
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 March 2023
Sec. Cardiovascular Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1140670
This article is part of the Research Topic Data Driven and Model Based Computational Futures in Cardiovascular Practice View all 9 articles
Objectives: To evaluate the efficacy of the Cox-Maze IV procedure (CMP-IV) in combination with valve surgery in patients with both atrial fibrillation (AF) and valvular disease and use machine learning algorithms to identify potential risk factors of AF recurrence.
Methods: A total of 1,026 patients with AF and valvular disease from two hospitals were included in the study. 555 patients received the CMP-IV procedure in addition to valve surgery and left atrial appendage ligation (CMP-IV group), while 471 patients only received valve surgery and left atrial appendage ligation (Non-CMP-IV group). Kaplan–Meier analysis was used to calculate the sinus rhythm maintenance rate. 58 variables were selected as variables for each group and 10 machine learning models were developed respectively. The performance of the models was evaluated using five-fold cross-validation and metrics including F1 score, accuracy, precision, and recall. The four best-performing models for each group were selected for further analysis, including feature importance evaluation and SHAP analysis.
Results: The 5-year sinus rhythm maintenance rate in the CMP-IV group was 82.13% (95% CI: 78.51%, 85.93%), while in the Non-CMP-IV group, it was 13.40% (95% CI: 10.44%, 17.20%). The eXtreme Gradient Boosting (XGBoost), LightGBM, Category Boosting (CatBoost) and Random Fores (RF) models performed the best in the CMP-IV group, with area under the curve (AUC) values of 0.768 (95% CI: 0.742, 0.786), 0.766 (95% CI: 0.744, 0.792), 0.762 (95% CI: 0.723, 0.801), and 0.732 (95% CI: 0.701, 0.763), respectively. In the Non-CMP-IV group, the LightGBM, XGBoost, CatBoost and RF models performed the best, with AUC values of 0.738 (95% CI: 0.699, 0.777), 0.732 (95% CI: 0.694, 0.770), 0.724 (95% CI: 0.668, 0.789), and 0.716 (95% CI: 0.656, 0.774), respectively. Analysis of feature importance and SHAP revealed that duration of AF, preoperative left ventricular ejection fraction, postoperative heart rhythm, preoperative neutrophil-lymphocyte ratio, preoperative left atrial diameter and heart rate were significant factors in AF recurrence.
Conclusion: CMP-IV is effective in treating AF and multiple machine learning models were successfully developed, and several risk factors were identified for AF recurrence, which may aid clinical decision-making and optimize the individual surgical management of AF.
Atrial fibrillation (AF) is a common clinical tachyarrhythmia and is the most common arrhythmia in adult cardiac surgery patients, representing about one-third of all arrhythmias with a global incidence of nearly 1% (1, 2). It increases the risk of ischemic stroke (3, 4), myocardial infarction (5), and renal insufficiency (6).
Cox-Maze Procedure (CMP) is currently considered the most effective surgical treatment for AF and is the gold standard for surgical treatment of AF (7, 8). Cox-Maze IV Procedure (CMP-IV) replaces the “cut-and-sew” technique of the original CMP with lines of ablation created using bipolar radiofrequency energy (9–11). Some risk factors for the recurrence of AF after CMP have been identified, including enlarged left atrial diameter (LAD), failure to isolate the entire posterior left atrium, longer valvular disease duration, coronary artery disease, and larger right atrial diameter (12–15). However, there is still a lack of large-scale, long-term controlled studies, and the risk factors for AF recurrence after CMP-IV need further verification and evaluation. If further risk factors that influence the efficacy of CMP-IV can be identified, it may be possible to enhance the effectiveness of CMP-IV in treating AF.
Previous clinical studies have mainly used traditional statistical analysis methods such as linear and logistic regression, linear discriminant analysis, and correlation analysis to investigate relationships between variables and analyze clinical data (14, 15). However, these methods have limitations in terms of their ability to handle large amounts of data and explore nonlinear relationships (16, 17). Machine learning is a branch of artificial intelligence, which can learn from data and extract features for tasks such as classification, prediction, and clustering (18). Currently, machine learning is increasingly integrated into clinical practice, with applications ranging from preclinical data processing to patient stratification and treatment decision-making, mainly including disease diagnosis, treatment risk assessment, drug production and medical data analysis (19–22). The purpose of this study is to evaluate the efficacy of CMP-IV in patients with chronic valvular disease and AF through a retrospective cohort control study and to construct machine learning models to evaluate the risk factors for the recurrence of AF after CMP-IV.
This clinical study has been registered on the Chinese clinical trial website (ChiCTR1900023775). This research was funded by the National Key Research and Development Program of China (2018YFC1311204). The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and Xinqiao Hospital Affiliated to Army Medical University. Written consent was obtained from all patients before surgery.
In this study, we enrolled a total of 1,026 eligible patients with AF and chronic valvular disease at the Second Xiangya Hospital of Central South University and Xinqiao Hospital Affiliated to Army Medical University between January 2012 and December 2019. Among them, 555 patients underwent CMP-IV with valve surgery and left atrial appendage ligation (CMP-IV group), as well as 471 patients only underwent valve surgery and left atrial appendage ligation without CMP-IV at the same hospitals during the same period (Non-CMP-IV group). The inclusion and exclusion criteria for the study are detailed in Supplementary Material.
AF recurrence included the continuous occurrence of AF, atrial flutter and atrial tachycardia recorded on the electrocardiogram (ECG) for more than 30 s. Non-AF recurrent conditions include sinus rhythm, junctional rhythm, atrial premature beats, and ventricular premature beats. Diagnostic records: physical examination and auscultation, ECG, 24-h dynamic ECG, and related medical records. The postoperative follow-up period for included patients began at 6 months after surgery and continued for at least 12 months. Follow-up records were collected every 12 months. During follow-up visits, symptoms and signs were recorded, and routine examinations such as cardiac ultrasound and ECG were performed. In cases where patients were unable to come to the hospital for a follow-up visit due to personal reasons, they were contacted by phone and recommended to complete the examination at a local hospital and report the results to their follow-up recorder. If a patient experienced palpitations or suspected recurrent AF, an ECG should be performed at any time and the results reported to the follow-up recorder.
58 features were selected as variables in each group, including demographics, medical history, laboratory test results, and clinically relevant variables. Data were collected from the electronic medical records of the patients and underwent data cleaning and preprocessing to ensure quality and consistency. Specifically, we checked for and imputed missing values using the median value for numerical features and the mode for categorical features. We also checked for outliers and transformed skewed features using log transformation. By performing these data processing steps, we ensured the quality and consistency of the data used in our analysis. Both centers in this study performed data collection, data quality control, and unified data entry and storage in accordance with a uniform standard to ensure the compatibility of data.
We developed ten common machine learning models for the two groups respectively, including Support Vector Machine (SVM) (23), Logistic Regression (LR) (24), eXtreme Gradient Boosting (XGBoost) (25), Random Fores (RF) (26), Category Boosting (CatBoost) (27), Adaptive Boosting (AdaBoost) (28), Bootstrapped aggregation (Bagging) (29), Gradient boosting decision trees (GBDT) (30), Light Gradient Boosting Machine (LightGBM) (31) and Multilayer perceptron (MLP) (32). The optimal hyperparameter combination in machine learning models was determined through grid search. To evaluate the performance of these models, we performed five-fold cross-validation on the training data and calculated evaluation metrics including F1 score, Accuracy, Recall, Precision, and area under the curve (AUC), along with 95% confidence intervals. These metrics allowed us to assess the overall performance of the models and determine their suitability for use in clinical decision-making. The F1 score is the harmonic mean of precision and recall and is a useful metric for balancing the trade-off between these two metrics. Accuracy measures the percentage of correct predictions. Recall measures the percentage of true positive predictions among all actual positive cases. Precision measures the percentage of true positive predictions among all positive predictions. The Receiver Operating Characteristic (ROC) curve for each model was plotted in CMP-IV group and Non-CMP-IV group, using a 7:3 split for training and testing, to more clearly visualize the trade-off between true positive rate and false positive rate and evaluate the AUC (33).
Based on the evaluation results, we selected four best-performing machine learning models in both the CMP-IV group and the Non-CMP-IV group. Feature importance analysis was performed on the selected models to identify the influence of the features on the predictions. The importance of each feature was calculated based on the internal mechanisms of the models. We analyzed the top 20 features in the 4 models of the two groups and analyzed their feature importance. The features were ranked based on their importance and visualized using feature importance plots. Additionally, we conducted SHapley Additive exPlanations (SHAP) analysis to interpret the results of our machine learning models for the two groups. Using SHAP force plots, we were able to examine the contribution of each feature to the model's prediction for each individual sample.
We performed statistical analysis using Python 3.8, the scikit-learn (sklearn) library 0.23.2, IBM SPSS Statistics 27 and R 4.0.2 to perform statistical analysis. Normally distributed continuous data have been expressed as mean ± standard deviation. Skewed distributed continuous data have been described as medians with interquartile ranges and were logarithmically transformed when necessary. Comparisons between categorical data were performed with the chi-square test, while continuous variables were assessed by t-test (for normal distribution) or nonparametric tests (for skewed distribution). A difference was considered statistically significant if p ≤ 0.01.
Baseline, hospitalization and follow-up characteristics for the CMP-IV group and Non-CMP-IV group are shown in Table 1. In a total of 1,026 patients, AF recurred in 117 of 555 (21.08%) in the CMP-IV group (the detailed information of baseline characteristics is presented in Supplementary Table S1) and 402 of 471 (85.35%) in the Non-CMP-IV group (the detailed information of baseline characteristics is presented in Supplementary Table S2). The median follow-up time was 5 years. There was no significant difference in age, duration of AF, and follow-up time between the two groups.
We drew the sinus rhythm maintenance curves of the two groups, as shown in Figure 1. The results of our study showed that the sinus rhythm maintenance rate in the CMP-IV group was significantly higher than that in the Non-CMP-IV group. We used the Kaplan–Meier method to analyze the data and found that there was a statistically significant difference in the probability of recurrence between the two groups (p < 0.0001). The 5-year sinus rhythm maintenance rate in the CMP-IV group was 82.13% (95% CI: 78.51%, 85.93%), while in the Non-CMP-IV group, it was 13.40% (95% CI: 10.44%, 17.20%).
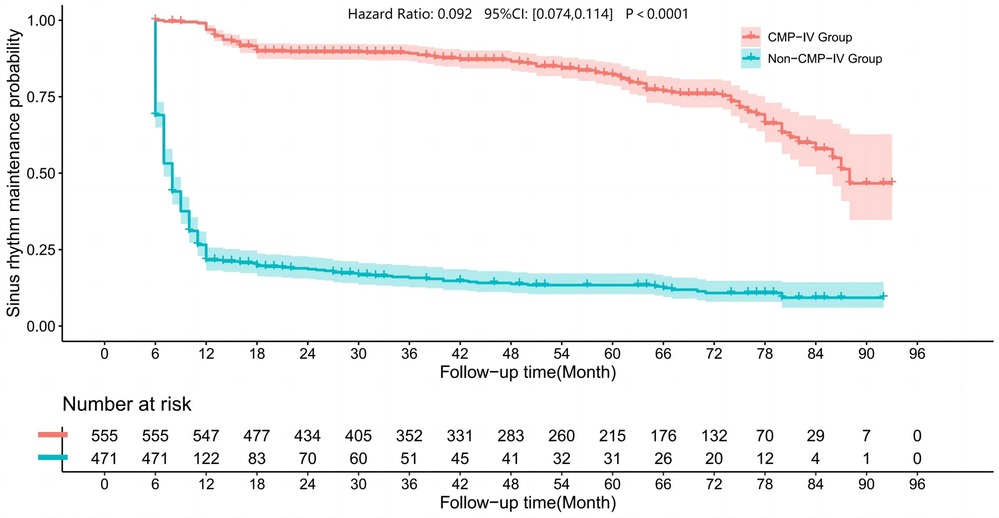
Figure 1. Kaplan–Meier curves were used to visualize the sinus rhythm maintenance rate in the two groups. The Log Rank rank sum test statistic for the comparison of survival time between the two groups was 673.932, indicating a significant difference in sinus rhythm maintenance rate between the CMP-IV group and the Non-CMP-IV group (p < 0.001). The median sinus rhythm maintenance time for the CMP-IV group was 88 months (95% CI: 84, NA) and for the Non-CMP-IV group was 8 months (95% CI: 7, 8). The hazard ratio for the Cox-Maze IV group compared to the Non-Cox-Maze IV group was 0.092 (95% CI: 0.074, 0.114). The follow-up time was plotted in months, and the number of patients at risk was shown below the graph.
To evaluate the performance of the machine learning models, we conducted a five-fold cross-validation by dividing the data into training and test sets at a ratio of 8:2. The results of the model evaluation are shown in Table 2. In order to provide a more intuitive comparison, we also plotted the ROC curves of the models using data sets divided at a ratio of 7:3, as shown in Figure 2. Based on the ROC curves and the model evaluation results, we found that the CatBoost, LightGBM, XGBoost, and RF models in the CMP-IV group had the highest AUC of 0.768 (95% CI:0.742, 0.786), 0.766 (95% CI:0.744, 0.792), 0.762 (95% CI:0.723, 0.801), 0.732 (95% CI:0.701, 0.763), respectively. Among the 10 models in the Non-CMP-IV group, the LightGBM, XGBoost, RF, and CatBoost models performed the best (as shown in Table 3), with AUC of 0.714 (95% CI: 0.699, 0.813), 0.712 (95% CI: 0.669, 0.836), 0.711 (95% CI: 0.666, 0.849), and 0.699 (95% CI: 0.669, 0.831), respectively. Other model evaluation metrics including F1, ACC, Recall, and Precision are also at good performances in the 4 models of the two groups.
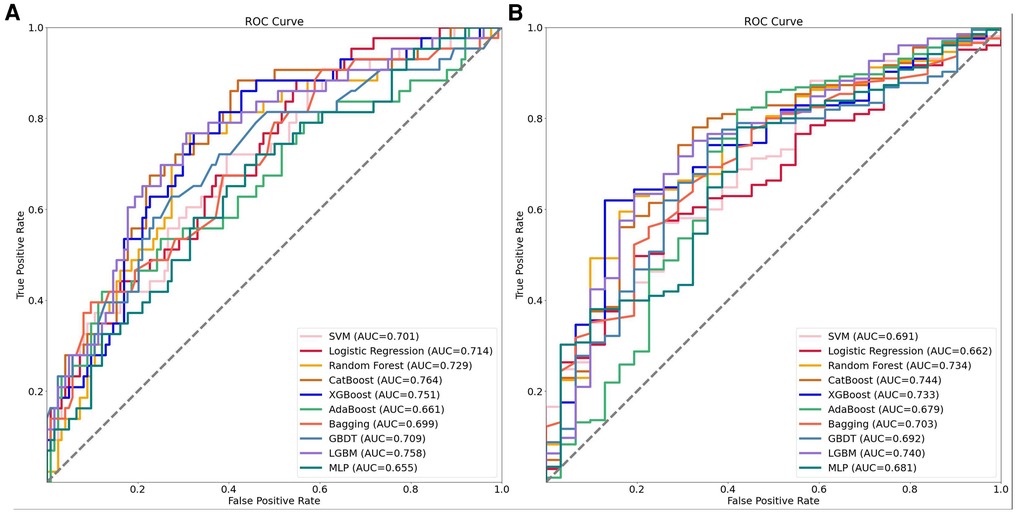
Figure 2. The receiver operating characteristic (ROC) curves of the 10 machine learning models established in the two groups. Panel A shows the ROC curves for the CMP-IV group, while Panel B shows the ROC curves for the No CMP-IV group. The acronyms in the legend stand for the following: SVM, support vector machine; Catboost, categorical boosting; XGBoost, extreme gradient boosting; AdaBoost, adaptive boosting; Bagging, bootstrapped aggregation; GBDT, gradient boosting decision trees; LightGBM, light gradient boosting machine; MLP, multilayer perceptron.
Four machine learning models (LightGBM, XGBoost, RF and CatBoost) were selected to analyze the risk factors for AF recurrence in the CMP-IV group and Non-CMP-IV group based on the model evaluation. The top 20 feature importance of the 4 models in the CMP-IV group is shown in Figure 3. The feature importance of the 4 models for the Non-CMP-IV group is shown in Figure 4. The SHAP force plots in Figure 5 allow us to evaluate the risk of AF recurrence for individual samples, which shows high-risk and low-risk examples of AF recurrence in both the CMP-IV and Non-CMP-IV groups. Figure 6 shows the SHAP summary diagrams for the analysis of the XGBoost model and the Catboost model.
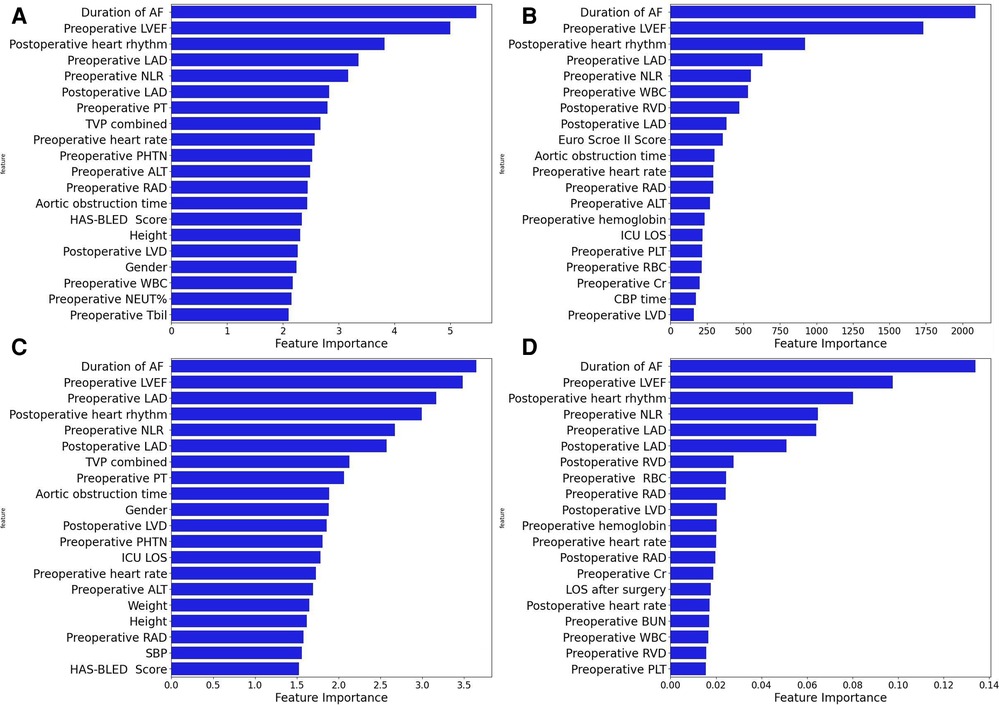
Figure 3. The feature importance plots of 4 machine learning models from the CMP-IV group are shown in A–D. Each plot is a bar graph with the feature importance on the x-axis and the names of each feature on the y-axis. The x-axis represents the feature importance, with the values indicating the contribution of the feature to the model. A higher value on the x-axis indicates a higher importance of the feature in the respective model. The models used are XGBoost (A), LightGBM (B), CatBoost (C), and Random Forest (D).
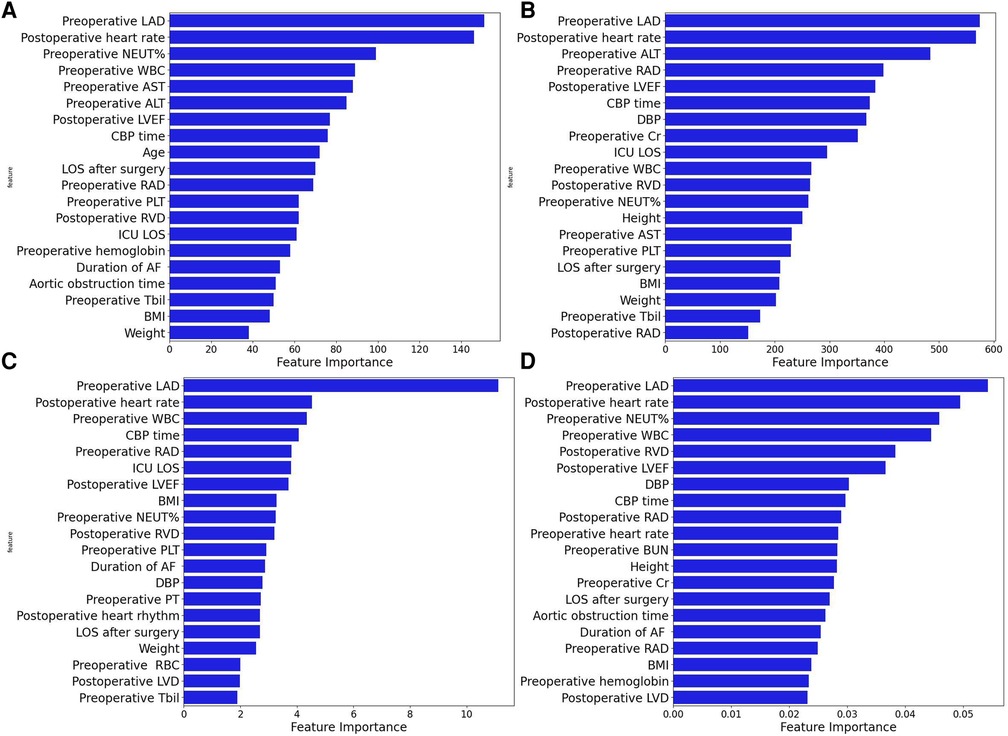
Figure 4. The feature importance plots of 4 machine learning models from the Non-CMP-IV group are shown in A–D. The models used are XGBoost (A), LightGBM (B), CatBoost (C), and Random Forest (D).
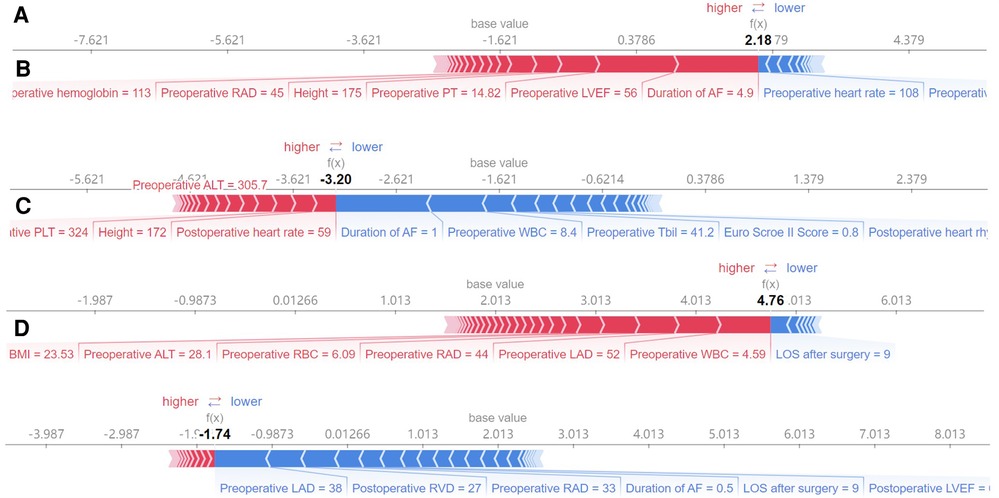
Figure 5. The SHAP force plots shown in A–D correspond to model-predicted risk scores for particular instances. The SHAP force plot is a graphical representation of the feature importance for a single sample in a machine learning model. The x-axis represents the different features, arranged from left to right. Each feature has a corresponding weight value, which indicates the impact of that feature on the prediction outcome. The size of the weight value indicates the magnitude of the impact of the feature on the prediction. The reference line represents the expected value of the prediction outcome if all feature values were set to their average value. The f(x) value represents the predicted outcome for the given sample. A,B show high and low risk examples for the CMP-IV group, respectively. C,D show high and low risk examples for the non-CMP-IV group, respectively.
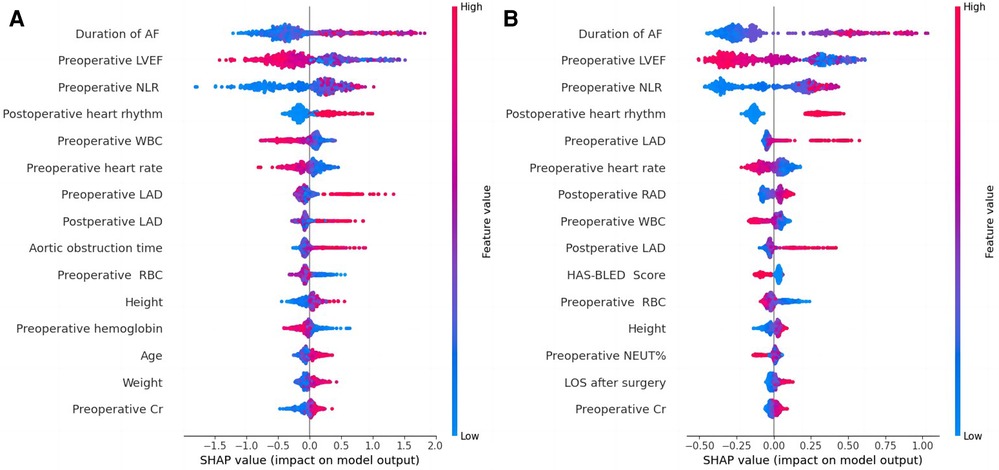
Figure 6. SHAP summary plots based on XGBoost model output (A) and catBoost model output (B) from the CMP-IV group. SHAP summary plots are used to visualize the impact of each feature on the model output. The plot includes a list of features on the left side and the corresponding feature values on the right side. The middle part of the plot is a cluster of coloured dots, where the red dots represent features that have a positive impact on the model output, the blue dots represent features that have a negative impact, and the purple dots represent features that have a neutral impact. The size of the dots represents the magnitude of the impact of each feature, with larger dots indicating a greater impact. The influence of each feature on the outcome can be discerned by comparing the position of the dots to the reference line.
We conducted a retrospective cohort study of the CMP-IV group and the Non-CMP-IV group and found that the CMP-IV resulted in a significantly higher rate of sinus rhythm maintenance at 5 years (82.13% in the CMP-IV group compared to 13.40% in the Non-CMP-IV group). This suggests that CMP-IV treatment for AF is highly effective and consistent with previous literature reports (34, 35). Left atrial appendage ligation can reduce the risk of thrombosis formation (36–38). Kawamura et al. found that left atrial appendage ligation may lead to a decrease in atrial dispersion, thereby improving the rate of sinus rhythm maintenance (39). We found that most patients in the Non-CMP-IV group who underwent valve surgery combined with left atrial appendage ligation experienced the recurrence of AF in the early postoperative period. A small number of patients in the Non-CMP-IV group regained sinus rhythm, which may be related to drug treatment and paroxysmal AF (40–42).
We successfully developed 10 machine learning models in each group. CatBoost, XGBoost, LightGBM, and Random Forest outperformed the others in predicting and classifying this data set. Using feature importance analysis and SHAP interpretability analysis, we identified several risk factors for AF recurrence by ranking the obtained features according to their importance and the number of occurrences in each model. In the CMP-IV group, the most significant risk factors were the duration of AF and preoperative LVEF. Other important risk factors included postoperative heart rhythm, preoperative LAD, preoperative NLR, preoperative heart rate, and preoperative WBC. In the Non-CMP-IV group, the most important risk factors were preoperative LAD and postoperative heart rate. Other potentially influential risk factors included preoperative WBC and preoperative NEUT%, preoperative ALT, and postoperative LVEF.
In our study, all four of our machine learning models in the CMP-IV group found that the duration of AF is the most important feature for AF recurrence. This is similar to a Meta-analysis result of Chew et al. which found that the duration between the first diagnosis of AF and ablation, or diagnosis-to-ablation time (DAT), is associated with AF recurrence following catheter ablation (43). Andrade et al. also found that AF episode duration is associated with post-AF outcomes, in which longer episodes are linked to higher AF burden (44). Our study further suggests that the risk of AF recurrence increases significantly with a prolonged duration of AF. We found that preoperative LVEF is also an important risk factor for AF recurrence in the CMP-IV group. The SHAP summary plot of the two models showed that as preoperative LVEF decreases, the risk of AF recurrence increases. While this feature was not identified in previous studies, its importance in our models ranked second among all four models, indicating a high level of influence on the outcome. One possible reason is that the use of machine learning methods allowed us to identify novel risk factors that were not previously known to be associated with AF recurrence. This provided us with a deeper understanding of the relationships between the various risk factors and AF recurrence. Further research is needed to confirm the role of preoperative LVEF in AF recurrence. Our multiple models also found that LAD is closely related to AF recurrence. The SHAP interpreter clearly showed that the risk of AF recurrence increases with increasing preoperative or intraoperative LAD, which is consistent with previous studies (45–47). The feature importance analysis of the XGBoost and Catboost models found that combined tricuspid valve surgery is also a risk factor for AF recurrence. Previous studies have found that long-term AF is associated with functional tricuspid regurgitation, and severe tricuspid regurgitation may be associated with AF (48, 49), which suggests that preoperative tricuspid insufficiency may be associated with the risk of AF recurrence.
WBC has been found to correlate with cardiovascular risk (50), we also found that preoperative neutrophil-lymphocyte ratio (NLR) was significantly associated with AF recurrence after CMP-IV. NLR is linked to cardiovascular risk and can predict the prognosis of various clinical diseases (51, 52), including hypertension and heart failure (53, 54). High NLR could potentially be used as a predictor for AF recurrence (55, 56), which is in line with our machine learning model's further findings, as the SHAP summary plot shows that as NLR increases, the risk of AF recurrence also increases. In addition, our study found that early postoperative AF recurrence is correlated with final AF recurrence. Specifically, the presence of postoperative early recurrence, which may be related to the postoperative excessive inflammatory response (57, 58), significantly increases the risk of late AF recurrence. This finding is consistent with previous research on radiofrequency catheter ablation by Kim et al. (59). Prothrombin time (PT) and international normalized ratio (INR) are used to measure the effectiveness of anticoagulants in patients with AF. Combined with the analysis results of multiple models, Has-bled (60), EuroSCore II (61) and CHA2DS2VASc (62) which are commonly used surgical scoring tools, were not found to be significant risk factors in predicting AF recurrence in this study. Additionally, gender, age, and the main type of valve surgery were not found to be significant risk factors.
In the feature importance analysis of the Non-CMP-IV group, we found that the LAD was consistently identified as the most important factor associated with AF recurrence in all four models. However, as most patients in this group still had AF postoperatively and AF was detected early, the characteristic value gaps for most features were small, leading to potentially weakened correlations between these risk factors and AF recurrence.
Our study found that the four groups of models had high consistency in identifying the most influential risk factors for AF recurrence, but the results for risk factors with lower feature importance varied among the models. Besides, the differences in feature importance rankings can be attributed to the different algorithms and approaches used to calculate feature importance. This suggests that the machine learning models were effective in identifying key risk factors, but there may be room for further optimization in the assessment of overall risk factors. Similar to the SHAP force plot analysis based on a single sample in Figure 5, it is helpful for individualized risk assessment of AF recurrence for each patient. Previous studies on risk factors for recurrence of atrial fibrillation after CMP-IV have been conducted (14, 15, 63), but they were based on traditional statistical analyses. Our study expanded the sample size and length of follow-up compared to previous studies and used machine learning algorithms to visualize the risk factors for recurrence of atrial fibrillation for the overall study and for individual patients.
Our study has a few limitations. One is that the sample size of our study may not be sufficient to fully capture the complexity of AF recurrence. Additionally, our study was conducted at two hospitals and may not be generalizable to other settings. Furthermore, the effectiveness of machine learning techniques depends on the quality and relevance of input features. In this study, we only considered a limited number of features and did not analyze more detailed intraoperative data or evaluate the transmurality of ablation in patients. There may be other risk factors for AF recurrence that have not been identified. The clinical application of this study will help to establish a predictive model for AF recurrence after CMP-IV. This will serve as precision therapy by helping to individualize patient treatment. It will allow surgeons to improve treatment plans in a timely manner, thereby improving the prognosis of patients. Further research can continue to study individualized risk factors of AF recurrence with larger-scale data sets and specific values, such as developing an individualized assessment system for AF recurrence to help surgeons to assess the prognosis of AF surgical treatment based on individual patient data.
In conclusion, our study found that Cox-Maze IV procedure is effective in treating AF and maintaining sinus rhythm in patients with AF and valvular disease. We have successfully developed multiple machine learning models and identified several clinical risk factors for AF recurrence, including duration of AF, preoperative LVEF, postoperative heart rhythm, preoperative LAD, preoperative NLR, preoperative heart rate, postoperative LAD, preoperative WBC and postoperative AST. CatBoost, XGBoost, LightGBM, and Random Forest performed the best in predicting AF recurrence in this data set. Machine learning models can be used to identify risk factors and may be useful in predicting and preventing AF recurrence in clinical practice. Further studies are needed to confirm our findings and to determine the generalizability of these results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Xiangya Hospital of Central South University and Xinqiao Hospital Affiliated to Army Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LL: conceptualization, project administration, supervision, data acquisition. ZJ: conception and design of the research, experiment performance, data analysis, manuscript writing. LS, CL and LL: investigation. ZJ, LS and CL: data acquisition and data interpretation. YS and HT: resources. HZ and RG: technical assistance. All authors contributed to the article and approved the submitted version.
This study was supported by the National Key Research and Development Program of China (No. 2018YFC1311204 to LL).
We thank all participating patients and relevant clinicians.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1140670/full#supplementary-material.
1. Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. (2016) 374(20):1911–21. doi: 10.1056/NEJMoa1602002
2. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in olmsted county, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. (2006) 114(2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140
3. Paciaroni M, Agnelli G, Micheli S, Caso V. Efficacy and safety of anticoagulant treatment in acute cardioembolic stroke: a meta-analysis of randomized controlled trials. Stroke. (2007) 38(2):423–30. doi: 10.1161/01.STR.0000254600.92975.1f
4. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46(10):2981–6. doi: 10.1161/STROKEAHA.115.010049
5. Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: a systematic review and meta-analysis. Eur J Prev Cardiol. (2017) 24(14):1555–66. doi: 10.1177/2047487317715769
6. Bohm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. (2015) 65(23):2481–93. doi: 10.1016/j.jacc.2015.03.577
7. Garcia-Villarreal OA. The cox-maze procedure: rules not to be broken. Ann Thorac Surg. (2021) 112(6):2109. doi: 10.1016/j.athoracsur.2021.02.026
8. Ruaengsri C, Schill MR, Khiabani AJ, Schuessler RB, Melby SJ, Damiano RJ Jr. The cox-maze IV procedure in its second decade: still the gold standard? Eur J Cardiothorac Surg. (2018) 53(suppl_1):i19–25. doi: 10.1093/ejcts/ezx326
9. Ad N, Henry L, Hunt S, Holmes SD. The outcome of the cox maze procedure in patients with previous percutaneous catheter ablation to treat atrial fibrillation. Ann Thorac Surg. (2011) 91(5):1371–7; discussion 1377. doi: 10.1016/j.athoracsur.2011.01.026
10. Robertson JO, Saint LL, Leidenfrost JE, Damiano RJ Jr. Illustrated techniques for performing the cox-maze IV procedure through a right mini-thoracotomy. Ann Cardiothorac Surg. (2014) 3(1):105–16. doi: 10.3978/j.issn.2225-319X.2013.12.11
11. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. (2012) 14(4):528–606. doi: 10.1093/europace/eus027
12. Winkle RA, Mead RH, Engel G, Patrawala RA. Long-term results of atrial fibrillation ablation: the importance of all initial ablation failures undergoing a repeat ablation. Am Heart J. (2011) 162(1):193–200. doi: 10.1016/j.ahj.2011.04.013
13. Kim JS, Lee SA, Park JB, Chee HK, Chung JW. Preoperative risk factor analysis of postoperative stroke after cox-maze procedure with mitral valve repair. BMC Cardiovasc Disord. (2014) 14:116. doi: 10.1186/1471-2261-14-116
14. Takagaki M, Yamaguchi H, Ikeda N, Yamakage H, Nakamura H, Kadowaki T, et al. Risk factors for atrial fibrillation recurrence after cox maze IV performed without pre-exclusion. Ann Thorac Surg. (2020) 109(3):771–9. doi: 10.1016/j.athoracsur.2019.07.016
15. Damiano RJ Jr., Schwartz FH, Bailey MS, Maniar HS, Munfakh NA, Moon MR, et al. The cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg. (2011) 141(1):113–21. doi: 10.1016/j.jtcvs.2010.08.067
16. Ij H. Statistics versus machine learning. Nat Methods. (2018) 15(4):233. doi: 10.1038/nmeth.4642
17. Rajula HSR, Verlato G, Manchia M, Antonucci N, Fanos V. Comparison of conventional statistical methods with machine learning in medicine: diagnosis, drug development, and treatment. Medicina. (2020) 56(9):455. doi: 10.3390/medicina56090455
18. Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. (2015) 349(6245):255–60. doi: 10.1126/science.aaa8415
19. Verma AA, Murray J, Greiner R, Cohen JP, Shojania KG, Ghassemi M, et al. Implementing machine learning in medicine. Can Med Assoc J. (2021) 193(34):E1351–7. doi: 10.1503/cmaj.202434
20. Peiffer-Smadja N, Rawson TM, Ahmad R, Buchard A, Georgiou P, Lescure FX, et al. Machine learning for clinical decision support in infectious diseases: a narrative review of current applications. Clin Microbiol Infect. (2020) 26(5):584–95. doi: 10.1016/j.cmi.2019.09.009
21. van Kooten RT, Bahadoer RR, Ter Buurkes de Vries B, Wouters M, Tollenaar R, Hartgrink HH, et al. Conventional regression analysis and machine learning in prediction of anastomotic leakage and pulmonary complications after esophagogastric cancer surgery. J Surg Oncol. (2022) 126(3):490–501. doi: 10.1002/jso.26910
22. Wagner M, Brandenburg JM, Bodenstedt S, Schulze A, Jenke AC, Stern A, et al. Surgomics: personalized prediction of morbidity, mortality and long-term outcome in surgery using machine learning on multimodal data. Surg Endosc. (2022) 36(11):8568–91. doi: 10.1007/s00464-022-09611-1
23. Noble WS. What is a support vector machine? Nat Biotechnol. (2006) 24(12):1565–7. doi: 10.1038/nbt1206-1565
25. Chen T, Guestrin C. Xgboost: a scalable tree boosting system. Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining (2016).
27. Prokhorenkova L, Gusev G, Vorobev A, Dorogush AV, Gulin A. CatBoost: unbiased boosting with categorical features. Advances in neural information processing systems (2018). p. 31.
28. Schapire RE. Explaining adaboost. In: Schölkopf B, editor. Empirical inference. Berlin: Springer (2013). p. 37–52.
29. Lee T-H, Ullah A, Wang R. Bootstrap aggregating and random forest. In: Fuleky P, Kilian L, editors. Macroeconomic forecasting in the era of big data. Cham: Springer (2020). p. 389–429.
30. Friedman JH. Stochastic gradient boosting. Comput Stat Data Anal. (2002) 38(4):367–78. doi: 10.1016/S0167-9473(01)00065-2
31. Ke G, Meng Q, Finley T, Wang T, Chen W, Ma W, et al. Lightgbm: a highly efficient gradient boosting decision tree. Adv Neural Inf Process Syst. (2017) 30:3149–57. doi: 10.5555/3294996.3295074
32. Tang J, Deng C, Huang GB. Extreme learning machine for multilayer perceptron. IEEE Trans Neural Netw Learn Syst. (2016) 27(4):809–21. doi: 10.1109/TNNLS.2015.2424995
33. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44(3):837–45. doi: 10.2307/2531595
34. Khiabani AJ, MacGregor RM, Bakir NH, Manghelli JL, Sinn LA, Maniar HS, et al. The long-term outcomes and durability of the cox-maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. (2022) 163(2):629–641.e7. doi: 10.1016/j.jtcvs.2020.04.100
35. Philpott JM, Zemlin CW, Cox JL, Stirling M, Mack M, Hooker RL, et al. The ABLATE trial: safety and efficacy of cox maze-IV using a bipolar radiofrequency ablation system. Ann Thorac Surg. (2015) 100(5):1541–6; discussion 1547–8. doi: 10.1016/j.athoracsur.2015.07.006
36. Kim R, Baumgartner N, Clements J. Routine left atrial appendage ligation during cardiac surgery may prevent postoperative atrial fibrillation-related cerebrovascular accident. J Thorac Cardiovasc Surg. (2013) 145(2):582–9; discussion 589. doi: 10.1016/j.jtcvs.2012.10.016
37. Ando M, Funamoto M, Cameron DE, Sundt TM. Concomitant surgical closure of left atrial appendage: a systematic review and meta-analysis. J Thorac Cardiovasc Surg. (2018) 156(3):1071–1080.e2. doi: 10.1016/j.jtcvs.2018.03.017
38. Melduni RM, Schaff HV, Lee HC, Gersh BJ, Noseworthy PA, Bailey KR, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score-matched analysis of 10 633 patients. Circulation. (2017) 135(4):366–78. doi: 10.1161/CIRCULATIONAHA.116.021952
39. Kawamura M, Scheinman MM, Lee RJ, Badhwar N. Left atrial appendage ligation in patients with atrial fibrillation leads to a decrease in atrial dispersion. J Am Heart Assoc. (2015) 4(5):e001581. doi: 10.1161/JAHA.114.001581
40. Sardar MR, Saeed W, Kowey PR. Antiarrhythmic drug therapy for atrial fibrillation. Heart Fail Clin. (2016) 12(2):205–21. doi: 10.1016/j.hfc.2015.08.017
41. Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol. (2020) 75(25):3105–18. doi: 10.1016/j.jacc.2020.04.065
42. Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. (2012) 125(2):381–9. doi: 10.1161/CIRCULATIONAHA.111.019927
43. Chew DS, Black-Maier E, Loring Z, Noseworthy PA, Packer DL, Exner DV, et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. (2020) 13(4):e008128. doi: 10.1161/CIRCEP.119.008128
44. Andrade JG, Deyell MW, Verma A, Macle L, Champagne J, Leong-Sit P, et al. Association of atrial fibrillation episode duration with arrhythmia recurrence following ablation: a secondary analysis of a randomized clinical trial. JAMA Netw Open. (2020) 3(7):e208748. doi: 10.1001/jamanetworkopen.2020.8748
45. Minami K, Kabata D, Kakuta T, Fukushima S, Fujita T, Shintani A, et al. U-shaped association between intraoperative net fluid balance and risk of postoperative recurrent atrial tachyarrhythmia among patients undergoing the cryo-maze procedure: an observational study. J Cardiothorac Vasc Anesth. (2021) 35(8):2392–6. doi: 10.1053/j.jvca.2020.10.023
46. Hwang SK, Yoo JS, Kim JB, Jung SH, Choo SJ, Chung CH, et al. Long-term outcomes of the maze procedure combined with mitral valve repair: risk of thromboembolism without anticoagulation therapy. Ann Thorac Surg. (2015) 100(3):840–3; discussion 843–4. doi: 10.1016/j.athoracsur.2015.02.073
47. Jin X, Pan J, Wu H, Xu D. Are left ventricular ejection fraction and left atrial diameter related to atrial fibrillation recurrence after catheter ablation?: a meta-analysis. Medicine. (2018) 97(20):e10822. doi: 10.1097/MD.0000000000010822
48. Muraru D, Caravita S, Guta AC, Mihalcea D, Branzi G, Parati G, et al. Functional tricuspid regurgitation and atrial fibrillation: which comes first, the chicken or the egg? CASE. (2020) 4(5):458–63. doi: 10.1016/j.case.2020.04.011
49. Bak M, Jeong DS, Park S-J, Park B, Seo JH, Park I, et al. Predictor of atrial fibrillation recurrence in patients who underwent a tricuspid valve operation with modified cox maze procedure. Echocardiography. (2022) 39(3):447–56. doi: 10.1111/echo.15315
50. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. (2005) 45(10):1638–43. doi: 10.1016/j.jacc.2005.02.054
51. Khiabani AJ, Schuessler RB, Damiano RJ Jr. Surgical ablation of atrial fibrillation in patients with heart failure. J Thorac Cardiovasc Surg. (2021) 162(4):1100–5. doi: 10.1016/j.jtcvs.2020.05.125
52. Yayla C, Akboga MK, Gayretli Yayla K, Ertem AG, Efe TH, Sen F, et al. A novel marker of inflammation in patients with slow coronary flow: lymphocyte-to-monocyte ratio. Biomark Med. (2016) 10(5):485–93. doi: 10.2217/bmm-2016-0022
53. Fici F, Celik T, Balta S, Iyisoy A, Unlu M, Demitkol S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. (2013) 62(4):388–93. doi: 10.1097/FJC.0b013e31829f716a
54. Uthamalingam S, Patvardhan EA, Subramanian S, Ahmed W, Martin W, Daley M, et al. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am J Cardiol. (2011) 107(3):433–8. doi: 10.1016/j.amjcard.2010.09.039
55. Silberman S, Fink D. Neutrophil to lymphocyte ratio and risk of atrial fibrillation. Am J Cardiol. (2019) 123(11):1889. doi: 10.1016/j.amjcard.2019.03.033
56. Min K, Kwon S, Cho S-Y, Choi WJ, Park S-U, Jung W-S, et al. Atrial fibrillation is strongly associated with the neutrophil to lymphocyte ratio in acute ischemic stroke patients: a retrospective study. J Clin Lab Anal. (2017) 31(2):e22041. doi: 10.1002/jcla.22041
57. Wei Y, Bao Y, Lin C, Xie Y, Luo Q, Zhang N, et al. Early recurrence after cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: mechanism and implication in long-term outcome. BMC Cardiovasc Disord. (2022) 22(1):400. doi: 10.1186/s12872-022-02816-1
58. McCabe JM, Smith LM, Tseng ZH, Badhwar N, Lee BK, Lee RJ, et al. Protracted CRP elevation after atrial fibrillation ablation. Pacing Clin Electrophysiol. (2008) 31(9):1146–51. doi: 10.1111/j.1540-8159.2008.01155.x
59. Kim YG, Boo KY, Choi J-I, Choi YY, Choi HY, Roh S-Y, et al. Early recurrence is reliable predictor of late recurrence after radiofrequency catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. (2021) 7(3):343–51. doi: 10.1016/j.jacep.2020.09.029
60. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the euro heart survey. Chest. (2010) 138(5):1093–100. doi: 10.1378/chest.10-0134
61. Nashef SAM, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. (2012) 41(4):734–45. doi: 10.1093/ejcts/ezs043.22378855
62. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European society of cardiology (ESC). Eur Heart J. (2010) 31(19):2369–429. doi: 10.1093/eurheartj/ehq278
Keywords: Cox-Maze IV procedure, atrial fibrillation, machine learning, recurrence prediction, risk factors, feature importance analysis
Citation: Jiang Z, Song L, Liang C, Zhang H, Tan H, Sun Y, Guo R and Liu L (2023) Machine learning-based analysis of risk factors for atrial fibrillation recurrence after Cox-Maze IV procedure in patients with atrial fibrillation and chronic valvular disease: A retrospective cohort study with a control group. Front. Cardiovasc. Med. 10:1140670. doi: 10.3389/fcvm.2023.1140670
Received: 9 January 2023; Accepted: 13 March 2023;
Published: 24 March 2023.
Edited by:
Marco Manca, SCImPULSE Foundation, NetherlandsReviewed by:
Taghi Aliyev, SCImPULSE Foundation, Netherlands© 2023 Jiang, Song, Liang, Zhang, Tan, Sun, Guo and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liming Liu bGl1bGltaW5namlhQGNzdS5lZHUuY24=
Specialty Section: This article was submitted to Heart Surgery, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.