- 1School of Medicine, Ningbo University, Ningbo, China
- 2Department of Cardiology, Ningbo First Hospital, Ningbo, China
- 3Department of Cardiology, Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, Ningbo, China
- 4Clinical Medicine Research Centre for Cardiovascular Disease of Ningbo, Ningbo, China
Background and aims: The association between sleep traits and coronary artery disease (CAD) in patients with diabetes has been reported in previous observational studies. However, whether these potential relationships are causal remains unclear. We aim to assess the causal relationship between sleep traits and CAD in diabetic.
Methods: Genetic instrumental variables associated with five sleep-related traits (insomnia, sleep duration, ease of getting up, morningness and snoring) were extracted from corresponding genome-wide association studies (GWAS). The associations of genetic variants with CAD were based on 15,666 individuals with diabetes (3,968 CAD cases and 11,696 controls). The primary analysis was derived using the inverse variance weighting method. Further sensitivity analysis was conducted to confirm the robustness and consistency of the main results.
Results: Genetic liability to insomnia was significantly related to the increased risk of CAD in individuals with diabetes [odds ratio (OR): 1.163; 95% CI: 1.072–1.254; p = 0.001]. Suggestive evidence was found for the borderline associations between both sleep duration (OR: 0.629; 95% CI: 0.380–1.042, p = 0.072) and snoring (OR: 1.010, 95% CI: 1.000–1.020, p = 0.050) with CAD risk. However, no consistent evidence was found for the association between ease of getting up and morningness with the risk of CAD in diabetic. Similar results can be verified in most sensitivity analyses.
Conclusions: We provide consistent evidence for the causal effect of insomnia on the increased risk of CAD in individuals with diabetes. The management of sleep health should be emphasized to prevent CAD in diabetic patients.
Introduction
Cardiovascular disease (CVD) is the leading cause of global mortality and a principle contributor to disability (1). Meanwhile, CVD remains a major cause of death among patients with diabetes (2). The latest diabetes management guidelines published by ESC/ESAD elevated the status of cardiovascular risk in the management of type 2 diabetes mellitus (T2DM) (3). Several clinical studies have been published on cardiovascular risk management in adults with T2DM, involving lifestyle, blood pressure, blood glucose, cholesterol management and sleep in primary and secondary prevention of CVD (4–8).
Sleep disorders are increasingly prevalent modifiable risk factors for CVD (9). Plenty of epidemiological studies suggested that sleep duration was associated with an increased risk of CVD events and higher mortality risk (10–14). A recent prospective study including 18,876 patients with T2DM reported that short and long sleep durations were both independently associated with the increased incidence and mortality of CVD (15). Another study based on 36,058 Korean new-onset T2DM patients indicated that sleep disturbance was significantly associated with an increased risk of CVD mortality (16). Besides, a cross-sectional study with small samples found that the effect of poor sleep on the risk of CVD in patients with T2DM may be mediated by some inflammatory factors (17). Involvement of the endothelial function, autonomic nervous system, regulation of metabolism and inflammation have been proposed as possible mechanistic linked between sleep disorders and CVD (18). Some studies also suggested that sleep disorders contributed to the risk of T2DM by affecting insulin production (19–22). However, the causality between these associations remains unclear, especially in individuals with diabetes.
Mendelian randomization (MR) uses genetic variation as a natural experiment to study the causal relationship between potentially modifiable risk factors and health outcomes (23). As alleles follow random assignment during gamete formation, the estimations would not be affected by confounding factors and reverse causality compared to observational studies (24). Several previous studies have provided genetic evidence for a causal association of insomnia and sleep duration with increased risk of CAD in general population (13, 25). However, the causal association pattern in the diabetics remains unclear. Besides, the causal effect of other sleep characteristics, such as ease of getting up, morningness (being a morning person rather than an evening person) and snoring needs to be further investigated. As the sleep traits are driven by genetic risk, the MR study could provide long-term stable genetic evidence, which is not affected by lifestyle factors.
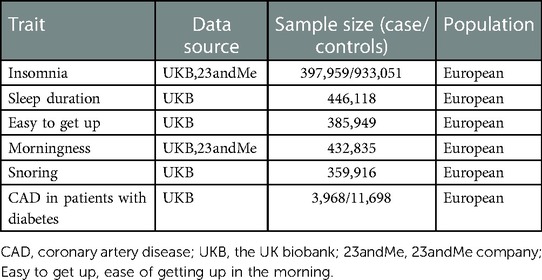
In the current study, we conducted a comprehensive MR study to evaluate the causal associations between five sleep traits and genetic susceptibility to CAD in individuals with diabetes (Table 1).
Methods
Study design
The study design overview was shown in Figure 1. The single nucleotide polymorphisms (SNPs) identified as genetic instrumental variables (IVs) for sleep traits depend on the following three core assumptions: (I) The genetic variation should be strongly related to sleep traits, (II) genetic variation is not influenced by potential confounding factors, as well as (III) genetic variation should only be related to the risk of CAD in diabetic patients through sleep traits (26). Ethical review approval and informed consent were obtained from participants for all included original studies.
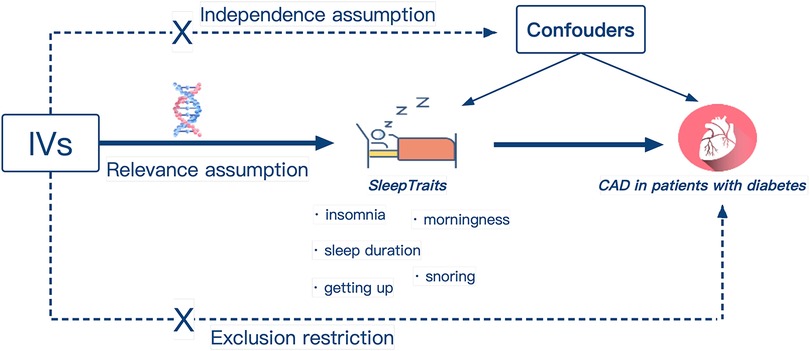
Figure 1. Design of the Mendelian randomization study. Three core assumptions were as follows: Relevance assumption, the genetic IVs must be associated with sleep traits; Independence assumption, IVs should not be associated with confounders; Exclusion restriction, IVs must influence CAD in patients with diabetes only via sleep traits. IVs, instrumental variables; Easy to get up, ease of getting up in the morning; CAD, coronary artery disease.
Genetic instrument selection
Insomnia
Genetic association with insomnia was obtained from the largest GWAS to date including 1,331,010 individuals of European ancestry (27). Insomnia was measured by a standardized question: “Do you often fall asleep late at night or wake up at midnight?”. There were four answer options: “never/rarely”, “sometimes”, “usually”, or “prefer not to answer”. Among them, the participants who answered “usually” were defined as suffering from insomnia, while the participants who answered “never or rarely” and “sometimes” were included in the control group.
Sleep duration
The sleep duration GWAS analysis identified 78 independent SNPs associated with sleep duration (p < 5 × 10−8), involving 446,118 individuals of European ancestry (28). The sleep duration was determined in the form of self-report. Subjects were asked “How long do you sleep every 24 h, including naps”, and the answers increased in hours. Subjects who responded to extreme values (less than 3 h or more than 18 h) or who reported using any sleep-regulating medication were excluded. Sleep duration was examined continuously.
Ease of getting up in the morning
The ease of getting up was investigated in a study including 1,331,010 participants by asking: “On an average day, how easy do you find getting up in the morning?”. The possible responses included “not at all easy”, “not very easy”, “fairly easy” and “very easy”. The ease of getting up was divided into four categories and examined as a continuous scale. The corresponding GWAS extracted 70 independent pilot SNPs located in 62 different genomic loci (27).
Morningness
Morningness was evaluated by asking: “Do you consider yourself to be?”. There were five possible responses “Definitely a ‘morning’ person”, “More a ‘morning’ than ‘evening’ person”, “More an ‘evening’ than a ‘morning’ person”, “Definitely an ‘evening’ person”, and “Do not know”. The corresponding GWAS identified 274 independent pilot SNPs located in 207 different genomic loci (27).
Snoring
Snoring was evaluated based on asking: “Does your partner or a close relative or friend complain about your snoring?”. People would reply with “yes” or “no”. A total of 3,59,916 subjects were included in the genome-wide analysis of snoring. The corresponding GWAS analysis revealed 3,416 GWS SNPs (p < 5 × 10−8), resulting in the identification of 42 SNPs associated with snoring, which were located in 36 different genomic loci (27).
CAD in patients with diabetes
The summary statistical data of CAD in diabetic were obtained from the latest GWAS (29). That study was conducted on the basis of the UK Biobank cohorts in 2018, including 15,666 European individuals with diabetes (3,968 CAD cases and 11,696 non-cases). The diabetes and CAD were defined based on linked data from hospital admissions and death registries, and verbal health interview (see Supplementary Methods). The average age at visit was 62.7 ± 5.6 and 60.2 ± 7.0 for CAD group and non-CAD group, respectively. 74.0% of CAD group and 60.2% of non-CAD group were male. In the CAD group, 268 (6.8%) individuals were with type I diabetes, while in the non-CAD group 945 (8.1%) individuals were with type I diabetes. Linkage disequilibrium (LD) testing was performed based on 1,000 genomes LD reference panel of Europeans only (30).
Statistical analysis
The inverse variance weighting (IVW) method was applied to evaluate the influence of genetically predicted sleep traits on the risk of CAD in diabetic populations in the main analysis (31). The Wald estimator was used to generate a causal estimate for each SNP and the standard error was obtained using the Delta method. The overall effect value was then obtained by combining these estimates (32).
Sensitivity analysis was performed using the maximum likelihood method, the weighted median method, the MR-Egger method, and the Mendelian Randomization Pleiotropy Residual Sum and Outlier (MR-PRESSO) method to evaluate the robustness of the results. The weighted median method allows accurate calculation of causal association effects, even when less than 50% of the genetic variation violates the core assumptions (33). The MR-PRESSO can exclude specific outliers to obtain estimates closer to the true value and detect horizontal gene pleiotropy (34). The MR-Egger intercept test was used to evaluate possible horizontal pleiotropic effects and to visually examine potential multidirectional effects by generating funnel plots (35). The causal association between exposure and health outcomes was described by using scatter plots. Subsequently, the leave-one-out sensitivity analyses were performed to assess whether the casual relationship was dramatically driven by any single SNP. All the statistical analyses were conducted using the TwoSampleMR and MR-PRESSO packages in the R software (Version 4.3.1).
Results
Insomnia
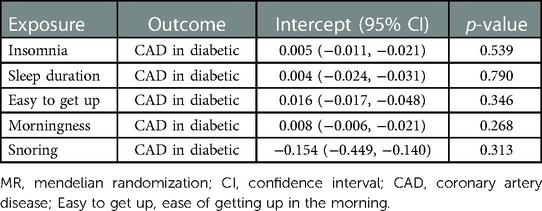
In total, 208 SNPs were identified and used as the genetic IVs for insomnia. The genetic liability to insomnia in diabetic patients showed a significant association with an increased risk of CAD in the IVW analysis (OR: 1.163; 95% CI: 1.072–1.254, p = 0.001; Figure 2). The main results remained consistent in the sensitivity analysis using the weighted median and maximum likelihood method (Supplementary Table S7). MR-Egger regression analysis showed no overall pleiotropy or heterogeneity between insomnia and CAD in diabetic patients (p > 0.05) (Table 2). In addition, scatter plots also showed that there was no directional polymorphism on CAD in diabetic patients (Figure 3). The leave-one-out sensitivity analysis indicated that the causal association was not greatly driven by any single SNP (Supplementary Figure S1).
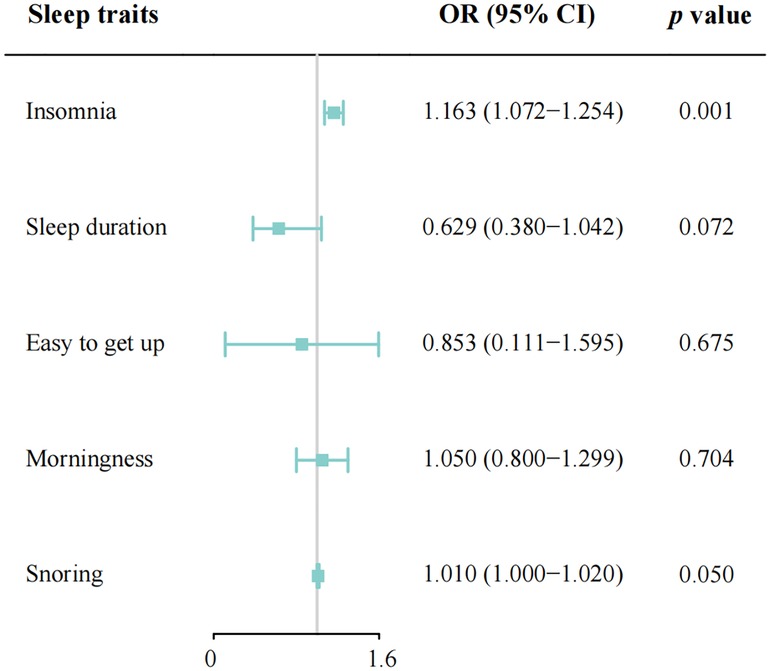
Figure 2. Mendelian randomization estimates of genetically predicted sleep disorders on coronary artery disease in patients with diabetes. Easy to get up, ease of getting up in the morning; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted.
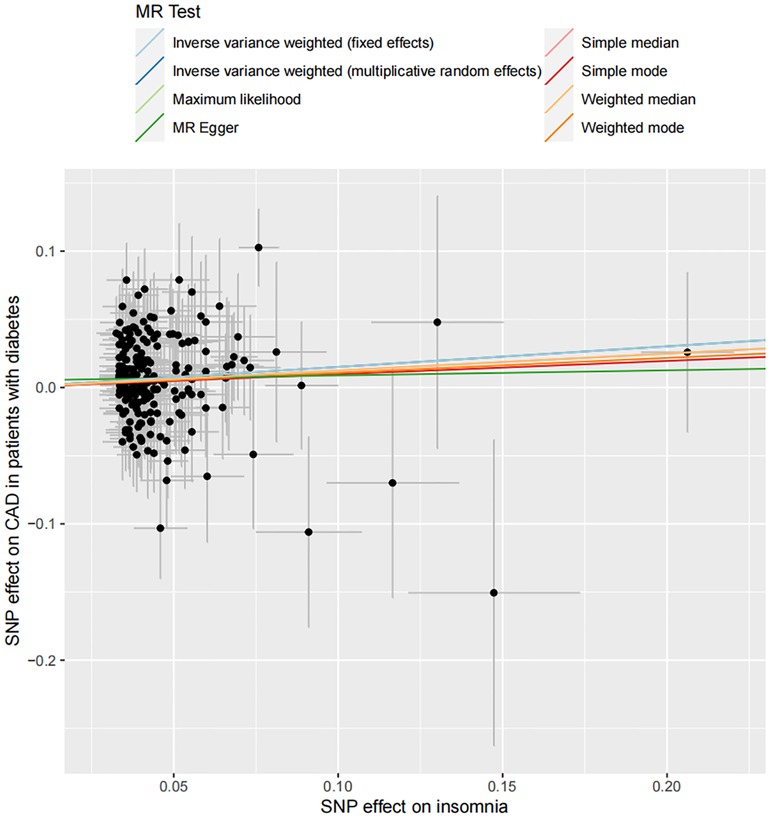
Figure 3. Scatter plot of the association between insomnia and the risk of CAD in patients with diabetes. Each dot indicates a SNP; each line indicates the estimate of association between insomnia and the risk of CAD in patients with diabetes using corresponding methods.
Other sleep traits
With the IVW analysis, the current study found suggestive evidence for the borderline associations between both sleep duration (OR: 0.629; 95% CI: 0.380–1.042, p = 0.072; Figure 2) and snoring (OR: 1.010, 95% CI: 1.000–1.020, p = 0.050) with CAD risk in individuals with diabetes, which needed to be further investigated. Horizontal pleiotropy was not detected using MR-Egger intercept testing (Supplementary Table S7). However, no consistent evidence was found for the causal associations between ease of getting up (OR: 0.853; 95% CI: 0.111–1.595; p = 0.675) and morningness (OR: 1.050; 95% CI: 0.800–1.299; p = 0.704) with the risk of CAD in diabetic patients. Besides, in the MR-Egger analysis, no pleiotropy was detected between ease of getting up and morningness with CAD risk (Supplementary Table S7). The association patterns kept consistent and robust in the other three statistical models (Supplementary Table S7).
Discussion
In this study, we investigated the causal effects of five sleep traits including insomnia, sleep duration, ease of getting up, morningness and snoring on CAD in patients with diabetes. Genetic liability to insomnia was closely related to the increased CAD risk in individuals with diabetes. Besides, suggestive evidence was found for the borderline associations between both sleep duration and snoring with the risk of CAD, which needed to be further investigated. However, no valid evidence for the causal effects of morningness and ease of getting up was found.
In regards to the directionality of the observed effects, we found a significant association between insomnia and increased CAD risk in patients with diabetes. Besides, the results suggested the borderline associations between sleep duration with reduced CAD risk and snoring with increased CAD risk, which were in line with previous observational studies. The easy to get up showed a trend of protective factors, however no consistent evidence was found. There were large differences in variation between the ORs of different sleep traits, partly due to the difference between binary variable (e.g., insomnia) and continuous variable (e.g., sleep duration). Besides, the causal effects of morningness and snoring on CAD risk may be relatively limited, compared to insomnia.
In the recent years, increasing observational evidence supported that insomnia was an important risk factor for the progression of CVD. Previous studies reported that people with significant insomnia symptoms have a 41%–55% increased risk of myocardial infarction and coronary heart disease, as well as a higher risk of cardiovascular and cerebrovascular related death. Suzanne M.Bertisch et al. found that insomnia or poor sleep quality combined with frequent short sleep increased the incidence of CVD events by 29% compared with controls in a propensity matching model (36). A large population cohort study showed that short or long sleep duration, insomnia and snoring were all related to an increased risk of CVD in a multivariate model (14). In a prospective cohort study involving 4,07,500 individuals, the incidence of CVD was significantly associated with a 7%, 26%, and 20% increased risk of snoring, insomnia, and narcolepsy, respectively (37).
Shorter sleep duration was independently associated with increased risk of subclinical multiple atherosclerosis (38). Among patients younger than 40 years of age, insomniacs had a higher risk of T2DM than controls (HR: 1.31; 95% CI: 1.14–1.55) (39). Insomnia symptoms may lead to an increase in glycated hemoglobin levels, suggesting a causal link between insomnia and T2DM (40). In addition, short and long sleep duration would increase the risk of T2DM (41). Another prospective cohort study also indicated that insomnia was associated with a higher risk of T2DM (42). And a meta-analysis of 13 prospective trials demonstrated that insomnia would increase the risk of CVD (43).
The pathophysiological basis and mechanisms underlying the association of insomnia with CVD have not been fully clarified. Possible mechanisms may include dysregulation of the hypothalamic-pituitary axis (HPA) (44), dysregulation of the autonomic nervous system (45), systemic inflammatory activation (46), and acceleration of atherosclerosis (47). First, sleep was linked to haematopoiesis and atherosclerosis in mice, and sleep fragmentation would lead to more Ly-6Chigh monocytes, larger atherosclerotic lesions and less hypocretin, which controls myelopoiesis. Second, insomnia may affect the endothelial function of coronary arteries through the autonomic nervous system, thereby accelerating coronary atherosclerosis (48). Third, sleep disturbance could induce increased NF-κB, proinflammatory cytokine production, and systemic inflammation through activation of β-adrenergic signaling pathways (49). Forth, sleep disorders can also lead to the occurrence of CAD by affecting the endocrine mechanism, inhibiting the metabolism of blood lipids and the regulation of blood glucose (50).
The main strength was the study design using multiple SNPs as genetic IVs for sleep characteristics, which minimized confounding and reverse causality. In the current study, sleep traits were driven by genetic risk and not modifiable, thus providing stable genetic evidence, which was not affected by lifestyle factors. In addition, as appropriate genetic IVs were screened by a large amount of genetic associations, the summary of data guaranteed the estimation of causal effect on the basis of the large sample. Besides, we evaluated the causal effect of five sleep traits on CAD in individuals with diabetes using a comprehensive analysis, which could greatly expand the scope of our discovery.
Several limitations of the current study should be pointed out. First, the selection of genetic instruments was based on the GWAS without hypothesis, which may lack a comprehensive understanding of the potential association mechanism between genetic variation and diseases. Second, it was impossible to completely exclude the potential effects of pleiotropy, thus the results may be affected. Third, our findings could not be generalized to other populations, as only European participants were included. Therefore, further studies were needed to investigate the causal association pattern in other populations. Forth, all the sleep traits were obtained through subjective description of patients, so it was difficult to avoid misclassification. Besides, due to lack of individual-level genetic data, stratification analysis was not available in the current study to assess the gender and/or age differences in the association between sleep and CAD risk. Likewise, we failed to perform the analysis on if the insomnia treatments influence the association with CAD, which needed to be further investigated.
Conclusion
In conclusion, we provided consistent evidence for the causal effect of insomnia on the increased CAD risk in diabetic patients. The borderline associations between both sleep duration and snoring with the risk of CAD needed to be further investigated. Increased attention should be paid to sleep health and better prevention of sleep disorder, which may reduce the risk of CAD in diabetic patients.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MT, HM, TH, and HC: made contributions to the design of the study. MT, HM, JS and HC: made contributions to the obtaining, analysis, or explanation of data for the study. MT: drafted the manuscript with critical revisions from JS and NH. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82200489), Key Laboratory of Precision Medicine for Atherosclerotic Diseases of Zhejiang Province, China (grant no. 2022E10026), Key research and development project of Zhejiang Province, China (grant no. 2021C03096) and Public Science and Technology Projects of Ningbo (grant no. 202002N3175).
Acknowledgments
The authors thank all the investigators of UK Biobank study and the 23andMe company for providing the data publicly.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1132281/full#supplementary-material.
References
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus – mechanisms, management, and clinical considerations. Circulation. (2016) 133:2459–502. doi: 10.1161/CIRCULATIONAHA.116.022194
3. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. (2019) 2020(41):255–323. doi: 10.1093/eurheartj/ehz486
4. Walli-Attaei M, Rosengren A, Rangarajan S, Breet Y, Abdul-Razak S, Sharief WA, et al. Metabolic, behavioural, and psychosocial risk factors and cardiovascular disease in women compared with men in 21 high-income, middle-income, and low-income countries: an analysis of the PURE study. Lancet. (2022) 400:811–21. doi: 10.1016/S0140-6736(22)01441-6
5. Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O'Connor PJ, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. (2018) 320:1570–82. doi: 10.1001/jama.2018.14619
6. Li X, Xue Q, Wang M, Zhou T, Ma H, Heianza Y, et al. Adherence to a healthy sleep pattern and incident heart failure: a prospective study of 408 802 UK biobank participants. Circulation. (2021) 143:97–9. doi: 10.1161/CIRCULATIONAHA.120.050792
7. Li J, Atasoy S, Fang X, Angerer P, Ladwig K-H. Combined effect of work stress and impaired sleep on coronary and cardiovascular mortality in hypertensive workers: the MONICA/KORA cohort study. Eur J Prev Cardiol. (2021) 28:220–6. doi: 10.1177/2047487319839183
8. Mariam A, Miller-Atkins G, Pantalone KM, Zimmerman RS, Barnard J, Kattan MW, et al. A type 2 diabetes subtype responsive to ACCORD intensive glycemia treatment. Diabetes Care. (2021) 44:1410–8. doi: 10.2337/dc20-2700
9. Schloss MJ, Swirski FK, Nahrendorf M. Modifiable cardiovascular risk, hematopoiesis, and innate immunity. Circ Res. (2020) 126:1242–59. doi: 10.1161/CIRCRESAHA.120.315936
10. Huang T, Mariani S, Redline S. Sleep irregularity and risk of cardiovascular events. J Am Coll Cardiol. (2020) 75:991–9. doi: 10.1016/j.jacc.2019.12.054
11. Jin Q, Yang N, Dai J, Zhao Y, Zhang X, Yin J, et al. Association of sleep duration with all-cause and cardiovascular mortality: a prospective cohort study. Front Public Health. (2022) 10:880276. doi: 10.3389/fpubh.2022.880276
12. Wang C, Bangdiwala SI, Rangarajan S, Lear SA, AlHabib KF, Mohan V, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J. (2019) 40:1620–9. doi: 10.1093/eurheartj/ehy695
13. Ai S, Zhang J, Zhao G, Wang N, Li G, So H-C, et al. Causal associations of short and long sleep durations with 12 cardiovascular diseases: linear and nonlinear Mendelian randomization analyses in UK biobank. Eur Heart J. (2021) 42:3349–57. doi: 10.1093/eurheartj/ehab170
14. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. (2020) 41:1182–9. doi: 10.1093/eurheartj/ehz849
15. Han H, Wang Y, Li T, Feng C, Kaliszewski C, Su Y, et al. Sleep duration and risks of incident cardiovascular disease and mortality among people with type 2 diabetes. Diabetes Care. (2023) 46:101–10. doi: 10.2337/dc22-1127
16. Choi Y, Choi JW. Association of sleep disturbance with risk of cardiovascular disease and all-cause mortality in patients with new-onset type 2 diabetes: data from the Korean NHIS-HEALS. Cardiovasc Diabetol. (2020) 19:61. doi: 10.1186/s12933-020-01032-5
17. Meng L-L, Tang Y-Z, Ni C-L, Yang M, Song H-N, Wang G, et al. Impact of inflammatory markers on the relationship between sleep quality and incident cardiovascular events in type 2 diabetes. J Diabetes Complications. (2015) 29:882–6. doi: 10.1016/j.jdiacomp.2015.06.011
18. Tobaldini E, Fiorelli EM, Solbiati M, Costantino G, Nobili L, Montano N. Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat Rev Cardiol. (2019) 16:213–24. doi: 10.1038/s41569-018-0109-6
19. Brady EM, Bodicoat DH, Hall AP, Khunti K, Yates T, Edwardson C, et al. Sleep duration, obesity and insulin resistance in a multi-ethnic UK population at high risk of diabetes. Diabetes Res Clin Pract. (2018) 139:195–202. doi: 10.1016/j.diabres.2018.03.010
20. Arora T, Chen MZ, Cooper AR, Andrews RC, Taheri S. The impact of sleep debt on excess adiposity and insulin sensitivity in patients with early type 2 diabetes mellitus. J Clin Sleep Med. (2016) 12:673–80. doi: 10.5664/jcsm.5792
21. van Dijk D, Balkau B, Segrestin B, Gottsäter M, Gabriel R, Hatunic M, et al. Associations between sleep duration and sleep debt with insulin sensitivity and insulin secretion in the EGIR-RISC study. Diabetes Metab. (2019) 45:375–81. doi: 10.1016/j.diabet.2018.11.001
22. Liu H, Chen G, Wen J, Wang A, Mu Y, Dou J, et al. Association between sleep duration and incidence of type 2 diabetes in China: the REACTION study. Chin Med J. (2022) 135:1242–8. doi: 10.1097/CM9.0000000000001835
23. Davies NM, Holmes MV, Davey Smith G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. Br Med J. (2018) 362:k601. doi: 10.1136/bmj.k601
24. Latvala A, Ollikainen M. Mendelian Randomization in (epi)genetic epidemiology: an effective tool to be handled with care. Genome Biol. (2016) 17:156. doi: 10.1186/s13059-016-1018-9
25. Yuan S, Mason AM, Burgess S, Larsson SC. Genetic liability to insomnia in relation to cardiovascular diseases: a Mendelian randomisation study. Eur J Epidemiol. (2021) 36:393–400. doi: 10.1007/s10654-021-00737-5
26. Holmes MV, Ala-Korpela M, Smith GD. Mendelian Randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. (2017) 14:577–90. doi: 10.1038/nrcardio.2017.78
27. The 23andMe Research Team, Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. (2019) 51:394–403. doi: 10.1038/s41588-018-0333-3
28. Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. (2019) 10:1100. doi: 10.1038/s41467-019-08917-4
29. Fall T, Gustafsson S, Orho-Melander M, Ingelsson E. Genome-wide association study of coronary artery disease among individuals with diabetes: the UK biobank. Diabetologia. (2018) 61:2174–9. doi: 10.1007/s00125-018-4686-z
30. The 1000 Genomes Project Consortium, Clarke L, Zheng-Bradley X, Smith R, Kulesha E, Xiao C, Toneva I, et al. The 1000 genomes project: data management and community access. Nat Methods. (2012) 9:459–62. doi: 10.1038/nmeth.1974
31. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization: a framework for two-sample summary data MR. Stat Med. (2017) 36:1783–802. doi: 10.1002/sim.7221
32. Zuber V, Colijn JM, Klaver C, Burgess S. Selecting likely causal risk factors from high-throughput experiments using multivariable Mendelian randomization. Nat Commun. (2020) 11:29. doi: 10.1038/s41467-019-13870-3
33. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
34. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
35. Burgess S, Thompson SG. Erratum to: interpreting findings from Mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32:391–2. doi: 10.1007/s10654-017-0276-5
36. Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep heart health study. Sleep. (2018) 41(6):zsy047. doi: 10.1093/sleep/zsy047
37. Tao F, Cao Z, Jiang Y, Fan N, Xu F, Yang H, et al. Associations of sleep duration and quality with incident cardiovascular disease, cancer, and mortality: a prospective cohort study of 407,500 UK biobank participants. Sleep Med. (2021) 81:401–9. doi: 10.1016/j.sleep.2021.03.015
38. Domínguez F, Fuster V, Fernández-Alvira JM, Fernández-Friera L, López-Melgar B, Blanco-Rojo R, et al. Association of sleep duration and quality with subclinical atherosclerosis. J Am Coll Cardiol. (2019) 73:134–44. doi: 10.1016/j.jacc.2018.10.060
39. Lin C-L, Chien W-C, Chung C-H, Wu F-L. Risk of type 2 diabetes in patients with insomnia: a population-based historical cohort study. Diabetes Metab Res Rev. (2018) 34:e2930. doi: 10.1002/dmrr.2930
40. Liu J, Richmond RC, Bowden J, Barry C, Dashti HS, Daghlas I, et al. Assessing the causal role of sleep traits on glycated hemoglobin: a Mendelian randomization study. Diabetes Care. (2022) 45:772–81. doi: 10.2337/dc21-0089
41. Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. (2015) 38:529–37. doi: 10.2337/dc14-2073
42. Green MJ, Espie CA, Popham F, Robertson T, Benzeval M. Insomnia symptoms as a cause of type 2 diabetes incidence: a 20 year cohort study. BMC Psychiatry. (2017) 17:94. doi: 10.1186/s12888-017-1268-4
43. Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. (2014) 21:57–64. doi: 10.1177/2047487312460020
44. Castro-Diehl C, Diez Roux AV, Redline S, Seeman T, Shrager SE, Shea S. Association of sleep duration and quality with alterations in the hypothalamic-pituitary adrenocortical axis: The Multi-Ethnic Study of Atherosclerosis (MESA). J Clin Endocrinol Metab. (2015) 100:3149–58. doi: 10.1210/jc.2015-1198
45. Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. (2015) 66:143–72. doi: 10.1146/annurev-psych-010213-115205
46. Floam S, Simpson N, Nemeth E, Scott-Sutherland J, Gautam S, Haack M. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. J Sleep Res. (2015) 24:296–304. doi: 10.1111/jsr.12259
47. Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. (2017) 74:321–9. doi: 10.1016/j.neubiorev.2016.07.004
48. Calandra-Buonaura G, Provini F, Guaraldi P, Plazzi G, Cortelli P. Cardiovascular autonomic dysfunctions and sleep disorders. Sleep Med Rev. (2016) 26:43–56. doi: 10.1016/j.smrv.2015.05.005
49. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
Keywords: sleep traits, coronary artery disease, diabetes, causal association, insomnia
Citation: Tian M, Ma H, Shen J, Hu T, Cui H and Huangfu N (2023) Causal association between sleep traits and the risk of coronary artery disease in patients with diabetes. Front. Cardiovasc. Med. 10:1132281. doi: 10.3389/fcvm.2023.1132281
Received: 27 December 2022; Accepted: 16 February 2023;
Published: 3 March 2023.
Edited by:
Gary David Lopaschuk, University of Alberta, CanadaReviewed by:
Cameron McAlpine, Icahn School of Medicine at Mount Sinai, United StatesMaaike Schilperoort, Columbia University Irving Medical Center, United States
© 2023 Tian, Ma, Shen, Hu, Cui and Huangfu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Huangfu bmluZ2h1YW5nZnVAMTI2LmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Cardiovascular Metabolism, a section of the journal Frontiers in Cardiovascular Medicine
 Mengyun Tian1,†
Mengyun Tian1,† Hanbin Cui
Hanbin Cui Ning Huangfu
Ning Huangfu