- 1Department of Cardiothoracic Surgery, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, China
- 3National Clinical Research Center for Child Health and Disorders, Chongqing, China
- 4China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Chongqing, China
- 5Chongqing Key Laboratory of Pediatrics, Chongqing, China
- 6Department of Cardiology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 7Department of Radiology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 8Intelligence Medical of Science and Technology Commission of Chongqing, Chongqing, China
Background: Coarctation of the aorta (CoA), is a congenital malformation, often combined with several cardiac abnormalities. At present, the operation effect is satisfactory, but postoperative restenosis is still a matter. Identification of risk factors for restenosis and prompt therapy adjustments may improve patient outcomes.
Materials and methods: A retrospective clinical study of patients under 12 who had CoA repair in 2012–2021, with a randomized cohort population of 475 patients.
Results: A total of 51 patients (M/F: 30/21) with a mean age of 5.33 (2.00–15.00) months and a median weight of 5.60 (4.20–10.00) kg. The mean follow-up was 8.93 (3.77–19.37) months. Patients were divided into 2 groups: no-restenosis (n-reCoA) (G1, 38 patients) and restenosis (reCoA) (G2, 13 patients). ReCoA was defined as a restenosis requiring interventional or surgery or a pressure gradient >20 mmHg at the repair site as reported by B-ultrasound with the presence of an upper and lower limb blood pressure gradient or growing dysplasia. The overall reCoA incidence was 25% (13/51). In multivariate COX regression, smaller preoperative z-score of the ascending aorta (P = 0.009, HR = 0.68) and transverse aortic arch (P = 0.015, HR = 0.66), arm-leg systolic pressure gradient ≥12.5 mmHg at discharge (P = 0.003, HR = 1.09) were independent risk factors for reCoA.
Conclusion: The overall outcome of CoA surgery is successful. Smaller preoperative z-score of the ascending aorta and transverse aortic arch, and an arm-leg systolic pressure gradient ≥12.5 mmHg at discharge increase reCoA risk, and closer follow-up for such patients are required especially within 1 postoperative year.
Introduction
Coarctation of the aorta (CoA), a congenital abnormality, often occurs in the arterial catheter or arterial ligament region, which has an incidence rate of about 0.287‰ and accounts for 3.57% of all congenital cardiovascular malformations (1). It can be isolated or with additional cardiac abnormalities such as ventricular septal defect and patent ductus arteriosus (2). End-to-end anastomosis (EEA) was first proposed by Crafoord and Nylin in 1945 (3), but with a significant rate of restenosis (reCoA) (4–6). People have used a variety of methods to reduce postoperative mortality and restenosis rate to improve patient survival time and quality, including patch aortoplasty (PAP), subclavian artery valvuloplasty, expanded end-to-end anastomosis (EEEA), end-to-side anastomosis (ESA), and balloon angioplasty. Advancements in science and technology have dramatically reduced mortality (7), therefore reCoA has attracted more focus. Several studies have shown contradictory outcomes on age and weight at the surgical procedure and other variables (8–12). This research aimed to collect data and examine risk factors of reCoA to guide clinical practice.
Materials and methods
Study population
This is a retrospective study of patients who underwent CoA surgical procedures at the Children's Hospital of Chongqing Medical University between 2012 and 2021. Patients were eligible if they met the following criteria: (1) age ≤12 years old, (2) had preoperative echocardiography and cardiac CT scan and three-dimensional reconstruction, (3) CoA is the main diagnosis, and (4) successful follow-up after discharge. Evaluate available data (all obtained by gathering clinical records, surgery reports, and discharge records) to detect reCoA risk factors. A total cohort population of 51 people was enrolled randomly from 475 patients. Demographic data (such as gender, age, and weight at the time of surgical procedure), perioperative data [such as whether cardiotonic agents were used before surgery, operation procedures, time of cardiopulmonary bypass (CPB), and so on], and follow-up data were gathered. The surgical treatment was considered a success if patients did not die after the operation, the Doppler pressure gradient across the repair site <20 mmHg during the follow-up, and the blood pressure of the upper limb was lower than that of the lower limb, and no evidence of hypertension. The hypertension diagnostic criteria were based on the China Guidelines for Prevention and Treatment of Hypertension, the systolic blood pressure (mmHg) for boys over 100 + 2× age (years), and girls over 100 + 1.5× age (years), or taking antihypertensive agents. When the z-score of the transverse arch < −2, hypoplastic aortic arch (HAA) can be diagnosed. The pressure gradient obtained by echocardiography varies depending on the location of the constriction. Use echocardiography to determine the flow rate at the constriction, and the pressure was estimated using the simplified Bernoulli equation P = 4V2. The Children's Hospital of Chongqing Medical University's Ethics Committee authorized the study, and all patients signed the informed consent form.
Surgical technique
The suitable surgical technique was chosen based on the patient's clinical state, including combined intracardiac malformations, preoperative cuff blood pressure, medical imaging data such as ultrasonic cardiogram and CT scan, and requests of their parents. A median sternotomy method is chosen for patients with intracardiac abnormalities that require simultaneous repair and CPB. A lateral thoracotomy method is chosen for patients with intracardiac abnormalities that can be treated minimally invasively without CPB. EEEA, ESA, and PAP are the three main categories of surgical techniques. The anastomotic strain encountered throughout the procedure determines the use of certain surgical techniques. In general, patients with simple isthmus aortic coarctation are treated with EEEA, whereas those with hypoplastic aortic arch may be treated with ESA and PAP. It should be highlighted that SAR was chosen by two patients. After performing SAR on the two patients during the operation, the surgeon noticed that the invasive systolic blood pressure difference between the brachial artery and the femoral artery had returned to normal (upper limb < lower limb). The surgeon then immediately informed the patients' families of this finding and they decided to change the preoperatively decided surgical plan and adopt SAR.
Statistical methods
Kolmogorov-Smirnov test examines the normality of continuous variables. Normal distribution variables were shown as mean ± standard deviation (SD), compared by Student's t-test or ANOVA. The skewed distribution variables were shown as medians (P25, P75), compared by the Wilcoxon signed-rank test. Chi-square test, corrected Chi-square test, or Fisher exact test compared rates or component ratios. Echocardiographic data and demographics were potential predictors. Only variables (P < 0.2) with univariate COX regression were included in multivariable COX regression operated by stepwise regression. ROC curve was used to determine the best cutoff (Jordan index-based) with the maximum sensitivity and specificity for a critical continuous risk factor. AUC measured accuracy. ReCoA-free survival rate was conducted by Kaplan–Meier curve. The log-rank test compared patient subgroups' differences in freedom from event (reCoA vs. n-reCoA) rates. The statistically significant difference was defined as P < 0.05, and all statistical analyses were performed using SPSS26.0 software.
Results
The study cohort included 51 patients (30 males, 21 females) with a 25% (13/51) incidence of reCoA. The median weight was 5.60 kg (interquartile range, 4.20–10.00 kg) and the mean age was 5.33 months (interquartile range, 2.00–15.00 months). The mean follow-up was 8.93 months (interquartile range, 3.77–19.37 months). Patients were divided into two groups: n-reCoA (G1, 38 patients) and reCoA (G2, 13 patients). Table 1 compares demographics and surgical variables. Concomitant hypoplastic aortic arch appeared to be a reCoA risk factor with 10 patients (26%) in G1 and 8 patients (62%) in G2, respectively (P = 0.050). As for gender, 10 males and 3 females had reCoA (P = 0.125). And 6 of 26 VSD patients (23%) and 7 of 25 non-VSD patients (28%) had ReCoA (P = 0.687). Surgical age shows no significant difference in <1 month compared with the other two groups (P = 0.751). Variables including surgical weight and CPB time did not statistically differ (P > 0.05).
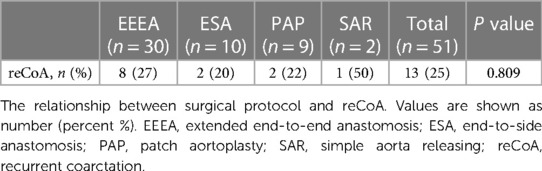
Table 2 shows the relationship between surgical protocol and reCoA. The six surgical regimens were statistically comparable (P > 0.05) (Figure 1A). We may hypothesize that selecting the optimal surgical strategy for each case is acceptable since reCoA rates after surgery are not significantly different. Meantime, in certain patients, simple aorta releasing (SAR) may achieve the therapeutic goal (1/2, 50%), highlighting the importance of fluid dynamics even with Artificial Intelligence technology in CoA diagnosis and therapy (Figure 2).
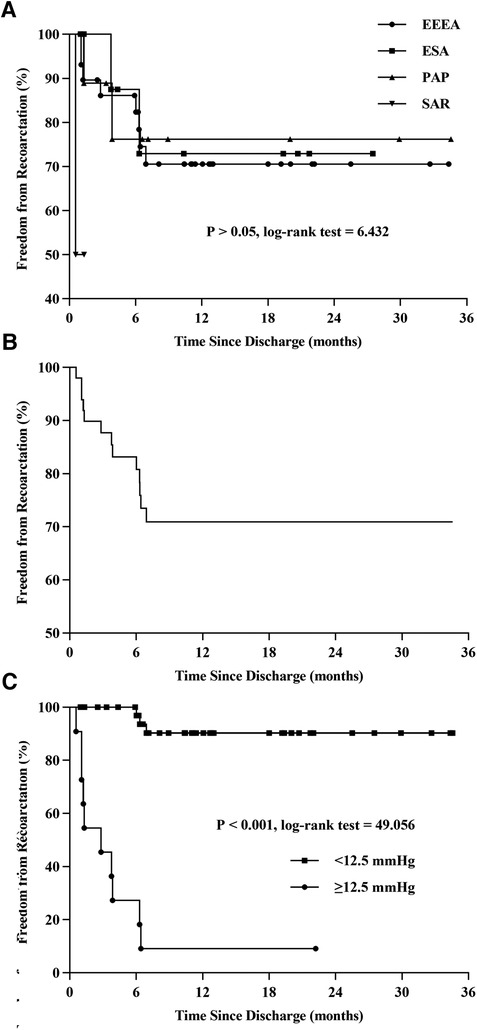
Figure 1. Kaplan-Meier curve. Compare the differences of reCoA among the six surgical options (A). Freedom from reCoA after discharge (B). Patients with a blood pressure gradient ≥12.5 mmHg at discharge have a significantly higher rate of reCoA (C). EEEA, extended end-to-end anastomosis; ESA, end-to-side anastomosis; PAP, patch aortoplasty; SAR, simple aorta releasing; reCoA, recurrent coarctation.
Figure 3 compares data for different time nodes of G1 and G2. Preoperatively, the mean gradient of G1 was slightly higher than that of G2 (29.45 ± 2.87 vs. 27.46 ± 4.81, P = 0.727). Although the mean gradient of G1 was almost identical to that of G2 one day after surgery (8.50 ± 2.49 vs. 7.54 ± 1.30, P = 0.733), it was significantly higher in G2 than G1 three days after surgery (10.46 ± 2.06 vs. 3.37 ± 1.45, P = 0.013) and at discharge (18, 11.5–21 vs. 1.79 ± 1.12, P < 0.001). The gradient of G2 at discharge was about 9 times that of G1 and gradually increased during the period (1 d after surgery, 3 d after surgery, and at discharge), while the gradient of G1 at discharge was lower than that during hospitalization (Figure 3A).

Figure 3. Changes in patient data from postoperative to discharge. Systolic blood pressure gradient (arm-leg) (A). Doppler peak flow velocity across the repair site (B). The Doppler pressure gradient across the repair site (C). Diameter of constriction (D). *Statistical significance.
The mean peak Doppler flow velocity at the repair site: G1 was significantly lower than G2 at discharge (2.40 ± 0.09 vs. 2.83 ± 0.14, P = 0.019) while almost similar preoperatively (3.37 ± 0.14 vs. 3.41 ± 0.17, P = 0.863). Both groups had significantly lower velocity after the operation (G1: P < 0.001; G2: P = 0.006) (Figure 3B).
G1 had a significantly lower mean pressure gradient at the repair site than G2 at discharge (20.63, 16.24–30.30 vs. 32.85 ± 3.14, P = 0.009), which was almost comparable to G2 before surgery (43.50, 32.00–62.25 vs. 48.07 ± 4.52, P = 0.673). Gradient made a significant reduction in both groups (G1: P < 0.001; G2: P = 0.009) (Figure 3C).
The average diameters of repair site in G1 and G2 were almost identical before and after surgery (Preoperative: G1: 0.36 ± 0.02, G2: 0.38 ± 0.03, P = 0.539; Postoperative: G1: 0.60 ± 0.03, G2: 0.58 ± 0.03, P = 0.732). The diameter increased significantly in both groups at discharge (G1: P < 0.001; G2: P = 0.001) (Figure 3D).
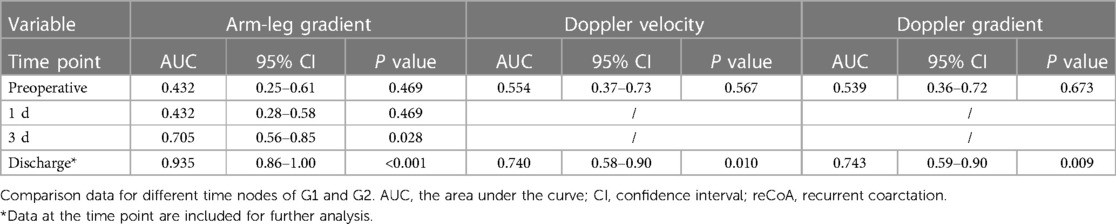
Use ROC curve to determine which time point between pre-surgery and discharge most affected reCoA and was then included in COX regression. The arm-leg systolic blood pressure gradient, Doppler peak blood flow velocity, and pressure gradient across the repair site (at discharge) were all well affected (Table 3).
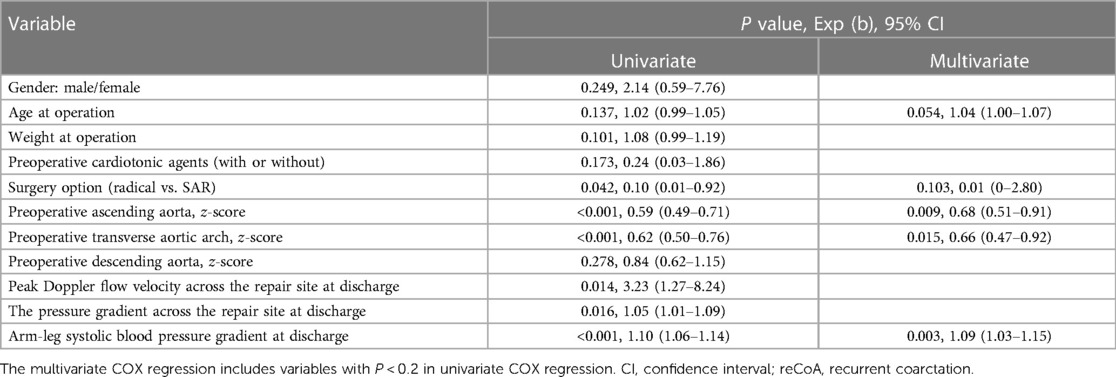
The multivariate COX regression includes variables with P < 0.2 in univariate COX regression (Table 4). Among the variable examined, the smaller preoperative z-score of the ascending aorta, the smaller preoperative z-score of the transverse aortic arch, and the higher arm-leg systolic blood pressure gradient at discharge were independent risk factors for reCoA (P < 0.05).
The Kaplan-Meier curve predicted freedom from reCoA was 70.9% after an average follow-up of 8.93 months (interquartile range, 3.77–19.37 months) (Figure 1B).
The median time from discharge to reCoA was 3.77 months (interquartile range, 1.15–6.32 months). All 13 patients had reCoA within one year, 1 had balloon angioplasty one year after discharge, and 1 is taking antihypertensive agents now, the other 11 patients are currently under close follow-up at the discretion of their parents.
The optimum cutoff for arm-leg systolic pressure gradient at discharge, based on the ROC curve and AUC area, was 12.5 mmHg, with a sensitivity of 76.9% (G2: 10/13) and specificity of 97.4% (G1: 1/38). Thus, patients with a gradient of 12.5 mmHg or higher at discharge had a significantly increased risk of reCoA during follow-up (Figure 1C, log-rank = 49.06, P < 0.001).
Discussion
Coarctation of the aorta (CoA), the sixth most common CHD (13), may occur anywhere in the aorta, mainly distal to the left subclavian artery (14, 15). Morgagni reported CoA in 1760. It could be isolated or related to long-segment stenosis or hypoplastic aortic arch (16). Various CoA treatments now exist, including balloon angioplasty reported by Singer in the 1980s (17). With the decline in surgical mortality, reCoA has received increasing attention with a rate of 5.9–46.6% (18). ReCoA risk factor analysis provides conflicting findings. Young age, low weight, hypoplastic aortic arch, and pressure gradient may increase risk (8–11, 19). In this study, 13 of 51 children developed reCoA within a year, and 1 got balloon angioplasty with satisfactory results. The smaller preoperative z-score of the ascending aorta, the smaller preoperative z-score of the transverse aortic arch, and the arm-leg systolic blood pressure gradient ≥12.5 mmHg at discharge have an increased risk of reCoA in our patients.
Our research found no significant difference between gender and reCoA (P = 0.249), which consists of multiple studies (9, 20, 21). In a study of 167 patients by Burch (10), female showed significant difference (P = 0.04, HR = 2.77). 11 females had reCoA (1 Turner's syndrome), which they considered difficult to explain. It has been reported that 7%–12% of Turner syndrome girls in childhood have COA (22). Gene loss on the short arm of the X chromosome may cause isolated CoA (23).
Age and weight at surgery did not increase the risk of reCoA. This finding matched Adamson's study (age, P = 0.73) (19). Bacha showed that surgical weight <1.5 kg increased the risk of reCoA (24). It has previously been argued that delaying surgery properly may lower the risk in well-controlled patients. That may be because the aortic arch and constrictions expand steadily as the patient ages, making it easier to detect and remove aberrant areas during operation. With the improved surgical ability and perioperative control, we believe age and weight have lessened their effect on reCoA.
PGE1 has been proven to keep arterial ducts even constrictions open to sustain life. Liberman found ectopic ductal-like tissue in the aorta may induce CoA (25). PGE1 relieves blockage even arterial catheter is closed. Ajay suggested that high-dose PGE1 may treat serious patients with CoA who were unsuccessful in the standard dose (26). However, Burch found that 14 of 105 PGE1-treated patients had reCoA, compared to 1 of 41 non-PGE1-treated patients (P = 0.07) (10). We did not use PGE1 preoperatively, thus this factor has not been studied in this study and may be explored in the future. Similar to our findings, reCoA has not been connected to cardiac abnormalities (8, 9, 19, 27).
We believed preoperative cardiotonic agents do not increase the risk of reCoA. But Truong found that did (P = 0.04, HR = 5.57), while multivariate analyses did not (12). We hypothesized that probably because most young PGE1 recipients were treated with cardiotonic agents preoperatively.
Many studies have examined how surgery option affects reCoA. Several procedures have been developed to reduce risk. However, the optimal one is still debated (28, 29). Crafoord proposed EEA in 1944 (3), despite a decreased mortality (6), the reCoA rate was high (4–6), probably because of incomplete resection of the catheter tissue, which partially grow into the normal-appearing aorta wall, lack of growth at the circular anastomosis and the hypoplastic aortic arch. Thus, patch aortoplasty has gradually replaced EEA (30). Patches materials include artificial materials, allogeneic blood vessels, autologous pulmonary artery, or pericardium. The anastomotic stoma is tension-free, collateral vessels do not need to be ligated or disconnected, and the hypoplastic region of the arch can be extended at the same time. But Adamson linked patch material to reCoA (P = 0.014, OR = 9.26) (19), and long-term patch's contralateral aortic posterior wall aneurysm is another potential risk (31). EEEA can better manage remaining catheter tissue, protect the left subclavian artery, avoid artificial materials, retain natural vascular architecture, lower aneurysm risk, and repair transverse arch and isthmus dysplasia (32). Meantime, EEEA reduces mortality and reCoA rate (33, 34) and promotes long-term aortic compliance (35), so it may be the best potential surgery. Patients with other CHD may accept one-period surgery through median sternal, with satisfactory outcomes (36, 37). Results from the analysis of ESA were similar (33, 37, 38). Balloon angioplasty can cure reCoA well (10, 17, 39), with a 93% success rate (40). One reCoA patient in this study got balloon angioplasty without any additional intervention.
Each strategy has pros and cons. EEEA or ESA may cause patients to suffer from large-scale surgery, which is psychologically and financially taxing. Nearly 60% of patients in this research had successful EEEA. Meantime, if we took EEEA, ESA, and PAP as radical surgery whereas SAR was considered a relative surgery. Neither was proven to be reCoA risk factor (P = 0.075). Although fewer patients had SAR, we assumed that careful selection of operation according to ease patients may even prevent major surgery. Therefore, in the future, we may take more in-depth research on the morphology and hydrodynamics of the aortic arch during the perioperative period, and even pre-do the operation with Virtual Reality (VR), to allow surgeons to better determine the therapy for patients.
Multiple studies have explored aortic arch morphology and whether systolic blood pressure gradient affects reCoA. We found that the preoperative z-score of the ascending aorta impacted reCoA (P = 0.009), which matched Kumar (34). In this 10-variable research, reCoA was only associated with a small preoperative ascending aorta (P < 0.01). McElhinney agreed (P = 0.02, HR = 2.1), but disagreed when body weight was the indicator (P = 0.48, HR = 1.34) (9). This may be because children with lower body weight in cohorts of patients had a lesser ascending aorta. Unfortunately, maybe because of the limited sample size in the cohort, we could not identify an optimal cutoff for the ascending aorta z-score linked with reCoA. Future research in larger cohorts is conceivable.
A smaller preoperative z-score of the transverse aortic arch increased the risk of reCoA (P = 0.015). Burch found that for every 1 mm increase in aortic arch transverse diameter, reCoA risk was reduced (P = 0.04, RR = 0.57) (10). However, Kumar (34) cannot relate transverse arch dysplasia to reCoA. Truong found that preoperative aortic arch measurements and transverse aortic arch abnormalities were not reCoA risk factors in thoracotomy patients (12). We believe our findings are reliable, cardiac surgeons should choose the approach carefully for patients with smaller preoperative z-score of the transverse aortic arch to improve their outcomes.
Arm-leg systolic blood pressure gradient ≥12.5 mmHg at discharge affected reCoA (P < 0.001, log-rank = 49.06). Kumar examined blood pressure gradients at 24 h, 48 h, and 72 h following surgery and at discharge, finding that the gradient at discharge was significant compared with other points (34), reCoA was more likely in patients with a gradient >13 mmHg (P < 0.001, log-rank = 19.49). Although we considered that in the early postoperative stage, blood pressure was unstable due to operation, anesthesia, and other factors, we think the conclusion is reliable. In the future, more precise pressure measurements can help further explore its relationship with reCoA.
About 60% (8/13) developed reCoA within 6 postoperative months, and all within one year. Therefore, we suggested that closer follow-up is necessary for the first year postoperatively.
Limitations
This work is unusual because we evaluated the impacts of several parameters on reCoA and found the blood pressure cutoff and its specificity and sensitivity to better predict reCoA, which has great practical application. However, our study had several limitations. This is a retrospective study that has the inherent limitations of any retrospective study. Five congenital cardiac surgeons operated the surgical procedures and at least three different echocardiography specialists were included to take the measurements of aortic arch morphology preoperatively and postoperatively. Meanwhile, several issues need to be explored, including the best preoperative z-score cutoff of the ascending aorta and transverse aortic arch, which may be examined in a larger sample cohort. For reCoA, because the follow-up did not exactly follow the plan, the accurate reCoA time may be earlier, but all reCoA can be found within 1 year after the surgical procedure, so we think it does not affect the study results.
Conclusion
In conclusion, the smaller preoperative z-score of the ascending aorta, the smaller preoperative z-score of the transverse aortic arch, or the discharge arm-leg systolic blood pressure gradient ≥12.5 mmHg make an increased risk of reCoA. We suggested more active follow-up for such patients, especially within 1 postoperative year, to detect reCoA timely.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Children’s Hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
ZZ, ZP, and XJ contributed to the conception and design of the study and the critical revision of the manuscript. ZZ and ZP contributed to the interpretation of the data. ZZ and CW contributed to the manuscript draft. JT, JQ, and YZ contributed to the acquisition and analysis of the data. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by project of Chongqing general project (No. cstc2020jcyj-msxm0282), and the project of intelligent medicine of Chongqing Medical University (No. ZHYX202122).
Acknowledgment
We would like to appreciate all the doctors, nurses, and social workers who contributed to this study for giving so much advice and help.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Chen S, Zühlke L, Black GC, Choy M, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48(2):455–63. doi: 10.1093/ije/dyz009
2. Dijkema EJ, Leiner T, Grotenhuis HB. Diagnosis, imaging and clinical management of aortic coarctation. Heart. (2017) 103(15):1148–55. doi: 10.1136/heartjnl-2017-311173x
3. Crafoord C, Nylin G. Congenital coarctation of the aorta and its surgical treatment. J Thorac Surg. (1945) 14:347–61. doi: 10.1016/S0096-5588(20)31801-8
4. Williams WG, Shindo G, Trusler GA, Dische MR, Olley PM. Results of repair of coarctation of the aorta during infancy. J Thorac Cardiovasc Surg. (1980) 79(4):603. doi: 10.1016/S0022-5223(19)37927-9
5. Quaegebeur JM, Jonas RA, Weinberg AD, Blackstone EH, Kirklin JW. Outcomes in seriously ill neonates with coarctation of the aorta. A multiinstitutional study. J Thorac Cardiovasc Surg. (1994) 108(5):841–51. 852–4. doi: 10.1016/s0022-5223(94)70182-2
6. Korfer R, Meyer H, Kleikamp G, Bircks W. Early and late results after resection and End-to-End anastomosis of coarctation of the thoracic aorta in early infancy. J Thorac Cardiovasc Surg. (1985) 89(4):616–22. doi: 10.1016/s0022-5223(19)38767-7
7. St. Louis J.D, Harvey BA, Menk JS, O'Brien JJ, Kochilas LK. Mortality and operative management for patients undergoing repair of coarctation of the aorta: a retrospective review of the pediatric cardiac care consortium. World J Pediatr Congenit Heart Surg. (2015) 6(3):431–7. doi: 10.1177/2150135115590458
8. Lehnert A, Villemain O, Gaudin R, Méot M, Raisky O, Bonnet D. Risk factors of mortality and recoarctation after coarctation repair in infancy. Interact Cardiovasc Thorac Surg. (2019) 29(3):469–75. doi: 10.1093/icvts/ivz117
9. McElhinney Doff B., Yang Song-Gui, Hogarty Alexa N., Rychik Jack. Recurrent arch obstruction after repair of isolated coarctation of the aorta in neonates and young infants: Is low weight a risk factor. 2001. doi: 10.1067/mtc.2001.116316
10. Burch PT, Cowley CG, Holubkov R, Null D, Lambert LM, Kouretas PC, et al. Coarctation repair in neonates and young infants: is small size or low weight still a risk factor? J Thorac Cardiovasc Surg. (2009) 138(3):547–52. doi: 10.1016/j.jtcvs.2009.04.046
11. Gorbatykh AV, Soynov IA, Nichai NR, Ivantsov SM, Voitov AV, Kulyabin YY, et al. Risk factors for aortic coarctation development in young children. Pediatria (Bucur). (2017) 96:118–24. doi: 10.24110/0031-403x-2017-96-3-118-124
12. Truong DT, Tani LY, Minich LL, Burch PT, Bardsley TR, Menon SC. Factors associated with recoarctation after surgical repair of coarctation of the aorta by way of thoracotomy in young infants. Pediatr Cardiol. (2014) 35(1):164–70. doi: 10.1007/s00246-013-0757-6
13. Tanous D, Benson LN, Horlick EM. Coarctation of the aorta: evaluation and management. Curr Opin Cardiol. (2009) 24(6):509–15. doi: 10.1097/HCO.0b013e328330cc22
14. Forbes TJ, Kim DW, Du W, Turner DR, Holzer R, Amin Z, et al. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the ccisc (congenital cardiovascular interventional study consortium). J Am Coll Cardiol. (2011) 58(25):2664–74. doi: 10.1016/j.jacc.2011.08.053
15. Butera G, Manica JL, Marini D, Piazza L, Chessa M, Filho RI, et al. From bare to covered: 15-year single center experience and follow-up in trans-catheter stent implantation for aortic coarctation. Catheter Cardiovasc Interv. (2014) 83(6):953–63. doi: 10.1002/ccd.25404
16. Zani A, Cozzi DA. Giovanni battista morgagni and his contribution to pediatric surgery. J Pediatr Surg. (2008) 43(4):729–33. doi: 10.1016/j.jpedsurg.2007.12.065
17. Singer MI, Rowen M, Dorsey TJ. Transluminal aortic balloon angioplasty for coarctation of the aorta in the newborn. Am Heart J. (1982) 103(1):131–2. doi: 10.1016/0002-8703(82)90539-7
18. Dias MQ, Barros A, Leite-Moreira A, Miranda JO. Risk factors for recoarctation and mortality in infants submitted to aortic coarctation repair: a systematic review. Pediatr Cardiol. (2020) 41(3):561–75. doi: 10.1007/s00246-020-02319-w
19. Adamson G, Karamlou T, Moore P, Natal-Hernandez L, Tabbutt S, Peyvandi S. Coarctation Index predicts recurrent aortic arch obstruction following surgical repair of coarctation of the aorta in infants. Pediatr Cardiol. (2017) 38(6):1241–6. doi: 10.1007/s00246-017-1651-4
20. Hager A, Schreiber C, Nützl S, Hess J. Mortality and restenosis rate of surgical coarctation repair in infancy: a study of 191 patients. Cardiology. (2008) 112(1):36–41. doi: 10.1159/000137697
21. Weismann CG, Grell BS, Odermarsky M, Mellander M, Liuba P. Echocardiographic predictors of recoarctation after surgical repair: a Swedish national study. Ann Thorac Surg. (2021) 111(4):1380–6. doi: 10.1016/j.athoracsur.2020.05.062
22. Bondy CA. Congenital cardiovascular disease in turner syndrome. Congenit Heart Dis. (2008) 3(1):2–15. doi: 10.1111/j.1747-0803.2007.00163.x
23. Bondy C, Bakalov VK, Cheng C, Olivieri L, Rosing DR, Arai AE. Bicuspid aortic valve and aortic coarctation are linked to deletion of the X chromosome short arm in turner syndrome. J Med Genet. (2013) 50(10):662–5. doi: 10.1136/jmedgenet-2013-101720
24. Bacha EA, Almodovar M, Wessel DL, Zurakowski D, Mayer JJ, Jonas RA, et al. Surgery for coarctation of the aorta in infants weighing less than 2 kg. Ann Thorac Surg. (2001) 71(4):1260–4. doi: 10.1016/s0003-4975(00)02664-3
25. Liberman L, Gersony WM, Flynn PA, Lamberti JJ, Cooper RS, Starc TJ. Effectiveness of prostaglandin E 1 in relieving obstruction in coarctation of the aorta without opening the ductus arteriosus. Pediatr Cardiol. (2004) 25(1):49–52. doi: 10.1007/s00246-003-0549-5
26. Desai AR, Maiya S, Inwald D, Slavik Z. Prostaglandin in aortic coarctation and closed arterial duct-treatment beyond ductal Re-opening. Cor Vasa. (2013) 55(5):e460–2. doi: 10.1016/j.crvasa.2013.04.004
27. Welke KF, Diggs BS, Karamlou T, Ungerleider RM. Comparison of pediatric cardiac surgical mortality rates from national administrative data to contemporary clinical standards. Ann Thorac Surg. (2009) 87(1):216–22. 222–3. doi: 10.1016/j.athoracsur.2008.10.032
28. Gropler M, Marino BS, Carr MR, Russell WW, Gu H, Eltayeb OM, et al. Long-Term outcomes of coarctation repair through left thoracotomy. Ann Thorac Surg. (2019) 107(1):157–64. doi: 10.1016/j.athoracsur.2018.07.027
29. Kozyrev IA, Kotin NA, Averkin II, Ivanov AA, Latypov AA, Gordeev ML, et al. Modified technique for coarctation of aorta with hypoplastic distal aortic arch. J Card Surg. (2021) 36(6):2063–9. doi: 10.1111/jocs.15492
30. Vossschulte K. Plastic surgery of the isthmus in aortic isthmus stenosis. Thoraxchirurgie. (1957) 4(5):443–50.13422410
31. Maxey TS, Serfontein SJ, Reece TB, Rheuban KS, Kron IL. Transverse arch hypoplasia may predispose patients to aneurysm formation after patch repair of aortic coarctation. Ann Thorac Surg. (2003) 76(4):1090–3. doi: 10.1016/s0003-4975(03)00822-1
32. Amato JJ, Rheinlander HF, Cleveland RJ. A method of enlarging the distal transverse arch in infants with hypoplasia and coarctation of the aorta. Ann Thorac Surg. (1977) 23(3):261–3. doi: 10.1016/s0003-4975(10)64121-5
33. Tabbutt S, Nicolson SC, Dominguez TE, Wells W, Backer CL, Tweddell JS, et al. Perioperative course in 118 infants and children undergoing coarctation repair via a thoracotomy: a prospective, multicenter experience. J Thorac Cardiovasc Surg. (2008) 136(5):1229–36. doi: 10.1016/j.jtcvs.2008.06.035
34. Kumar TKS, Zurakowski D, Sharma R, Saini S, Jonas RA. Prediction of recurrent coarctation by early postoperative blood pressure gradient. J Thorac Cardiovasc Surg. (2011) 142(5):1130–6. doi: 10.1016/j.jtcvs.2011.02.048
35. Bassareo PP, Marras AR, Manai ME, Mercuro G. The influence of different surgical approaches on arterial rigidity in children after aortic coarctation repair. Pediatr Cardiol. (2009) 30(4):414–8. doi: 10.1007/s00246-008-9381-2
36. Padalino MA, Bagatin C, Bordin G, Tua L, Francescato A, Pradegan N, et al. Surgical repair of aortic coarctation in pediatric age: a single center two decades experience. J Card Surg. (2019) 34(5):256–65. doi: 10.1111/jocs.14019
37. Tulzer A, Mair R, Kreuzer M, Tulzer G. Outcome of aortic arch reconstruction in infants with coarctation: importance of operative approach. J Thorac Cardiovasc Surg. (2016) 152(6):1506–13. doi: 10.1016/j.jtcvs.2016.08.029
38. Zou MH, Ma L, Xia YS, Yang SC, Chen WD, Cao F, et al. End-to-Side anastomosis for interrupted aortic arch in neonates and infants. Zhonghua Wai Ke Za Zhi. (2018) 56(3):217–20. doi: 10.3760/cma.j.issn.0529-5815.2018.03.010
39. Wood AE, Javadpour H, Duff D, Oslizlok P, Walsh K. Is extended arch aortoplasty the operation of choice for infant aortic coarctation? Results of 15 Years’ experience in 181 patients. Ann Thorac Surg. (2004) 77(4):1353–7. 1357–8. doi: 10.1016/j.athoracsur.2003.07.045
Keywords: coarctation of the aorta (COA), congenital heart disease - cardiac, pediatrics - children, risk factor, surgical treatment
Citation: Zhao Z, Pan Z, Wu C, Tian J, Qin J, Zhang Y and Jin X (2023) Risk factors for recurrence after surgical repair of coarctation of the aorta in children: a single-center experience based on 51 children. Front. Cardiovasc. Med. 10:1144755. doi: 10.3389/fcvm.2023.1144755
Received: 15 January 2023; Accepted: 16 May 2023;
Published: 30 May 2023.
Edited by:
Konstantinos Spanos, University of Thessaly, GreeceReviewed by:
Vladimir Shipulin, Russian Academy of Sciences, RussiaLee Pyles, West Virginia University, United States
© 2023 Zhao, Pan, Wu, Tian, Qin, Zhang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Jin amlueGluY3FAMTYzLmNvbQ==
 Zhenjiang Zhao
Zhenjiang Zhao Zhengxia Pan1,2,3,4,5
Zhengxia Pan1,2,3,4,5 Jie Tian
Jie Tian Jinjie Qin
Jinjie Qin Xin Jin
Xin Jin