
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 17 March 2023
Sec. Cardiovascular Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1133724
Background: Numerous studies have shown that hyperuricemia (HUA) is associated with cardiovascular and renal outcomes, but few studies specifically explored the effect of age on this relationship. Therefore, our study aimed to explore the relationship between HUA and other cardiometabolic risk factors in different age groups.
Methods: This cross-section study used the data from Survey on uric acid in Chinese subjects with essential hypertension (SUCCESS). We performed multivariate logistic regressions in different age groups.
Results: After adjusting for potential confounders, among young and middle-aged adults less than 60, HUA was associated with higher body mass index (BMI, adjusted OR = 1.114, 95% CI: 1.057–1.174), higher fasting blood glucose (FBG, adjusted OR = 1.099, 95% CI: 1.003–1.205), triglycerides (TG, adjusted OR = 1.425, 95% CI: 1.247–1.629), higher low-density lipoprotein cholesterol (LDL-C, adjusted OR = 1.171, 95% CI: 1.025–1.337), and lower estimated glomerular filtration rate (eGFR, adjusted OR = 0.992, 95% CI: 0.988–0.996). Among elderly adults 60 years or older, HUA was associated with higher SBP (adjusted OR = 1.024, 95% CI: 1.005–1.042), higher TG (adjusted OR = 1.716, 95% CI: 1.466–2.009), and higher LDL-C (adjusted OR = 1.595, 95% CI: 1.366–1.863).
Conclusion: HUA is associated with more cardiometabolic risk factors in younger adults with hypertension (HT). Comprehensive management of HT with HUA is needed in clinical settings.
Hyperuricemia (HUA) is a metabolic disorder caused by abnormal purine catabolism and urate excretion via urate transporters (1, 2). Numerous epidemiological studies have explored the association between HUA and cardiovascular and renal outcomes (3, 4). It is suggested that HUA is associated with hypertension (HT) (5), cardiovascular death (6), diabetes (7), chronic kidney disease (CKD) (8) and stroke (9).
It is noteworthy that previous studies have shown the age-differential relationship between HUA and HT (10, 11) or CKD (12), which provides a new idea for studying the mechanism of uric acid (UA)-related metabolic disorders and the individualized management of HUA.
However, most studies adjusted age as a covariate in the analysis, and few studies specifically explored the effect of age on the relationship between elevated UA and other cardiometabolic risk factors, such as dyslipidemia, obesity, and glucose metabolism disorders in hypertensive population. Therefore, we aimed to explore the relationship between HUA and other cardiometabolic risk factors in different age groups.
The data analyzed in this article came from SUCCESS. It was a nationwide cross-sectional study, which included adult hypertensive patients from 17 provinces and cities across. Patients who took more than one oral antihypertensive drug and those who had gout or received urate-lowering treatment were excluded. Details of the research proposal have been published elsewhere (13).
In this study, only individuals from Beijing (n = 4,126) were included, and individuals with error values (n = 90), missing fundamental values (n = 49), and outliers (n = 64) were excluded. Finally, 3,923 individuals were analyzed. They were divided into two groups based on age, the young and middle-aged group (under 60 years) and the elderly group (60 years or older).
All participants provided written informed consent, and the Peking University First Hospital Central Institutional Review Board approved the study protocol.
Each participant's clinical information, including age, sex, education level, and medication history, was collected using standard questionnaires by the trained physicians. The education level was divided into junior high school or below, senior high school, junior college, and undergraduate or above. Medication history included the use of antihypertensive agents and aspirin.
The physical examination information of participants was also measured and calculated by professional doctors in the clinic room, including height (cm), weight (kg), waist circumference (cm), body mass index (BMI, kg/m2), SBP (mmHg), and diastolic blood pressure (DBP, mmHg).
All blood samples of the subjects were taken 8 h of fasting, and parameters such as FBG, total cholesterol (TC), LDL-C, high-density lipoprotein cholesterol (HDL-C), TG, UA, and serum creatinine (SCr) were obtained through routine biochemical tests. The eGFR was calculated according to the Cockcroft-Gault equation. This study defined HUA as uric acid of more than 420 µmol/L in men or 360 µmol/L in women. All individuals were divided into HUA and non-HUA groups.
Continuous variables were described as mean ± standard deviation (SD), and categorical variables were described as quantity (frequency or percentage). The t-test and the Chi-square test were used to compare the variables between different groups (young and middle-aged group and elderly group, HUA group and non-HUA group). Then we conducted multivariate logistic regressions in different age groups, adjusting for age, sex, education level, aspirin use, and antihypertensive agents by including these factors into the regression equation at the same time. All the analysis was completed with IBM SPSS for windows, version 24.0, and statistical significance was defined as p value of <0.05.
A total of 3,923 hypertensive adults were analyzed, including 2,240 adults in the young and middle-aged group and 1,683 adults in the elderly group. The demographic characteristics, medications and cardiometabolic profiles are shown in Tables 1, 2. The average age of the two groups was 50.3 and 68.3 years old, respectively.
Compared with the elderly group, the young and middle-aged group had a higher proportion of males, a higher education level (junior college and undergraduate or above), and a lower proportion used aspirin and antihypertensive agents.
Regarding cardiometabolic profiles, the levels of SBP and DBP in the young and middle-aged group were significantly higher than in the elderly group. However, waist circumference, FBG, TC, and TG levels were significantly lower. There was no significant difference in BMI, UA, LDL-C, HDL-C, SCr, and eGFR between the two groups.
The demographic characteristics, medications and cardiometabolic profiles of hypertensive adults with and without HUA in different age groups are presented in Tables 3, 4.
The young and middle-aged hypertensive adults with HUA had a higher proportion of females and used aspirin and a lower proportion of used antihypertensive agents than adults without HUA. What's more, the levels of SBP, BMI, UA, FBG, TG, LDL-C, HDL-C, and SCr were higher, and the level of eGFR was lower than adults without HUA.
Similar patterns were observed in elderly adults. Among adults with HUA, the proportion of females and the use of aspirin and the levels of SBP, UA, FBG, TG, LDL-C, and HDL-C were higher, and the proportion used antihypertensive agents was lower than adults without HUA. Unlike the young and middle-aged adults, the adults with HUA had higher TC levels than those without HUA in the elderly group. Moreover, there was no significant difference in BMI, Scr, and eGFR between adults with HUA and without HUA.
We conducted multivariate logistic regressions to explore the relationship between HUA and other cardiometabolic risk factors in the two groups (Figure 1 and Supplementary Table S1).
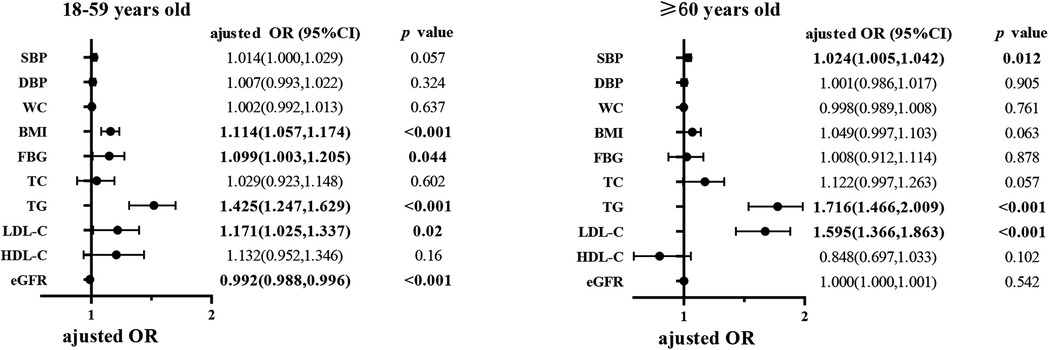
Figure 1. Multivariate logistic regression analysis of HUA in different age groups. The Odds ratios were adjusted by including age, sex, education level, use of aspirin, use of antihypertensive agents and all the above cardiometabolic factors into the regression equation at the same time. HUA, hyperuricemia; OR, odds ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; BMI, body mass index; FBG, fasting blood glucose; TC, total cholesterol; TG, triglycerides; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate. The bold value represents that the p < 0.05, and its corresponding mean ± standard deviation in table 1-4 or adjusted OR (95%CI) in figure 1.
Among young and middle-aged adults, HUA was associated with higher BMI (adjusted OR = 1.114, 95% CI: 1.057–1.174), higher FBG (adjusted OR = 1.099, 95% CI: 1.003–1.205), higher TG (adjusted OR = 1.425, 95% CI: 1.247–1.629), higher LDL-C (adjusted OR = 1.171, 95% CI: 1.025–1.337), and lower eGFR (adjusted OR = 0.992, 95% CI: 0.988–0.996), after adjusting for age, sex, education level, use of aspirin, and use of antihypertensive agents.
Among elderly adults, higher SBP (adjusted OR = 1.024, 95% CI: 1.005–1.042), higher TG (adjusted OR = 1.716, 95% CI: 1.466–2.009), and higher LDL-C (adjusted OR = 1.595, 95% CI: 1.366–1.863) were associated with HUA. However, unlike the young and middle-aged adults, BMI, FBG, and eGFR did not seem to relate to HUA among elderly hypertensive adults after adjusting for other potential confounders.
We found that HUA is closely associated with higher blood lipid profiles (containing TG and LDL-C), FBG, BMI, and lower eGFR in young and middle-aged hypertensive adults in Beijing population. In elderly hypertensive adults, HUA was associated with higher SBP, TG, and LDL-C. HUA was associated with more cardiometabolic risk factors in young and middle-aged hypertensive adults than in the elderly.
A series of epidemiological studies (14) have shown the association of HUA with cardiovascular diseases and risk factors like high LDL cholesterol and hypertriglyceridemia (15). A previous study has also shown that younger men with HUA had higher blood pressure (BP; a major risk factor for CVD) than older men (16). It is still unclear why agents that lower serum UA may not lower BP or may lower BP in only a select population (17). Our study finds the association of HUA with HT jointly predisposes young and middle-aged people to more cardiovascular risk factors than elderly people with HT and HUA. The mechanism of how HUA and HT jointly increase the risk of cardiovascular disease is unknown. HUA was shown to cause HT and renal injury in the rat via a crystal-independent mechanism, with stimulation of the renin-angiotensin system (RAS) and inhibition of neuronal NO synthase (18). HUA can cause thickening of the preglomerular arteries with smooth muscle cell proliferation and increase renal renin and COX-2 expression (19, 20) resulting in elevation of blood pressure in hyperuricemic rats that was reduced after allopurinol treatment (17). Dysregulation of the immune system by induction of COX-2, interleukin-1β expression and generation of superoxide radical (O2−) by xanthine oxidoreductase (XOR) in HUA (21) and impaired renal function (reduced GFR) (19) together constitute a risk factor for metabolic syndrome. Metabolic syndrome and cardiovascular diseases are epidemiologically and pathophysiologically intertwined.
Several studies have found the difference in association of HUA and other risk indicators between young people and the elderly, but few study has focused on HUA and cardiometabolic risk factors in hypertensive population. Brand et al. (22) found that the association between UA and the risk of coronary heart disease and future diabetes diminished with advanced age. A study by Sundström et al. (23) indicated that the relationship between elevated serum UA and HT wakened with increasing follow-up time by comparing previous cohort studies. A prospective nested Case-control study (10) demonstrated that elevated serum UA was significantly associated with HT in men <60 years after adjusting for other metabolic factors, whereas it was not significantly associated with HT in men ≥60 years. An 8-year prospective cohort study (24) recently suggested that HUA was more strongly associated with HT and hypertriglyceridemia in young adults. In addition, a meta-analysis (12) of 15 cohorts with 99,205 individuals specifically explored the effect of age on the association between UA and CKD in subgroup analyses. The results showed that HUA was significantly associated with CKD among adults younger than 60 years of age (RR = 1.26, p value = 0.022). In contrast, no significant association was observed in adults aged 60 years or older (RR = 1.04, p value = 0.409).
In this study, we found that HUA is closely associated with more cardiometabolic risk factors in hypertensive young and middle-aged Beijing people than hypertensive elderly. Age-related differences in inflammation and hemostasis response (25) might explain why HUA is closely correlated with more cardiometabolic risk factors in young and middle-aged people, as in younger age, critical illness is associated with a sharp increase in inflammation and hemostasis reactions (25) although along with a quick resolution. On the other hand, HUA-associated activation of RAS and insulin resistance (26, 27), which are pronounced in younger hypertensive people, might contribute to more cardiometabolic disorders in this population. Hypertension in elderly is mainly attributable to increased vascular stiffness, salt sensitivity and reduced renal function (10, 18, 28), and in that stage, hypertension is mainly driven by the kidney, and lowering UA levels is no longer protective (18, 28).
This study has several limitations. First, this study is cross-sectional, the information on correlation of the trajectory of UA and other risk factors with cardiovascular diseases are lacking, and the causal relationship cannot be determined. Second, this study only included the hypertensive population in Beijing, so we should be cautious when extending the conclusion to a broader population. Third, although we have included many demographic and clinical parameters in the analysis, more factors with predictive potential or affecting the correlation may need to be considered, such as lifestyles, and the use of lipid -lowering drugs, especially among young and middle-aged people.
In fact, the causal role of HUA for cardiovascular diseases is yet to be proven. The current convincing evidence for the causal role of UA exists only for gout and nephrolithiasis. More basic and clinical trials are needed to clarify the relationship between HUA and cardiovascular disease, and to determine the benefits of lowering UA levels in preventing or treating cardiovascular diseases. Besides HUA, the levels of testosterone (27) in male, estrogen in female (29) and melatonin (30, 31) might play important roles in cardiovascular diseases and warrants future studies.
The results of our study suggest that HUA is closely associated with more cardiometabolic disorders in young and middle-aged hypertensive adults in Beijing population. Comprehensive management of HT with HUA is needed in clinical settings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Central institutional review board of Peking University First Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XS: performed the statistical analysis and wrote the first draft of the article. JL: conceived the study, supervised and edited the article. NS and YH: supervised and edited the article. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1133724/full#supplementary-material.
1. Mandal AK, Mount DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
2. Mandal AK, Mercado A, Foster A, Zandi-Nejad K, Mount DB. Uricosuric targets of tranilast. Pharmacol Res Perspect. (2017) 5(2):e00291. doi: 10.1002/prp2.291
3. Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. (2017) 15(1):123. doi: 10.1186/s12916-017-0890-9
4. Richette P, Perez-Ruiz F, Doherty M, Jansen TL, Nuki G, Pascual E, et al. Improving cardiovascular and renal outcomes in gout: what should we target? Nat Rev Rheumatol. (2014) 10(11):654–61. doi: 10.1038/nrrheum.2014.124
5. Grayson PC, Kim SY, LaValley M, Choi HK. Hyperuricemia and incident hypertension: a systematic review and meta-analysis. Arthritis Care Res. (2011) 63(1):102–10. doi: 10.1002/acr.20344
6. Zhao G, Huang L, Song M, Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. (2013) 231(1):61–8. doi: 10.1016/j.atherosclerosis.2013.08.023
7. Krishnan E, Pandya BJ, Chung L, Hariri A, Dabbous O. Hyperuricemia in young adults and risk of insulin resistance, prediabetes, and diabetes: a 15-year follow-up study. Am J Epidemiol. (2012) 176(2):108–16. doi: 10.1093/aje/kws002
8. Xia X, Luo Q, Li B, Lin Z, Yu X, Huang F. Serum uric acid and mortality in chronic kidney disease: a systematic review and meta-analysis. Metab Clin Exp. (2016) 65(9):1326–41. doi: 10.1016/j.metabol.2016.05.009
9. Li M, Hou W, Zhang X, Hu L, Tang Z. Hyperuricemia and risk of stroke: a systematic review and meta-analysis of prospective studies. Atherosclerosis. (2014) 232(2):265–70. doi: 10.1016/j.atherosclerosis.2013.11.051
10. Forman JP, Choi H, Curhan GC. Plasma uric acid level and risk for incident hypertension among men. J Am Soc Nephrol. (2007) 18(1):287–92. doi: 10.1681/asn.2006080865
11. Lee SW, Kim HC, Nam C, Lee HY, Ahn SV, Oh YA, et al. Age-differential association between serum uric acid and incident hypertension. Hypertens Res. (2019) 42(3):428–37. doi: 10.1038/s41440-018-0168-4
12. Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PloS ONE. (2014) 9(6):e100801. doi: 10.1371/journal.pone.0100801
13. Liu J, Chen L, Yuan H, Huang K, Li G, Sun N, et al. Survey on uric acid in Chinese subjects with essential hypertension (success): a nationwide cross-sectional study. Ann Transl Med. (2021) 9(1):27. doi: 10.21037/atm-20-3458
14. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. (2008) 359(17):1811–21. doi: 10.1056/NEJMra0800885
15. Kuwabara M, Borghi C, Cicero AFG, Hisatome I, Niwa K, Ohno M, et al. Elevated serum uric acid increases risks for developing high ldl cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int J Cardiol. (2018) 261:183–8. doi: 10.1016/j.ijcard.2018.03.045
16. Seki S, Tsutsui K, Fujii T, Yamazaki K, Anzawa R, Yoshimura M. Association of uric acid with risk factors for chronic kidney disease and metabolic syndrome in patients with essential hypertension. Clin Exp Hypertens. (2010) 32(5):270–7. doi: 10.3109/10641960903265220
17. Feig DI, Mazzali M, Kang DH, Nakagawa T, Price K, Kannelis J, et al. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol. (2006) 17(4 Suppl 2):S69–73. doi: 10.1681/asn.2005121331
18. Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. (2001) 38(5):1101–6. doi: 10.1161/hy1101.092839
19. Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. (2002) 13(12):2888–97. doi: 10.1097/01.asn.0000034910.58454.fd
20. Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. (2004) 94(7):932–5. doi: 10.1016/j.amjcard.2004.06.032
21. Schiattarella GG, Wang Y, Tian R, Hill JA. Metabolism and inflammation in cardiovascular health and diseases: mechanisms to therapies. J Mol Cell Cardiol. (2021) 157:113–4. doi: 10.1016/j.yjmcc.2021.02.011
22. Brand FN, McGee DL, Kannel WB, Stokes J 3rd, Castelli WP. Hyperuricemia as a risk factor of coronary heart disease: the framingham study. Am J Epidemiol. (1985) 121(1):11–8. doi: 10.1093/oxfordjournals.aje.a113972
23. Sundström J, Sullivan L, D'Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. (2005) 45(1):28–33. doi: 10.1161/01.HYP.0000150784.92944.9a
24. Zhang Y, Zhang M, Yu X, Wei F, Chen C, Zhang K, et al. Association of hypertension and hypertriglyceridemia on incident hyperuricemia: an 8-year prospective cohort study. J Transl Med. (2020) 18(1):409. doi: 10.1186/s12967-020-02590-8
25. Kale SS, Yende S. Effects of aging on inflammation and hemostasis through the continuum of critical illness. Aging Dis. (2011) 2(6):501–11.22396897
26. Battelli MG, Bortolotti M, Polito L, Bolognesi A. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim Biophys Acta Mol Basis Dis. (2018) 1864(8):2557–65. doi: 10.1016/j.bbadis.2018.05.003
27. Kanbay M, Jensen T, Solak Y, Le M, Roncal-Jimenez C, Rivard C, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. (2016) 29:3–8. doi: 10.1016/j.ejim.2015.11.026
28. Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan H, et al. Uric acid, hominoid evolution, and the pathogenesis of salt-sensitivity. Hypertension. (2002) 40(3):355–60. doi: 10.1161/01.hyp.0000028589.66335.aa
29. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. (2017) 8(1):33. doi: 10.1186/s13293-017-0152-8
30. Reiter RJ, Tan DX, Paredes SD, Fuentes-Broto L. Beneficial effects of melatonin in cardiovascular disease. Ann Med. (2010) 42(4):276–85. doi: 10.3109/07853890903485748
Keywords: hyperuricemia, cardiometabolic risk factors, hypertension, age-related, cross-section study
Citation: Su X, Liu J, Sun N, Huo Y and the SUCCESS Investigation Group (2023) Hyperuricemia is associated with more cardiometabolic risk factors in hypertensive younger Chinese adults than in elderly. Front. Cardiovasc. Med. 10:1133724. doi: 10.3389/fcvm.2023.1133724
Received: 29 December 2022; Accepted: 28 February 2023;
Published: 17 March 2023.
Edited by:
Tatsuya Morimoto, University of Shizuoka, JapanReviewed by:
Jidong Cheng, Xiamen University, China© 2023 Su, Liu, Sun, Huo and the SUCCESS Investigation Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Liu aGVhcnRjZW50ZXJAMTYzLmNvbQ== Yong Huo aHVveW9uZ0AyNjMubmV0LmNu
Specialty Section: This article was submitted to Cardiovascular Metabolism, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.