation. Afr J Tradit Complement Altern Med. (2011) 8:104–27. doi: 10.4314/ajtcam.v8i2.63195
45. Thu VT, Kim HK. Majonoside-R2 postconditioning protects cardiomyocytes against hypoxia/reoxygenation injury by attenuating the expression of HIF1α and activating RISK pathway. J Med Food. (2021) 24(11):1222–9. doi: 10.1089/jmf.2021.K.0083
46. Badalzadeh R, Yavari R, Chalabiani D. Mitochondrial ATP-sensitive K+ channels mediate the antioxidative influence of diosgenin on myocardial reperfusion injury in rat hearts. Gen Physiol Biophys. (2015) 34:323–9. doi: 10.4149/gpb_2015009
47. Nielsen J, Johnsen J, Pryds K, Ørtenblad N, Bøtker HE. Myocardial subcellular glycogen distribution and sarcoplasmic reticulum Ca2 + handling: effects of ischaemia, reperfusion and ischaemic preconditioning. J Muscle Res Cell Motil. (2021) 42:17–31. doi: 10.1007/s10974-019-09557-3
48. Chang X, Zhang T, Zhang W, Zhao Z, Sun J. Natural drugs as a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxidative Med Cell Longevity. (2020) 2020:5430407. doi: 10.1155/2020/5430407
49. Huang X, Wang Y, Wang Y, Yang L, Wang J, Gao Y. Ophiopogonin D reduces myocardial ischemia-reperfusion injury via upregulating CYP2J3/EETs in rats. Cell Physiol Biochem. (2018) 49:1646–58. doi: 10.1159/000493500
50. Liu X, Lu M, Zhong H, Wang L, Fu Y. Panax Notoginseng saponins attenuate myocardial ischemia-reperfusion injury through the HIF-1 alpha/BNIP3 pathway of autophagy. J Cardiovasc Pharmacol. (2019) 73:92–9. doi: 10.1097/FJC.0000000000000640
51. Liu X, Lu M, Zhong H, Liu J, Fu Y. Panax Notoginseng saponins protect H9c2 cells from hypoxia-reoxygenation injury through the forkhead box O3a hypoxia-inducible factor-1 alpha cell signaling pathway. J Cardiovasc Pharmacol. (2021) 78:e681–9. doi: 10.1097/FJC.0000000000001120
52. Zhaolin Z, Guohua L, Shiyuan W, Zuo W. Role of pyroptosis in cardiovascular disease. Cell Prolif. (2019) 52:e12563. doi: 10.1111/cpr.12563
53. Jia C, Chen H, Zhang J, Zhou K, Zhuge Y, Niu C, et al. Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol. (2019) 67:311–8. doi: 10.1016/j.intimp.2018.12.028
54. Ning N, Dang X, Bai C, Zhang C, Wang K. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol. (2012) 139:504–12. doi: 10.1016/j.jep.2011.11.040
55. Yu Y-H, Zhang P, Wang C-L, Liu J-G, Li P, Zhang D-W. Panax quinquefolium saponin optimizes energy homeostasis by modulating AMPK-activated metabolic pathways in hypoxia-reperfusion induced cardiomyocytes. Chin J Integr Med. (2021) 27:613–20. doi: 10.1007/s11655-020-3194-4
56. Guo M, Liu J, Guo F, Shi J, Wang C, Bible P, et al. Panax quinquefolium saponins attenuate myocardial dysfunction induced by chronic ischemia. Cell Physiol Biochem. (2018) 49:1277–88. doi: 10.1159/000493407
57. Wang J, Zeng L, Zhang Y, Qi W, Wang Z, Tian L, et al. Pharmacological properties, molecular mechanisms and therapeutic potential of ginsenoside Rg3 as an antioxidant and anti-inflammatory agent. Front Pharmacol. (2022) 13:975784. doi: 10.3389/fphar.2022.975784
58. Wang Y, Che J, Zhao H, Tang J, Shi G. Platycodin D inhibits oxidative stress and apoptosis in H9c2 cardiomyocytes following hypoxia/reoxygenation injury. Biochem Biophys Res Commun. (2018) 503:3219–24. doi: 10.1016/j.bbrc.2018.08.129
59. Yao H, Xie Q, He Q, Zeng L, Long J, Gong Y, et al. Pretreatment with panaxatriol saponin attenuates mitochondrial apoptosis and oxidative stress to facilitate treatment of myocardial ischemia-reperfusion injury via the regulation of Keap1/Nrf2 activity. Oxid Med Cell Longev. (2022) 2022:9626703. doi: 10.1155/2022/9626703
60. Sui DY, Qu SC, Yu XF, Chen YP, Ma XY. Protective effect of ASS on myocardial ischemia-reperfusion injury in rats. Zhongguo Zhong Yao Za Zhi. (2004) 29:71–4.15709388
61. Zhang S, Li H, Liang L, Wei Z-R, Yang S-J. Protective effect of GSTT preconditioning on myocardial ischemia-reperfusion injury in isolated rat heart. J Jilin Univ Med Ed. (2010) 36:229–32. doi: 10.13481/j.1671-587x.2010.02.012
62. Zhang Y-G, Song H-Y, Li Y, Jiao Y, Zhang S, Yang S-J. Protective effect of GSTT preconditioning on myocardial ischemia-reperfusion injury in rats. Chin Pharmacol Bull. (2010) 26:714–8.
63. Xia K-P, Cao H-M, Shao C-Z. Protective effect of notoginsenoside R1 in a rat model of myocardial ischemia reperfusion injury by regulation of vitamin D3 upregulated protein 1/NF-κB pathway. Pharmazie. (2015) 70:740–4. doi: 10.1691/ph.2015.5694
64. Wang M, Tian Y, Du Y-Y, Sun G-B, Xu X-D, Jiang H, et al. Protective effects of araloside C against myocardial ischaemia/reperfusion injury: potential involvement of heat shock protein 90. J Cell Mol Med. (2017) 21:1870–80. doi: 10.1111/jcmm.13107
65. Li H, Feng C-X, Guan F-Y, Yang S-J. Protective effects of gross saponins Tribulus Terrestris on myocardial ischemia-reperfusion injury in rats. J Jilin Univ Med Ed. (2009) 35:794–7. doi: 10.13481/j.1671-587x.2009.05.017
66. Lu H, Wang L-Y, Wang Q-J. Protective effects of panaxtriol saponin on myocardial ischemia-reperfusion injury in rats. Chin Trad Herbal Drugs. (2016) 47:275–80. doi: 10.7501/j.issn.0253-2670.2016.02.016
67. Wang S, Ye L, Wang L. Protective mechanism of shenmai on myocardial ischemia-reperfusion through the energy metabolism pathway. Am J Transl Res. (2019) 11:4046–62.31396317
68. Zhao X, Zhang F, Wang Y. Proteomic analysis reveals Xuesaitong injection attenuates myocardial ischemia/reperfusion injury by elevating pyruvate dehydrogenase-mediated aerobic metabolism. Mol Biosyst. (2017) 13:1504–11. doi: 10.1039/c7mb00140a
69. Xu H, Wang D, Peng C, Huang X, Ou M, Wang N, et al. Rabbit Sera containing compound danshen dripping pill attenuate leukocytes adhesion to TNF-alpha-activated human umbilical vein endothelial cells by suppressing endothelial ICAM-1 and VCAM-1 expression through NF-KappaB signaling pathway. J Cardiovasc Pharmacol. (2014) 63:323–32. doi: 10.1097/FJC.0000000000000046
70. Prem PN, Sivakumar B, Boovarahan SR, Kurian GA. Recent advances in potential of fisetin in the management of myocardial ischemia-reperfusion injury–A systematic review. Phytomedicine. (2022) 101:154123. doi: 10.1016/j.phymed.2022.154123
71. Chang X, Zhang W, Zhao Z, Ma C, Zhang T, Meng Q, et al. Regulation of mitochondrial quality control by natural drugs in the treatment of cardiovascular diseases: potential and advantages. Front Cell Dev Biol. (2020) 8. doi: 10.3389/fcell.2020.616139
Keywords: meta-analysis, saponins, traditional Chinese medicine, myocardial ischemia-reperfusion injury, experimental animal model, infarct size, cardiac function
Citation: Sun J, Fan J, Yang F, Su X, Li X, Tian L, Liu C and Xing Y (2023) Effect and possible mechanisms of saponins in Chinese herbal medicine exerts for the treatment of myocardial ischemia-reperfusion injury in experimental animal: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1147740. doi: 10.3389/fcvm.2023.1147740
Received: 19 January 2023; Accepted: 7 July 2023;
Published: 26 July 2023.
Edited by:
Rong-Rong He, Jinan University, ChinaReviewed by:
Shichao Lv, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, ChinaShaonan Liu, Guangzhou University of Chinese Medicine, China
Long Ge, Lanzhou University, China
© 2023 Sun, Fan, Yang, Su, Li, Tian, Liu and Xing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanwei Xing eGluZ3lhbndlaTEyMzQ1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Jiahao Sun
Jiahao Sun Jiarong Fan
Jiarong Fan Fan Yang
Fan Yang Xin Su
Xin Su Xinye Li
Xinye Li Li Tian2
Li Tian2 Can Liu
Can Liu Yanwei Xing
Yanwei Xing