
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 08 September 2023
Sec. Pediatric Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1131383
This article is part of the Research Topic Health Informatics in Pediatric Cardiology View all 6 articles
 Linhong Song1,2,3,4,5,†
Linhong Song1,2,3,4,5,† Yi Wang2,3,4,5,†
Yi Wang2,3,4,5,† Hui Wang2,3,4,5
Hui Wang2,3,4,5 Gang Wang2,3,4,5
Gang Wang2,3,4,5 Ning Ma2,3,4,5
Ning Ma2,3,4,5 Qiang Meng2,3,4,5
Qiang Meng2,3,4,5 Kunao Zhu2,3,4,5
Kunao Zhu2,3,4,5 Siqi Hu2,3,4,5*
Siqi Hu2,3,4,5* Gengxu Zhou1,2,3,4,5*
Gengxu Zhou1,2,3,4,5* Zhichun Feng1,2,3,4,5*
Zhichun Feng1,2,3,4,5*
Background: Congenital heart diseases (CHDs) are conditions that involve structural problems to the heart's structure existing at birth, with an incidence of approximately 8 per 1,000 live births globally. CHD is one of the leading causes of maternal, fetal, and neonatal morbidity and mortality worldwide. The present study sought to examine the clinical profiles of CHD patients and provide important implications for therapeutic interventions.
Methods: This was a retrospective, observational, cohort study. The medical records of all CHDs patients aged between 0 and 18 years were collected from July 1, 2021 to June 30, 2022. Clinical profiles and demographic data were collected from cardiology and pediatric department registers for analysis.
Results: Of the 265 children with CHDs, 201 were diagnosed with acyanotic CHD (ACHD), while 64 children had cyanotic CHD (CCHD). Based on the eleventh revision of the International Classification of Diseases (ICD-11), “congenital anomaly of a ventricle or the ventricular septum” was the most common CHD. The most common symptom was failure to thrive, accounting for 18.5% of all CHD cases. The most frequent symptom in ACHD was murmur (93.53%) and sweating (80.60%), whereas the most common symptom in CCHD was sweating (95.31%) and cyanosis (84.38%).
Conclusions: This study retrospectively analyzed CHD clinical characteristics from children receiving care at the seventh center, which forms a proper basis for appropriate clinical treatments and further studies.
Congenital heart diseases (CHDs) are the most common type of congenital birth defect and the leading cause of infant morbidity and mortality worldwide. The incidence of CHD in different studies varies from approximately 4/1,000 to 50/1,000 live births (1). Recently, a meta-analysis demonstrated that the prevalence (1970–2017) of CHD diagnoses first made in childhood was 1.384/1,000 (2). CHD is often classified into two types based on the pathophysiology and the affected heart structure: acyanotic CHD (ACHD) and cyanotic CHD (CCHD) (3). CCHD involves heart defects in which the blood delivered to the body contains less-than-normal amounts of oxygen. ACHDs are cardiac malformations that affect normal blood flow.
Until recently, the exact etiology of the majority of CHDs is unknown. To explain the pathogenesis of CHDs, several theories have been put forward. In 1968, Nora introduced the multifactorial inheritance hypothesis, which suggested that CHD results from genetic-environmental interaction (4). CHDs may be related to gross chromosomal or genetic aberrations, smoking or drinking, and illnesses in the mother during pregnancy. In recent times, the utilization of comprehensive techniques such as whole-exome sequencing (WES) and whole-genome sequencing (WGS), along with advanced bioinformatic tools and extensive, aggregated, population-based sequencing datasets, has facilitated the recognition of detrimental missense and loss-of-function (LOF) variants—collectively referred to as damaging variants. This approach also extends to the detection of small insertions or deletions, copy number variants (CNVs), and structural variants (5). These approaches identify damaging coding variants in definitive and candidate genes for CHD in 45% of patients with CHD (6–9). So far, more 400 genes have been implicated in the development and progression of CHD, encompassing transcription factors, cell signaling molecules, structural proteins, chromatin modifiers and cilia related proteins that are important for heart development (5, 10). CHD symptoms may present at birth or may appear later in life. Signs and symptoms of CHDs vary from mild to severe depending on the severity and type of the heart defect. Some heart defects might have little or no symptoms while others might have serious symptoms, including blueish skin, lips, or nails (cyanosis), hypersomnia, troubled breathing or fast breathing, poor circulation, getting unusually tired or breathing difficulty when exercising, heart murmur (a whooshing or swishing sound made by turbulent blood flow through the heart valves), and pounding heartbeat or weak pulse.
Psychological stress symptoms such as depression and anxiety are common among patients with CHDs. In addition, families with CHD patients are faced with financial hardships (11–13). Therefore, CHD is considered to be one of the leading diseases with the highest burden.
CHDs can further be categorized according to the degree of severity: mild CHDs with relatively little need for healthcare and severe or highly complex CHDs, which demand significantly greater expertise (14). Delayed diagnosis and intervention may promote relative complications such as severe cyanosis, heart failure, and other long-term sequelae in children (15–17). Recently, advances in cardiovascular diagnostics and medical treatments have critically improved the survival rate and the quality of life of patients with CHDs (18, 19). Thus, understanding the clinical profile of CHDs is important for better management of these diseases (20, 21).
CHD is a serious threat to the health of children and is one of the top five causes of death in children under 5 years of age in China. With rapid economic development in China, the mortality rate of CHD children under 5 years of age has decreased significantly in both rural and urban areas (22, 23). While the mortality rate of surgical treatment of simple CHD lesions is relatively lower in China than in European countries, the mortality rate of complex CHD lesions is significantly higher in China than in European countries (23). Regional disparities in health give rise to a significant future disease burden (24). Thus, data on the clinical symptoms, severity, urban-rural disparity and phenotypes of CHDs in China is urgently needed.
Hence, the current study was conducted to explore the clinical profiles and manifestations of CHD among patients receiving medical care at the Seventh Medical Center of the PLA General Hospital, which is a tertiary care center for patients from northern China, with a well-established pediatric cardiac surgery department. We established a clinical database to conduct further analysis of clinical profiles of CHDs in patients aged 0–18 years, which is of great significance in understanding the types and trends in CHDs in northern China. Findings from this study may help design more targeted treatment protocols and prevention measures.
This is a retrospective review of prospectively collected data. The study was performed at the Seventh Medical Center of the PLA General Hospital. Patients were identified by trained cardiologists in our center. All patients were enrolled between July 1, 2021 and June 30, 2022. A total of 265 patients with CHD aged 0–18 years were included in this study. This study was approved by the Ethics Committee of the Seventh Medical Center of PLA General Hospital (2022-195).
Clinical data were collected from the electronic medical record of the Seventh Medical Center of the PLA General Hospital. Clinical profiles and demographic data were obtained from cardiology and pediatric department registers.
Descriptive statistics, including age, region, International Classification of Diseases (ICD) diagnostic codes, and symptoms, were described as frequencies and percentages using GraphPad Prism version 7 for Windows (GraphPad, San Diego, CA). The chi-squared test was used to infer any differences between categorical variables. P-values of less than 0.05 were considered statistically significant. All statistical analyses were carried out using Statistical Package for the Social Sciences (SPSS) software version 20 (IBM Corp.; Armonk, NY, USA).
A total of 265 patients with CHD were enrolled, including 135 (50.9%) males and 130 (49.1%) females (Figure 1A), with a male-to-female ratio of 1.04:1. Of the 265 patients, 146 (55.1%) were from urban areas, 146 (44.2%) were from rural areas, and 2 (0.8%) were from Welfare center for children (Figure 1B). A total of 201 (75.8%) children were diagnosed with ACHD, while 64 (24.2%) children had CCHD (Figure 1C).
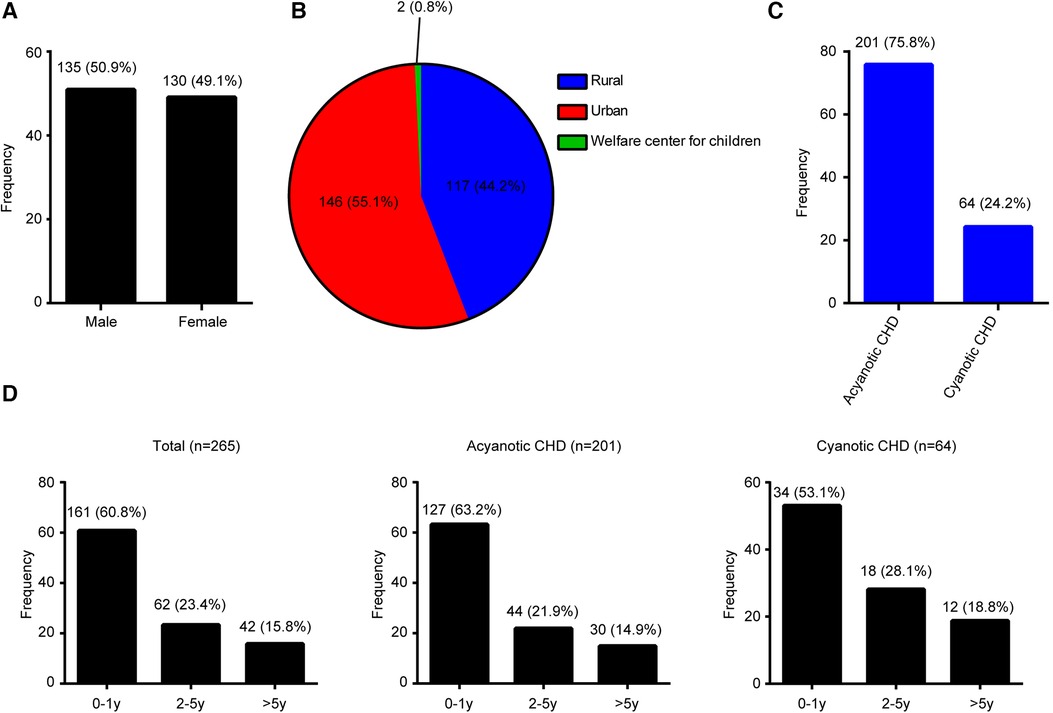
Figure 1. Disease distribution proportion in congenital heart disease patients. (A) Gender. (B) Location. (C) Type. (D) Age.
The age distribution of CHDs is shown in Figure 1D. The majority (60.8%, 161/265) of patients with CHDs were <1 year old, 23.4% (62/265) of patients were 1–5 years old, and 15.8% (42/265) were >5 years old. ACHD age distribution is shown in Figure 1D. About 63.2% (127/265) of patients were in the <1 year age group, 21.9% (44/265) were in the 1–5 years age group, and 14.9% (30/265) were in the >5 years age group. CCHD age distribution is shown in Figure 1D. Approximately 53.1% (34/265) of patients were in the <1 year age group, 28.1% (18/265) were in the 1–5 years age group, and 18.8% (12/265) were in the >5 years age group.
Based on the eleventh revision of the International Classification of Diseases (ICD-11), “congenital anomaly of a ventricle or the ventricular septum” constituted 124 cases, accounting for 46.80% of all CHD cases, followed by “congenital anomaly of a ventriculoarterial valve or adjacent regions” with 33 cases, accounting for 12.5% of all CHD cases, and “congenital anomaly of atrial septum” with 32 cases, accounting for 12.1% of all CHD cases. Other common types of CHD observed in CHD children at our hospital are listed in Table 1.
The prevalence of specific CHD types is prevented in Table 2. Our data share some variance with other studies (Table 2). Of all CHD types, ventricular septal defect (VSD) was the most common congenital cardiac anomaly in children (21, 25–30), accounting for 17.4% of all CHD cases in our study.
Information on the types, age distribution, and regional distribution of CHD is shown in Table 3. Out of 265 cases of CHD, 156 (58.9%) cases were simple lesions and 109 (41.1%) were complex lesions. About 22.3% of cases from rural areas were diagnosed as complex lesions after the first year of life, which was higher than those in urban areas (8.6%). This finding was statistically significant (P < 0.05). This may be because convenient and high-level medical conditions in urban areas enabled early detection of CHD.
The symptoms of CHDs were analyzed and the most common symptoms were murmur (239/265, 90.2%), followed by sweating (223/265, 84.2%), failure to thrive (183/265, 69.1%), fast breathing (181/265, 68.3%), cyanosis (140/265, 52.8%), difficulty during feeding (128/265, 48.3%), retraction of the chest (112/265, 42.3%), congestive heart failure (96/265, 36.2%), respiratory infections (64/265, 24.2%), cough (46/265, 17.4%), cyanotic spell (19/265, 7.2%), fever (10/265, 3.8%), chest stuffiness (5/265, 1.89%), edema (4/265, 1.51%), and hemoptysis (1/265, 0.4%) (Figure 2A).
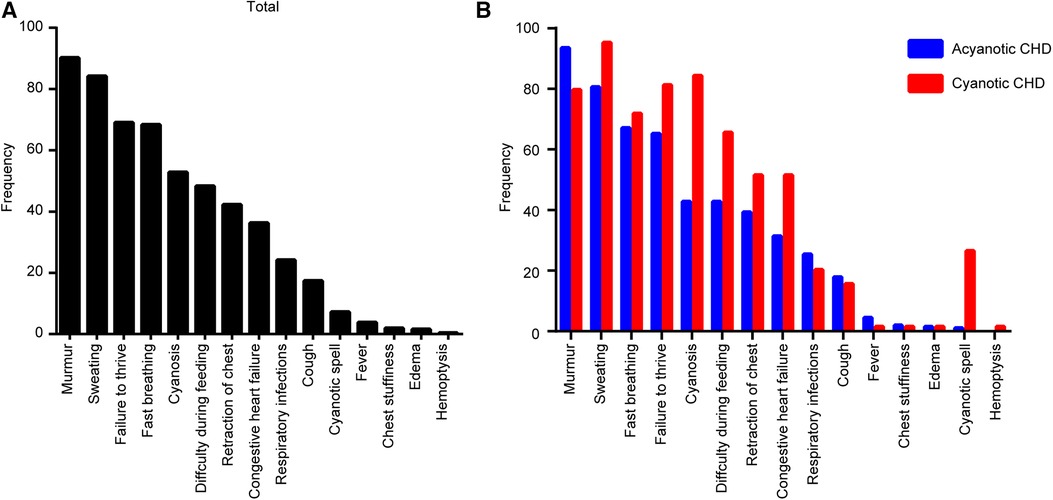
Figure 2. Clinical presentations associated with congenital heart disease (CHD) types in diagnosed children. (A) Total CHD. (B) Each type of CHD.
In the ACHD group, the most common symptoms included murmur (188/201, 93.5%), sweating (162/201, 80.6%), and fast breathing (135/201, 67.2%). In the CCHD group, the most common symptoms included sweating (61/64, 95.3%), cyanosis (54/64, 84.4%), and failure to thrive (52/64, 81.3%) (Figure 2B). Clinical symptoms of CHD are summarized in Table 4.
Clinical symptoms of CHD in our study and other studies are summarized in Table 5. Murmur was the most common symptom in the present study, which is consistent with the reported 49.7% and 81.4% in other studies (31, 32). Failure to thrive, fast breathing, and difficulty during feeding were the next common symptoms in other studies (21, 26, 31, 32).
A total of 265 patients with CHD were enrolled in this study, it showed that the majority of children with CHDs were below one-year-old, which is consistent with findings from previous studies (21, 33). The ratio between the number of males and females was approximately the same (1.04:1), which is comparable with that in previous reports (31, 34). However, this ratio was 2:1 in some studies (21, 35, 36). “Congenital anomaly of a ventricle or the ventricular septum” was the most common CHD according to ICD-11. Fast breathing and cyanosis were the most common symptoms in ACHD and CCHD, respectively.
Approximately 22.3% of patients hailing from rural regions presented with complex lesions during the initial year of their lives. This percentage significantly surpassed the corresponding figure for patients residing in urban localities, which stood at 8.6%. This data underscores the burden experienced by individuals living in rural areas with undetected complex CHD lesions. This suggests that restricted access to healthcare, coupled with insufficient resources and referral systems, likely contributed to the missed diagnosis of these lesions. Optimization of health care resource allocation in poor rural areas and low-income households to facilitate early screening is an urgent policy priority in reducing the rate of missed diagnosis of complex CHD lesions.
According to Mitchell et al. (37), CHD is a gross structural abnormality of the heart or intrathoracic great vessels that is actually or potentially of functional significance. CHD may be identified at virtually any age. Certain symptoms and signs generally appear in neonates while others are rarely diagnosed during infancy (38).
The clinical symptoms of CHD can vary by the type and severity of the defect. Based on the data obtained in our study and other studies published from 2014 to 2022 (21, 26, 31, 32), CHD symptoms include murmur, failure to thrive, fast breathing, and difficulty during feeding during infancy and early childhood, with murmur being the most common symptom in our study and some previous studies (31, 32). However, some studies reported breathing difficulty as the most common symptom (26, 31).
CHD is the leading cause of birth defect-related mortality, accounting for approximately 40% of all deaths in children with birth defects worldwide (39–41). Prenatal screening by fetal echocardiography and postnatal detection by pulse oximetry combined with clinical assessment are the most common methods for CHD screening, which enables early detection of CHD lesions and proper implementation of suitable treatments (18, 42–44).
With improvements in people's living standards, the selection of elective surgery is becoming more scientific and rational. In addition, patients' guardians can assist doctors in decision-making, based on a more scientific basis. CHD is one of the top five causes of death in children under 5 years of age in China. Based on China's large population, the burden of CHD will be formidable in terms of healthcare services and economic costs in the future. Thus, future research should primarily focus on improving the treatment of complex CHD lesions in China.
To the best of our knowledge, this is the first study to assess clinical profiles of CHDs in China with considerable sample size. Nevertheless, it is a single-center, hospital-based study and not a community-based study. This study is a retrospective study and may contain some incomplete or missing data. Furthermore, this study was not designed to investigate the potential risk factors as predictors of CHDs.
In summary, this study retrospectively analyzed the clinical characteristics of children with CHD diagnosed at a tertiary center in China. Approximately 75.8% of subjects were diagnosed with ACHD and 24.2% were diagnosed with CCHD. “Congenital anomaly of a ventricle or the ventricular septum”, “congenital anomaly of a ventriculoarterial valve or adjacent regions”, and “congenital anomaly of atrial septum” were the top three diseases based on ICD-11. VSD was the most common congenital cardiac anomaly and murmur was the most common symptom in children with CHD. This is one of the first studies in the pediatric age group in northern China and may help to better understand the clinical characteristics and prevalence of CHDs in this region. Moreover, our data may assist in formulating policies for better management of CHDs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by This study was approved by the Ethics Committee of the Seventh Medical Center of PLA General Hospital (2022-195). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because the retrospective design of the study.
All methods of this study were carried out in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Conceptualization, LS, YW, SH, GZ, and ZF; analysis, LS, YW, HW, and GW; resources, LS, HW, GW, NM, QM, KZ, and GZ; supervision and coordination, SH, GZ, and ZF; writing-original draft, LS, YW, SH, and GZ All authors provided discussed, participated in revising the manuscript. All author contributed to the article and approved the submitted version.
This study was supported by the Military Family Planning Special Research Project (20JSZ15).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CHD, congenital heart disease; ACHD, acyanotic congenital heart disease; CCHD, cyanotic congenital heart disease; ICD, International Classification of Diseases; VSD, Ventricular Septal Defect; ASD, Atrial Septal Defect; PDA, Patent Ductus Arteriosus; PS, Pulmonary Stenosis; COA, Coarctation of the Aorta; TGA, Transposition of Great Arteries; DORV, Double Outlet Right Ventricle; TAPVR, Total Anomalous Pulmonary Venous Return; AS, Aortic Stenosis; SV, Single Ventricle; PFO, Patent Foramen Ovale; TOF, Tetralogy of Fallot; TA, Truncus Arteriosus; IAA, Interrupted Aortic Arch; CECD, Complete Endocardial Cushion Defect; PMV, Prolapse of Mitral Valve; PA, Pulmonary Atresia; TR, Tricuspid Regurgitation; PI, Pulmonary Insufficiency; CCTGA, Congenitally Corrected Transposition of the Great Arteries; MV, Mitral Valve Insufficiency; MVS, Mitral Valve Stenosis; AI, Aortic Incompetence; PAS, Pulmonary Artery Sling; AOPA, Anomalous Origin of Pulmonary Artery from Ascending Aorta; CARP, Congenital Absence of Right Pulmonary Artery; TS, Tricuspid Stenosis; CAF, Coronary Artery Fistulae; CA, Common Atrium; CT, Cor Triatriatum; PH, Pulmonary Hypertension; ARSA, Aberrant Right Subclavian Arteries; RAA, Right Aortic Arch; PLSVC, Persistent Left Superior Vena Cava; AV malformation, Atrial Ventricular malformation.
1. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. (2002) 39:1890–900. doi: 10.1016/S0735-1097(02)01886-7
2. Liu Y, Chen S, Zuhlke L, Babu-Narayan SV, Black GC, Choy MK, et al. Global prevalence of congenital heart disease in school-age children: a meta-analysis and systematic review. BMC Cardiovasc Disord. (2020) 20:488. doi: 10.1186/s12872-020-01781-x
3. Tulloh RMR, Medrano-Lopez C, Checchia PA, Stapper C, Sumitomo N, Gorenflo M, et al. CHD and respiratory syncytial virus: global expert exchange recommendations. Cardiol Young. (2017) 27:1504–21. doi: 10.1017/S1047951117000609
4. Nora JJ. Multifactorial inheritance hypothesis for the etiology of congenital heart diseases. The genetic-environmental interaction. Circulation. (1968) 38:604–17. doi: 10.1161/01.CIR.38.3.604
5. Morton SU, Quiat D, Seidman JG, Seidman CE. Genomic frontiers in congenital heart disease. Nat Rev Cardiol. (2021) 19:26–42. doi: 10.1038/s41569-021-00587-4
6. Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease. Circ Res. (2013) 112:707–20. doi: 10.1161/CIRCRESAHA.112.300853
7. Jin SC, Homsy J, Zaidi S, Lu Q, Morton S, DePalma SR, et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat Genet. (2017) 49:1593–601. doi: 10.1038/ng.3970
8. Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. (2015) 350:1262–6. doi: 10.1126/science.aac9396
9. Sifrim A, Hitz M-P, Wilsdon A, Breckpot J, Turki SHA, Thienpont B, et al. Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat Genet. (2016) 48:1060–5. doi: 10.1038/ng.3627
10. Shabana NA, Shahid SU, Irfan U. Genetic contribution to congenital heart disease (CHD). Pediatr Cardiol. (2020) 41:12–23. doi: 10.1007/s00246-019-02271-4
11. O'Donovan CE, Painter L, Lowe B, Robinson H, Broadbent E. The impact of illness perceptions and disease severity on quality of life in congenital heart disease. Cardiol Young. (2016) 26:100–9. doi: 10.1017/S1047951114002728
12. McClung N, Glidewell J, Farr SL. Financial burdens and mental health needs in families of children with congenital heart disease. Congenit Heart Dis. (2018) 13:554–62. doi: 10.1111/chd.12605
13. Jackson AC, Frydenberg E, Liang RP, Higgins RO, Murphy BM. Familial impact and coping with child heart disease: a systematic review. Pediatr Cardiol. (2015) 36:695–712. doi: 10.1007/s00246-015-1121-9
14. Xie D, Fang J, Liu Z, Wang H, Yang T, Sun Z, et al. Epidemiology and major subtypes of congenital heart defects in Hunan Province, China. Medicine (Baltimore). (2018) 97:e11770. doi: 10.1097/MD.0000000000011770
15. Lui GK, Saidi A, Bhatt AB, Burchill LJ, Deen JF, Earing MG, et al. Diagnosis and management of noncardiac complications in adults with congenital heart disease: a scientific statement from the American heart association. Circulation. (2017) 136:e348–92. doi: 10.1161/CIR.0000000000000535
16. Mueller AS, McDonald DM, Singh HS, Ginns JN. Heart failure in adult congenital heart disease: tetralogy of fallot. Heart Fail Rev. (2020) 25:583–98. doi: 10.1007/s10741-019-09903-0
17. Oliver Ruiz JM. Congenital heart disease in adults: residua, sequelae, and complications of cardiac defects repaired at an early age. Rev Esp Cardiol. (2003) 56:73–88. doi: 10.1157/13042345
18. Krishna MR, Kumar RK. Diagnosis and management of critical congenital heart diseases in the newborn. Indian J Pediatr. (2020) 87:365–71. doi: 10.1007/s12098-019-03163-4
19. Bregman S, Frishman WH. Impact of improved survival in congenital heart disease on incidence of disease. Cardiol Rev. (2018) 26:82–5. doi: 10.1097/CRD.0000000000000178
20. Hoffman J. The global burden of congenital heart disease. Cardiovasc J Afr. (2013) 24:141–5. doi: 10.5830/CVJA-2013-028
21. Kishore S, Kumar M, Kumar A, Gupta A, Chandan C, Anshuman A, et al. Clinical and echocardiographic profile of congenital heart diseases in the 0−12-year age group in a tertiary care medical institute in eastern India: a retrospective, cross-sectional study. Cureus. (2022) 14:e26114. doi: 10.7759/cureus.26114
22. Liu Z, Liu XR, He CH, Miao L, Kang LN, Li XH, et al. Analysis of mortality and leading causes of death in Chinese children under 5-year-old between 2010 and 2016. Zhonghua Yu Fang Yi Xue Za Zhi. (2019) 53:411–4. doi: 10.3760/cma.j.issn.0253-9624.2019.04.016
23. Zheng G, Wang J, Chen P, Huang Z, Zhang L, Yang A, et al. Epidemiological characteristics and trends in postoperative death in children with congenital heart disease (CHD): a single-center retrospective study from 2005 to 2020. J Cardiothorac Surg. (2023) 18:165. doi: 10.1186/s13019-023-02224-2
24. Liu Z, Su Z, Li W, Zhang F, Ouyang W, Wang S, et al. Global, regional, and national time trends in disability-adjusted life years, mortality, and variable risk factors of non-rheumatic calcified aortic valve disease, 1990–2019: an age-period-cohort analysis of the global burden of disease 2019 study. J Thorac Dis. (2023) 15:2079–97. doi: 10.21037/jtd-23-480
25. Thomford NE, Biney RP, Okai E, Anyanful A, Nsiah P, Frimpong PG, et al. Clinical spectrum of congenital heart defects (CHD) detected at the child health clinic in a tertiary health facility in Ghana: a retrospective analysis. J Congenit Cardiol. (2020) 4:3. doi: 10.1186/s40949-020-00034-y
26. Mohammad N, Shaikh S, Memon S, Das H. Spectrum of heart disease in children under 5 years of age at Liaquat University Hospital, Hyderabad, Pakistan. Indian Heart J. (2014) 66:145–9. doi: 10.1016/j.ihj.2013.12.041
27. Ashraf M, Jan M, Rasool S, Shahzad N, Wanni K, Ahmed K. Prevalence and spectrum of congenital heart diseases in children. Heart India. (2014) 2:76. doi: 10.4103/2321-449X.140230
28. Bakhtyar Zahid S, Zeb Jan A, Ahmed S, Achakzai H. Spectrum of congenital heart disease in children admitted for cardiac surgery at Rehman Medical Institute, Peshawar, Pakistan. Pak J Med Sci. (2013) 29:173–6. doi: 10.12669/pjms.291.2910
29. Pate N, Jawed S, Nigar N, Junaid F, Wadood AA, Abdullah F. Frequency and pattern of congenital heart defects in a tertiary care cardiac hospital of Karachi. Pak J Med Sci. (2016) 32:79–84. doi: 10.12669/pjms.321.9029
30. Ekure EN, Kalu N, Sokunbi OJ, Kruszka P, Olusegun-Joseph AD, Ikebudu D, et al. Clinical epidemiology of congenital heart disease in Nigerian children, 2012−2017. Birth Defects Res. (2018) 110:1233–40. doi: 10.1002/bdr2.1361
31. Singh R, Rajaram Tawker N. The spectrum of congenital heart disease in children in the andaman and nicobar islands: a five-year retrospective study. Cureus. (2022) 14:e29109. doi: 10.7759/cureus.29109
32. Chelo D, Nguefack F, Menanga AP, Ngo Um S, Gody JC, Tatah SA, et al. Spectrum of heart diseases in children: an echocardiographic study of 1,666 subjects in a pediatric hospital, Yaounde, Cameroon. Cardiovasc Diagn Ther. (2016) 6:10–9. doi: 10.3978/j.issn.2223-3652.2015.11.04
33. Abqari S, Gupta A, Shahab T, Rabbani MU, Ali SM, Firdaus U. Profile and risk factors for congenital heart defects: a study in a tertiary care hospital. Ann Pediatr Cardiol. (2016) 9:216–21. doi: 10.4103/0974-2069.189119
34. Chinawa JM, Eze JC, Obi I, Arodiwe I, Ujunwa F, Daberechi AK, et al. Synopsis of congenital cardiac disease among children attending University of Nigeria Teaching Hospital Ituku Ozalla, Enugu. BMC Res Notes. (2013) 6:475. doi: 10.1186/1756-0500-6-475
35. Deeva Kumar B, Rami Reddy K, Elizabeth B. Study of incidence of congenital heart diseases in children of age group 1 month to 12 years. J Evol Med Dent Sci. (2015) 4:1151–9. doi: 10.14260/jemds/2015/162
36. Hussain M, Tahura S, Sayeed A, Rahman M, Rahman M, Kar SK. Past and present pattern of congenital heart disease at Dhaka Shishu Hospital: a situation analysis. Banglad J Child Health. (2012) 34:51–5. doi: 10.3329/bjch.v34i2.10217
37. Mitchell SC, Korones SB, Berendes HW. Congenital heart disease in 56,109 births. Incidence and natural history. Circulation. (1971) 43:323–32. doi: 10.1161/01.CIR.43.3.323
38. Silberbach M, Hannon D. Presentation of congenital heart disease in the neonate and young infant. Pediatr Rev. (2007) 28:123–31. doi: 10.1542/pir.28.4.123
39. Yang Q, Khoury MJ, Mannino D. Trends and patterns of mortality associated with birth defects and genetic diseases in the United States, 1979−1992: an analysis of multiple-cause mortality data. Genet Epidemiol. (1997) 14:493–505. doi: 10.1002/(SICI)1098-2272(1997)14:5%3C493::AID-GEPI4%3E3.0.CO;2-2
40. Petrini J, Damus K, Johnston RB Jr. An overview of infant mortality and birth defects in the United States. Teratology. (1997) 56:8–10. doi: 10.1002/(SICI)1096-9926(199707/08)56:1/2%3C8::AID-TERA3%3E3.0.CO;2-U
41. Roncancio CP, Misnaza SP, Pena IC, Prieto FE, Cannon MJ, Valencia D. Trends and characteristics of fetal and neonatal mortality due to congenital anomalies, Colombia 1999−2008. J Matern Fetal Neonatal Med. (2018) 31:1748–55. doi: 10.1080/14767058.2017.1326901
42. McGovern E, Sands AJ. Perinatal management of major congenital heart disease. Ulster Med J. (2014) 83:135–9.25484461
43. McClain MR, Hokanson JS, Grazel R, Van Naarden Braun K, Garg LF, Morris MR, et al. Critical congenital heart disease newborn screening implementation: lessons learned. Matern Child Health J. (2017) 21:1240–9. doi: 10.1007/s10995-017-2273-4
Keywords: congenital heart defects, clinical presentation, cardiovascular complications, pediatrics, pediatric cardiology
Citation: Song L, Wang Y, Wang H, Wang G, Ma N, Meng Q, Zhu K, Hu S, Zhou G and Feng Z (2023) Clinical profile of congenital heart diseases detected in a tertiary hospital in China: a retrospective analysis. Front. Cardiovasc. Med. 10:1131383. doi: 10.3389/fcvm.2023.1131383
Received: 25 December 2022; Accepted: 28 August 2023;
Published: 8 September 2023.
Edited by:
Xiangbin Pan, Peking Union Medical College, ChinaReviewed by:
Yuejuan Xu, Shanghai Jiao Tong University, China© 2023 Song, Wang, Wang, Wang, Ma, Meng, Zhu, Hu, Zhou and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siqi Hu aHVzaXFpXzIwMDBAMTYzLmNvbQ== Gengxu Zhou Y2FyZGlhY3N1cmdlb25AMTI2LmNvbQ== Zhichun Feng emhqZmVuZ3pjQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.