- 1Department of Emergency Medicine, School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 2Department of Emergency Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan
- 3Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 4Department of Anesthesiology, Changhua Christian Hospital, Changhua, Taiwan
- 5Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 6Department of Medical Research, Chung Shan Medical University Hospital, Taichung, Taiwan
- 7Department of Microbiology and Immunology, Chung Shan Medical University, Taichung, Taiwan
Introduction: Psoriasis (PSO) is a chronic skin condition that affects a variety of disorders, especially the cardiovascular system. This study investigated the association between PSO and peripheral arterial disease (PAOD).
Methods: A retrospective cohort study design was carried out between 2000 and 2018. The exposure subject was a newly diagnosed PSO. The diagnosis of PSO was never elaborated as a comparison subject. Balanced heterogeneity of the two groups was used by propensity score matching. The cumulative incidence of PAOD between the two groups was performed using Kaplan-Meier analysis. The Cox proportional hazard model was used to measure the risk of PAOD risk hazard ratio.
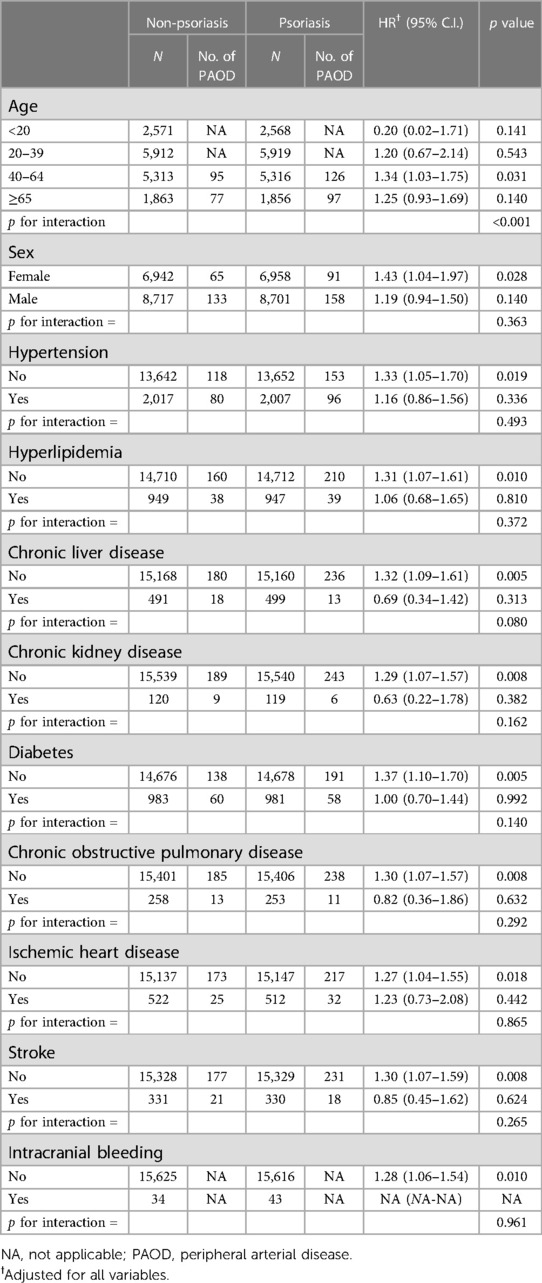
Results: After matching the 1: 1 propensity score, 15,696 subjects with PSO and the same number of subjects without the diagnosis of PSO were recruited. The PSO subject had a higher risk of PAOD than the non-PSO subject (adjusted HR = 1.25; 95% CI = 1.03-1.50). In the 40-64-year-old subgroup, the subject of PSO exhibited an increased risk of PAOD than the subject without PSO.
Conclusion: Psoriasis is associated with an increased risk of peripheral arterial disease and curative care is necessary to reduce the risk of PAOD..
Introduction
Psoriasis (PSO) is a chronic, recurrent, inflammatory disease. More than 125 million people, approximately 1%–2% of the general population worldwide, had PSO in 2020 (1, 2). However, the prevalence of PSO differs geographically. Generally, the incidence rate of PSO in Asian countries is less than in western countries. A survey in six cities in China showed a prevalence of PSO of only 0.47% (3). Although no specific survey has been conducted on the prevalence of PSO in Taiwan, a study using the National Health Insurance Research Database identified 53,761 PSO patients from a population of 23 million in 2011 (4). Therefore, the prevalence of PSO is approximately 0.24% in Taiwan. Based on its appearance in clinical presentations, PSO is classified into different forms, psoriasis vulgaris, inverse psoriasis, guttate psoriasis, pustular psoriasis, and erythrodermic psoriasis, while psoriasis vulgaris is a common form (1, 5).
Evidence showed that PSO can contribute to cardiovascular risk. For example, chronic inflammation in PSO patients increased the accumulation of visceral adipose tissue and the noncalcified burden of the coronary artery (6). PSO patients had a higher level of C-reactive protein, chemerin, fetuin-A, and osteopontin in the blood, which are predictors of cardiovascular risk (7). In addition, PSO can also promote severe vascular events such as myocardial infarction, stroke, and cardiovascular mortality (8). A higher portion of patients with PSO was found to have arterial stiffness compared to non-PSO patients (9). PSO can contribute to hypercoagulability in blood vessels to form thrombus (10).
Peripheral arterial occlusive disease (PAOD) is an atherosclerosis that usually occurs in the lower limb artery with the formation of arterial thrombosis or embolism. Initially, PAOD is not easily recognized unless intermittent claudication is evident (11). Severe PAOD can cause atherosclerotic stenosis, loss of limb, or even death (12). Ankle–brachial arterial index test, computed tomography, and magnetic resonance imaging were generally applied for the early detection of PAOD (13, 14).
Not only can PAOD develop into serious diseases, but it also increases the risk of developing other cardiovascular diseases (CVDs), such as coronary heart disease and myocardial infarction (MI) (15, 16). PSO is also associated with the risk of certain CVD, i.e., MI, hypertension, arrhythmia, and valvular disease (17, 18, 19).
Pathological changes in PSO and PAOD have certain common mechanisms based on the immune response. For example, significantly higher serum levels of cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 4 (IL-4), and interleukin 6 (IL-6) are observed in both diseases (20, 21). A β-galactoside-binding lectin, galectin-3, which is associated with cell proliferation, inflammation, and angiogenesis, plays a role in both PSO and PAOD (22, 23, 24).
Considering the increased risk of CVD and common immunological markers for PSO and PAOD, we enrolled both patients with and without PSO from the 2000 Longitudinal Health Insurance Database (LHID 2000) and investigated whether PSO influences the risk of PAOD. We hypothesized that patients with PSO would have a significantly higher risk of PAOD.
Materials and methods
Data sources
We retrieved a longitudinal health insurance database that was managed by the Health and Welfare Data Center (HWDC) of Taiwan. There are 2 million random sampling subjects from the registry of beneficiaries in the whole population for the year 2000. The database included data on outpatient, emergency, and hospitalization medical records, including medication, surgical operation, procedure, and expenditure data from 2000 to 2018. This study was certified by the institutional review board of Chung Shan Medical University Hospital (CS1-20056).
Study group and outcome
We conducted a retrospective cohort study. The study included individuals who were newly diagnosed with PSO (ICD-CM codes: 696.1, 696.2, 696.3, 696.4, 696.5, L30.5, L40.0–L40.4, L40.8, L40.9, L41.0, L41.1, L41.3–L41.5, L41.8, L41.9, L42, L44.0, L94.5) at onset time from 2002 to 2017. To ensure accuracy, patients were included if any only if their diagnosis was given by a rheumatologist or dermatologist for ≥3 times outpatient visits or ≥1 inpatient stay. The index date was defined as the date of initial diagnosed PSO. In order to ensure that all instances of PAOD would be new onset PAOD, patients with a diagnosis of PAOD before the index date were excluded. The non-PSO group was defined as never diagnosed psoriasis and similar disorders (ICD-CM codes: 696, L40, L41, L42, L44, L45, L30.5, L94.5) from 2000 to 2018.
The primary outcome was a diagnosis of PAOD (ICD-CM codes: 443.8, 443.9, 444, I73.81, I73.89, I73.9, I74.01, I74.09, I74.11, I74.2, I74.3, I74.4, I74.5, I74.8, I74.9, I79.1, I79.8). All samples were traced until the outcome of interest, death, or the end of 2018, whichever came first.
Covariates and matching
The demographic characteristics included in the analysis were age and sex and the comorbidities of hypertension (ICD-CM codes: 401–405, I10–I15), hyperlipidemia (ICD-CM codes: 272, E78), chronic liver disease (ICD-CM codes: 571, K70, K73, K74, K75.4, K75.81, K76.0, K76.89, K76.9), chronic kidney disease (ICD-CM codes: 585, N184, N185, N186, N189), diabetes (ICD-CM codes: 250, E10, E11, E13), chronic obstructive pulmonary disease (ICD-CM codes: 491, 492, 496, J41–J44), ischemic heart disease (ICD-CM codes: 410–414, I20–I25), stroke (ICD-CM codes: 433–438, I63, I64), and intracranial bleeding (ICD-CM codes: 430–432, I60–I62). An individual was defined as having these comorbidities if and only if they received a diagnosed within 1 year before the index date for ≥3 outpatient visits or ≥1 inpatient stay.
First, a 1:4 age and sex matching was performed to ensure an identical index date for any given PSO participant with their non-PSO counterparts. If the non-PSO group had a previous diagnosis of PAOD when matched to the corresponding index date, that non-PSO group would be excluded and rematched with a new comparison group. Subsequently, the propensity score matching (PSM) was carried out by age, sex, and comorbidities, including the presence of hypertension, hyperlipidemia, chronic liver disease, chronic kidney disease, diabetes, chronic obstructive pulmonary disease, ischemic heart disease, stroke, and intracranial bleeding comorbidities. The propensity score was calculated using a binary logistic regression. The binary variable was the diagnosis of PAOD. PSM ensured the balanced heterogeneity of the two groups.
Statistical analysis
The comparison of the PSO and non-PSO groups was performed using the absolute standardized difference (ASD). An ASD <0.1 indicated similarity in the characteristics of both groups (25). A Poisson regression model and Kaplan–Meier analysis were used to measure the relative risk (RR) and the cumulative incidence of PAOD between the two groups. To measure independent risk factors for PAOD, hazard ratios were evaluated using a Cox proportional hazards model. Moreover, sensitivity analysis was conducted in this study to reduce potential confounding factors by controlling for medications, including antiplatelet, anticoagulant, methotrexate, sulfasalazine, and corticosteroids. The statistical software was SAS version 9.4 (SAS Institute, NC, USA).
Results
Demographic characteristics
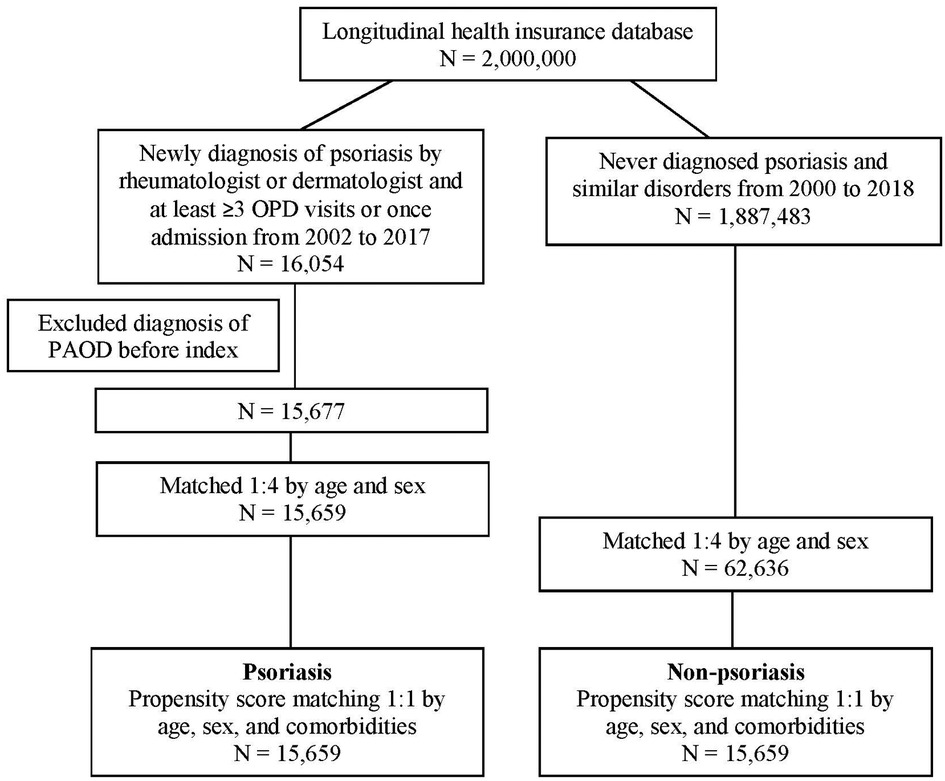
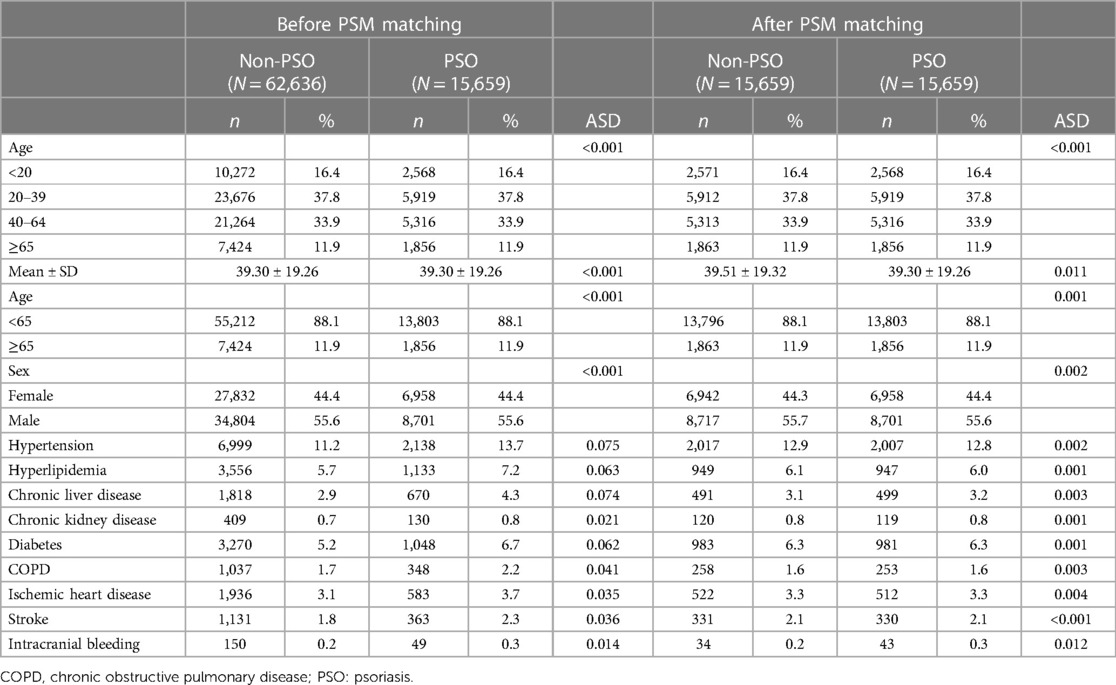
In total, 16,054 patients with PSO and 1,887,483 patients who had never been diagnosed with PSO were recruited. After excluding patients with a diagnosis of PAOD before the index date, 15,677 patients were in the PSO cohort. Subsequently, 1:1 PSM by age, sex, and comorbidity was analyzed. Finally, 15,659 patients in the PSO cohort and 15,659 patients without PSO in the matched cohort were analyzed for their risk of PAOD (Figure 1). The mean age of patients with and without PSO was 39.30 ± 19.26 and 39.51 ± 19.32, respectively. The male/female ratio was similar in both groups (55.7%:44.3% in the non-PSO group; 55.6%:44.4% in the PSO group). Before PSM, the PSO group compared with non-PSO group had slightly higher proportion in hypertension, hyperlipidemia, chronic liver disease, chronic kidney disease, diabetes, COPD, ischemic heart disease, stroke, and intracranial bleeding. After PSM, all ASD were <0.1. It represented that age, sex, and comorbidities were similar between both groups (Table 1).
Incidence of PAOD
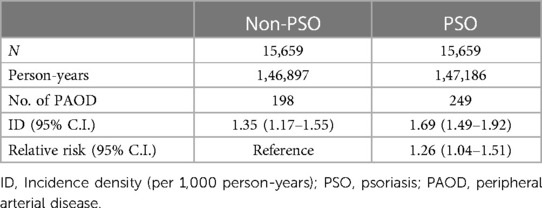
Subsequently, we used Poisson regression to compare the relative risk of PAOD between the two groups. The incidence density of the PSO group and the non-PSO group was 1.69 (95% C.I. = 1.49–1.92) and 1.35 (95% C.I. = 1.17–1.55), respectively. The relative risk of developing PAOD in PSO group compared to non-PSO group was 1.26 (95% C.I. = 1.04–1.51) (Table 2). The mean time of developing PAOD with and without PSO was 5.36 and 5.91 years. The log-rank test (p = 0.017) showed that the cumulative incidence of PAOD was higher in the PSO group than in the non-PSO group (Figure 2).
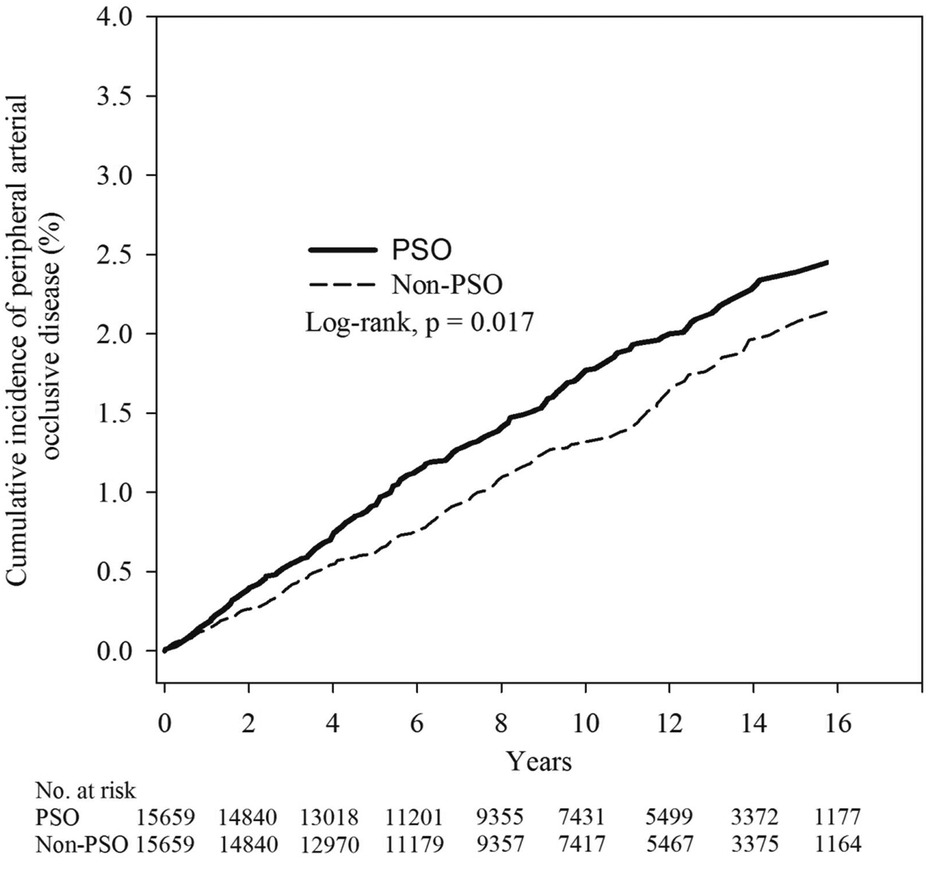
Figure 2. Kaplan–Meier curves of the cumulative proportions of PAOD in psoriasis and non- psoriasis patients.
Risk of PAOD between PSO and non-PSO groups
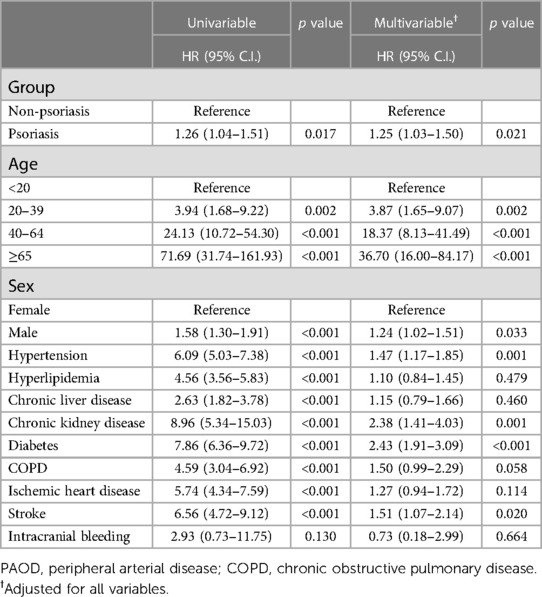
The PSO group had an increased risk of PAOD (adjusted HR = 1.25; 95% C.I. = 1.03–1.50). Patients aged 20 to 39 years (adjusted HR = 3.87 years; 95% C.I. = 1.65–9.07 years), 40 to 64 years (adjusted HR = 18.37 years; 95% C.I. = 8.13–41.49 years) and ≥65 years (adjusted HR = 36.70 years; 95% C.I. = 16.00–84.17 years) had a higher risk of PAOD. Men had an increasing risk of PAOD than women (adjusted HR = 1.24; 95% C.I. = 1.02–1.51). Furthermore, comorbidities such as hypertension, chronic kidney disease, diabetes, and stroke were also risk factors for PAOD (Table 3). In the 40 to 64-year-old subgroup, PSO had a significant interaction with PAOD risk in a subgroup analysis (adjusted HR = 1.34; 95% C.I. = 1.03–1.75; p for interaction < 0.001; Table 4). In a sensitivity analysis, after controlling for methotrexate, corticosteroids, anticoagulants, and antiplatelet agents, the PSO group had an increased risk of PAOD (adjusted HR = 1.52; 95% C.I. = 1.23–1.88) (Supplementary Table S1).
Discussion
We enrolled 16,054 patients with newly diagnosed PSO from LHID 2000 from 2002 to 2017 in this study. We analyzed the association between PSO and the risk of PAOD using PSM. Our results showed that patients with PSO had a higher risk of acquiring PAOD than the non-PSO group. However, few studies have evaluated the risk association between PSO and PAOD. A case report described the co-occurrence of PSO with occlusive vascular disease and even gangrene on the lower limb in one patient (26). A case–control study demonstrated that PSO was an independent risk factor for certain cerebrovascular and peripheral vascular diseases (27). Because PAOD involves atherosclerosis of the lower extremities, here is discussed the association between PSO and atherosclerotic conditions other than PAOD.
PSO has been shown to increase the risk of CVD such as myocardial infarction, hypertension, valvular disease, and arrhythmia (28–34). PSO and atherosclerosis share certain common inflammatory pathways, such as endothelial dysfunction, cytokine dysregulation, platelet upregulation, and dyslipidemia (17, 35). The available body of evidence suggests the pathogenesis of vascular damage. On the contrary, the administration of TNF, IL-17A, and IL-12/23p40 inhibitors for the treatment of PSO was shown to lower the risk of CVD (19). PSO may have similar mechanisms that increase the risk of PAOD in CVDs.
We also found that the PSO group had an increased risk of PAOD with increasing age using the Cox proportional hazards model. A subgroup analysis indicated that patients aged 40–64 years had an increased risk of PAOD in the PSO group. A cross-sectional study showed that, especially for symptomatic PAOD, prevalence rates were higher in elderly groups between 50 and >70 years (36). Elderly patients with chronic obstructive pulmonary disease (COPD) also exhibited an increased risk of PAOD (37). The reasons for the increased prevalence with age may be associated with poor peripheral artery drainage and tissue loss. Previous studies showed that patients had comorbidities such as hypertension, hypercholesterolemia, chronic kidney disease, history of heart disease, COPD, and CVDs that also increase the risk of PAOD (38). In this study, we also showed that age was a risk factor for PAOD in PSO patients.
In addition to age, sex was a risk factor for PAOD. Male patients had a higher risk of PAOD than female patients. Sex-based differences in PAOD have been demonstrated in different studies. A study found a close relationship between visceral adiposity index and peripheral artery disease (PAD) in normal weight adults with hypertension among men but not among women (39). In contrast, a study of sex differences in critical limb ischemia, a serious form of PAD, indicated that women had a higher incidence of adjusted in-hospital mortality, bleeding complications, and discharge from facilities compared to men. This may be because women had a higher prevalence of obesity, hypertension, heart failure, and prior stroke than men (40). Similar findings indicated that women showed clinical signs of popliteal artery lesions at an older age than men (41). Factors affecting sex differences in PAOD include environmental, pathophysiologic, genetic and epigenetic, and treatment-related factors (42).
PSO may increase the risk of different diseases. The development of uveitis after PSO diagnosis has been reported (43). PSO increases the risk of organ-related comorbidities, such as CVD and renal and liver diseases (33). This may be due to predisposition genes and immunological mechanisms that PSO has in common with these diseases. For example, a review found that PSO and inflammatory bowel disease shared at least 11 common genes (44).
Although both diseases have common inflammatory pathways, the increased risk of PAOD with PSO may be due to common factors for both diseases, such as the overproduction of pro-inflammatory factors. PSO causes inflammation of the blood vessels, which is a critical step in initiating atherogenesis and the development of atherosclerosis (45). Pro-inflammatory cytokines and chemokines are involved in this complex process. For example, TNF-α is a pro-inflammatory factor that increases in patients with PSO and PAD (46). Another mechanism of vascular inflammation in patients with PSO involves activation of the inflammasome signaling pathway. In an ex vivo study, up-regulation of a series of inflammatory transcripts such as IL-1β, CXCL1, CCL3, CXCL-8, CXCL10, COX-2, ICAM-1, lymphotoxin beta, and VCAM-1 was found in endothelial cells of the brachial vein in patients with PSO. Blood levels of IL-6 were also elevated in these patients (47). CCL20, IL-17A, and IL-6 are critical pro-inflammatory factors that affect vascular health and contribute to endothelial inflammation (48). Elevation of these transcripts promotes atherosclerosis and increases CVD risk and can also increase the risk of PAOD in PSO patients.
Regardless of the actions of pro-inflammatory factors in blood vessels, serum markers can also be factors in PSO and PAOD. For example, galectin-3, which belongs to a member of galectins, a group of β-galactoside-binding proteins that exhibit biological functions such as cell–cell interaction, cell proliferation, and differentiation. Galectin-3 is postulated to be involved in the pathogenesis of a series of diseases, including the formation of psoriatic plaques and arthrosclerosis (49). Furthermore, a high level of galectin-3 was detected in the serum of PSO patients (24). Galectin-3 is also a potential cardiovascular inflammatory factor and mediator of arthrosclerosis (23). Galectin-3 and hs-CRP are involved in fibrosis in PAD (22). Based on the above evidence, galactin-3 may be involved in the pathogenesis in both PSO and arthrosclerosis and supported our finding that PSO increases the risk of PAOD.
This study still has certain limitations. First, LHID does not provide information about the severity or different forms of PSO, which may affect the risk and onset of PAOD. Second, the database does not provide data on whether the lesion is located in the lower extremity. However, this could directly influence the risk of PAOD. Third, the database did not provide information on smoking, alcohol consumption, physical activity, diet, inflammatory markers, disease progression, disease markers, body mass index (BMI), and other areas were not provided by the database; these factors are potential confounders in this study. Regardless, by using the propensity score matching in the age, sex, and comorbidities, we can reduce the potential heterogeneity of the two groups.
Conclusion
In conclusion, our study demonstrates that having PSO significantly increases the risk of PAOD. Patients with PSO should be especially careful to prevent PAOD and other cardiovascular diseases. Additionally, age is a risk factor for PAOD, especially in patients with PSO 40–64 years of age.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The study was approved by the ethical review board of Chung Shan Medical University Hospital (CS1-20056). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CY, LY and CC: conceived and designed the experiments; SY, BW and YW: analyzed the data; CY, LY and CC: wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This study was partly based on data from the NHIRD provided by the NHI Administration, Ministry of Health and Welfare, and managed by the National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the NHI Administration, Ministry of Health and Welfare, or National Health Research Institutes. This study was funded by Chung Shan Medical University Hospital (CSH-2022-C-010 and CSH-2023-C-042).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1136540/full#supplementary-material
References
1. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. (2020) 323:1945–60. doi: 10.1001/jama.2020.4006
2. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. (2019) 20:1475. doi: 10.3390/ijms20061475
3. Ding X, Wang T, Shen Y, Wang X, Zhou C, Tian S, et al. Prevalence of psoriasis in China: a population-based study in six cities. Eur J Dermatol. (2012) 22:663–7. doi: 10.1684/ejd.2012.1802
4. Tsai TF, Wang TS, Hung ST, Tsai PI, Schenkel B, Zhang M, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. (2011) 63:40–6. doi: 10.1016/j.jdermsci.2011.03.002
6. Sajja A, Abdelrahman KM, Reddy AS, Dey AK, Uceda DE, Lateef SS, et al. Chronic inflammation in psoriasis promotes visceral adiposity associated with noncalcified coronary burden over time. JCI Insight. (2020) 5:e142534. doi: 10.1172/jci.insight.142534
7. Borsky P, Fiala Z, Andrys C, Beranek M, Hamakova K, Kremlacek J, et al. C-reactive protein, chemerin, fetuin-A and osteopontin as predictors of cardiovascular risks in persons with psoriasis vulgaris. Physiol Res. (2021) 70:383. doi: 10.33549/physiolres.934654
8. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. (2017) 18:2211. doi: 10.3390/ijms18102211
9. Yiu KH, Yeung CK, Chan HT, Wong RM, Tam S, Lam KF, et al. Increased arterial stiffness in patients with psoriasis is associated with active systemic inflammation. Br J Dermatol. (2011) 164:514–20. doi: 10.1111/j.1365-2133.2010.10107.x
10. Visser MJ, Tarr G, Pretorius E. Thrombosis in psoriasis: cutaneous cytokine production as a potential driving force of haemostatic dysregulation and subsequent cardiovascular risk. Front Immunol. (2021) 12:688861. doi: 10.3389/fimmu.2021.688861
11. Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for vascular surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. (2015) 61:2s–41s. doi: 10.1016/j.jvs.2014.12.009
12. Dissemond J. Peripheral occlusive arterial disease. Braun Falcó s Dermatol. (2020):1–10. doi: 10.1007/978-3-662-58713-3_65-2
13. Aschwanden M, Partovi S, Jacobi B, Fergus N, Schulte A-C, Robbin MR, et al. Assessing the end-organ in peripheral arterial occlusive disease—from contrast—enhanced ultrasound to blood-oxygen-level-dependent MR imaging. Cardiovasc Diagn Ther. (2014) 4:165. doi: 10.3978/j.issn.2223-3652.2014.03.02
14. Tanaka R, Yoshioka K, Takagi H, Schuijf J, Arakita K. Novel developments in non-invasive imaging of peripheral arterial disease with CT: experience with state-of-the-art, ultra-high-resolution CT and subtraction imaging. Clin Radiol. (2019) 74:51–8. doi: 10.1016/j.crad.2018.03.002
15. Subherwal S, Patel MR, Kober L, Peterson ED, Bhatt DL, Gislason GH, et al. Peripheral artery disease is a coronary heart disease risk equivalent among both men and women: results from a nationwide study. Eur J Prev Cardiol. (2015) 22:317–25. doi: 10.1177/2047487313519344
16. Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, et al. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index: uS preventive services task force recommendation statement. JAMA. (2018) 320:177–83. doi: 10.1001/jama.2018.8357
17. Doumas M, Katsiki N, Papademetriou V. Psoriasis and cardiovascular disease: two sides of the same coin? Angiology. (2018) 69:5–9. doi: 10.1177/0003319717702303
18. Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
19. Weber B, Merola JF, Husni ME, Di Carli M, Berger JS, Garshick MS. Psoriasis and cardiovascular disease: novel mechanisms and evolving therapeutics. Curr Atheroscler Rep. (2021) 23:67. doi: 10.1007/s11883-021-00963-y
20. Yan J-T, Wang Q-G, Liu X-Q, Li B, Ru Y, Hong S, et al. The immune status of patients with psoriasis vulgaris of blood-stasis syndrome and blood-dryness syndrome: a qualitative evidence synthesis. Ann Palliat Med. (2020) 9:1382–95. doi: 10.21037/apm-19-432
21. Saenz-Pipaon G, Martinez-Aguilar E, Orbe J, González Miqueo A, Fernandez-Alonso L, Paramo JA, et al. The role of circulating biomarkers in peripheral arterial disease. Int J Mol Sci. (2021) 22:3601. doi: 10.3390/ijms22073601
22. Ding N, Yang C, Ballew SH, Kalbaugh CA, McEvoy JW, Salameh M, et al. Fibrosis and inflammatory markers and long-term risk of peripheral artery disease: the ARIC study. Arterioscler Thromb Vasc Biol. (2020) 40:2322–31. doi: 10.1161/ATVBAHA.120.314824
23. Gao Z, Liu Z, Wang R, Zheng Y, Li H, Yang L. Galectin-3 is a potential mediator for atherosclerosis. J Immunol Res. (2020) 2020:5284728. doi: 10.1155/2020/5284728
24. Hayran Y, Allı N, Akpınar Ü, Öktem A, Yücel Ç, Fırat Oguz E, et al. Serum galectin-3 levels in patients with psoriasis. Int J Clin Pract. (2021) 75:e14545. doi: 10.1111/ijcp.14545
25. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28:3083–107. doi: 10.1002/sim.3697
26. Rao R, Prabhu S, Sripathi H. Co-occurrence of psoriasis and occlusive vascular disease. Indian J Dermatol Venereol Leprol. (2008) 74:399–401. doi: 10.4103/0378-6323.42923
27. Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. (2009) 145:700–3. doi: 10.1001/archdermatol.2009.94
28. Vizzardi E, Raddino R, Teli M, Gorga E, Brambilla G, Dei Cas L. Psoriasis and cardiovascular diseases. Acta Cardiol. (2010) 65:337–40. doi: 10.2143/AC.65.3.2050351
29. Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: a systematic review of the literature. J Gen Intern Med. (2011) 26:1036–49. doi: 10.1007/s11606-011-1698-5
30. Choudhary S, Patel R, Pradhan D, Deval R, Singh H, Thomas G, et al. Psoriasis and cardiovascular disorders: association or epiphenomenon? Meta-analysis of observational studies. Biotech. (2020) 10:1–15. doi: 10.1007/s13205-020-2089-6
31. Duan X, Liu J, Mu Y, Liu T, Chen Y, Yu R, et al. A systematic review and meta-analysis of the association between psoriasis and hypertension with adjustment for covariates. Medicine. (2020) 99:e19303. doi: 10.1097/MD.0000000000019303
32. Liu L, Cui S, Liu M, Huo X, Zhang G, Wang N. Psoriasis increased the risk of adverse cardiovascular outcomes: a new systematic review and meta-analysis of cohort study. Front Cardiovasc Med. (2022) 9:829709. doi: 10.3389/fcvm.2022.829709
33. Tang X, Chen L. The risk of organ-based comorbidities in psoriasis: a systematic review and meta-analysis. An Bras Dermatol. (2022) 97:612–23. doi: 10.1016/j.abd.2021.10.007
34. Ahlehoff O, Gislason GH, Charlot M, Jørgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: a danish nationwide cohort study. J Intern Med. (2011) 270:147–57. doi: 10.1111/j.1365-2796.2010.02310.x
35. Frieder J, Ryan C. Psoriasis and cardiovascular disorders. G Ital Dermatol Venereol. (2016) 151:678–93. PMID: 27627099.27627099
36. Hooi JD, Stoffers HE, Kester AD, Rinkens PE, Kaiser V, van Ree JW, et al. Risk factors and cardiovascular diseases associated with asymptomatic peripheral arterial occlusive disease. The limburg PAOD study. Peripheral arterial occlusive disease. Scand J Prim Health Care. (1998) 16:177–82. doi: 10.1080/028134398750003142
37. Liao KM, Kuo LT, Lu HY. Increased risk of peripheral arterial occlusive diseases in patients with chronic obstructive pulmonary disease: a nationwide study in Taiwan. Int J Chron Obstruct Pulmon Dis. (2019) 14:1455–64. doi: 10.2147/COPD.S202029
38. Smet N, Fourneau I, Roeleveld H, Boonman-de Winter L, Schraepen C, Favoreel M, et al. Age-Dependent outcome of first-line endovascular and surgical revascularization strategies in chronic limb-threatening ischemia. Ann Vasc Surg. (2022) 85:133–45. doi: 10.1016/j.avsg.2022.03.021
39. Shi Y, Yu C, Hu L, Li M, Zhou W, Wang T, et al. Visceral adiposity index and sex differences in relation to peripheral artery disease in normal-weight adults with hypertension. Biol Sex Differ. (2022) 13:22. doi: 10.1186/s13293-022-00432-4
40. Elbadawi A, Barssoum K, Megaly M, Rai D, Elsherbeeny A, Mansoor H, et al. Sex differences in trends and in-hospital outcomes among patients with critical limb ischemia: a nationwide analysis. J Am Heart Assoc. (2021) 10:e022043. doi: 10.1161/JAHA.121.022043
41. Özdemir-van Brunschot DMD, Torsello GB, Litterscheid S, Berchiolli R, Troisi N, Torsello GF. Sex differences in endovascular treatment of isolated popliteal lesions. Cardiovasc Intervent Radiol. (2022) 45:1267–75. doi: 10.1007/s00270-022-03216-w
42. Pabon M, Cheng S, Altin SE, Sethi SS, Nelson MD, Moreau KL, et al. Sex differences in peripheral artery disease. Circ Res. (2022) 130:496–511. doi: 10.1161/CIRCRESAHA.121.320702
43. Tseng S-T, Yao T-C, Huang J-L, Yeh K-W, Hwang Y-S. Clinical manifestations in uveitis patients with and without rheumatic disease in a Chinese population in Taiwan. J Microbiol Immunol Infect. (2017) 50:798–804. doi: 10.1016/j.jmii.2015.10.007
44. Cottone M, Sapienza C, Macaluso FS, Cannizzaro M. Psoriasis and inflammatory bowel disease. Dig Dis. (2019) 37:451–7. doi: 10.1159/000500116
45. Joshi AA, Lerman JB, Dey AK, Sajja AP, Belur AD, Elnabawi YA, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol. (2018) 3:949–56. doi: 10.1001/jamacardio.2018.2769
46. Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-α Antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. (2017) 137:1638–45. doi: 10.1016/j.jid.2017.02.977
47. Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. (2019) 39:787–98. doi: 10.1161/ATVBAHA.118.312246
48. Elnabawi YA, Garshick MS, Tawil M, Barrett TJ, Fisher EA, Lo Sicco K, et al. CCL20 In psoriasis: a potential biomarker of disease severity, inflammation, and impaired vascular health. J Am Acad Dermatol. (2021) 84:913–20. doi: 10.1016/j.jaad.2020.10.094
Keywords: psoriasis, peripheral arterial occlusive diseases, risks, retrospective cohort study, propensity score matching
Citation: Yeh C-B, Yeh L-T, Yang S-F, Wang B-Y, Wang Y-H and Chan C-H (2023) Association between psoriasis and peripheral artery occlusive disease: a population-based retrospective cohort study. Front. Cardiovasc. Med. 10:1136540. doi: 10.3389/fcvm.2023.1136540
Received: 26 January 2023; Accepted: 29 May 2023;
Published: 12 June 2023.
Edited by:
Karla Bianca Neves, University of Strathclyde, United KingdomReviewed by:
Renee Oliveira Da Silva, Loma Linda University, United StatesTiago J. Costa, Johns Hopkins University, United States
© 2023 Yeh, Yeh, Yang, Wang, Wang and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Ho Chan Y2hpaG9AY3NtdS5lZHUudHc=
†These authors have contributed equally to this work
 Chao-Bin Yeh
Chao-Bin Yeh Liang-Tsai Yeh
Liang-Tsai Yeh Shun-Fa Yang
Shun-Fa Yang Bo-Yuan Wang1,2,3
Bo-Yuan Wang1,2,3 Yu-Hsun Wang
Yu-Hsun Wang Chi-Ho Chan
Chi-Ho Chan