
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 17 May 2023
Sec. Cardiac Rhythmology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1130372
This article is part of the Research Topic Atrial Fibrillation: Selection of Management Strategy and Evaluation of Outcomes View all 39 articles
 Jihoon Kim1
Jihoon Kim1 Sung-Ji Park1*
Sung-Ji Park1* Dong Seop Jeong1*
Dong Seop Jeong1* Suryeun Chung2
Suryeun Chung2 Kina Jeon1
Kina Jeon1 Minjung Bak1
Minjung Bak1 Darae Kim1
Darae Kim1 Eun Kyoung Kim1
Eun Kyoung Kim1 Sung-A Chang1
Sung-A Chang1 Sang-Chol Lee1
Sang-Chol Lee1 Seung Woo Park1
Seung Woo Park1
Background: Left atrial (LA) fibrosis is related with development and severity of atrial fibrillation (AF). The aim of this study was to investigate the association between LA strain and LA fibrosis in patients undergoing totally thoracoscopic ablation (TTA) for AF.
Methods: Between February 2012 and March 2015, a total of 128 patients who underwent TTA were enrolled from a tertiary hospital. Left atrial appendage (LAA) was harvested during surgery to determine the degree of fibrosis. LAA fibrosis was classified as mild (1st quartile), moderate (2nd and 3rd quartile), or severe (4th quartile). Clinical outcome was 5-year recurrence rate of AF detected on electrocardiogram or 24 h Holter monitoring.
Results: The mean age was 54.3 ± 8.8 years and 18.8% had paroxysmal AF. Patients with mild LAA fibrosis had a significantly lower rate of recurrent AF (23.3%) at 5 years after TTA compared with those with moderate (51.4%; hazard ratio [HR] 2.69; 95% confidence interval [CI] 1.19–6.12) or severe (53.2%; HR 2.84; 95% CI 1.16–6.97) fibrosis. Among clinical and echocardiographic parameters, peak LA strain was the only predictor of mild LAA fibrosis (coefficient 0.10, p = 0.005) with the best cutoff value of 14.7% (area under the curve 0.732). The prevalence of mild LAA fibrosis was 40.6% in patients with peak LA strain ≥14.7%, but only 6.8% in those with peak LA strain <14.7%.
Conclusions: In patients undergoing TTA for AF, mild LAA fibrosis was associated with a lower risk of 5-year AF recurrence. LA strain was the only predictor of mild LAA fibrosis that reflects a lower risk of 5-year AF recurrence.
Atrial fibrillation (AF) is the most common arrhythmia and a growing disease affecting more than 3 million new cases each year (1). Structural atrial remodeling is a key element in AF development and progression. There are number of studies demonstrating the relationship between atrial fibrosis and AF (2, 3). Atrial fibrosis can be directly approached by invasive methods. Endomyocardial biopsy (4) or surgical biopsy during open heart surgery (5–7) allows pathological evaluation of atrial fibrosis. Electroanatomic mapping also can be used to detect areas of remodeling or fibrosis (8). As a noninvasive method, delayed enhancement magnetic resonance imaging (MRI) provides information on left atrial (LA) fibrosis and predicts outcome after catheter ablation of AF (9, 10).
Traditionally, echocardiographic assessment of LA remodeling has been limited to LA diameter and volume, or tissue velocity. LA strain by two-dimensional speckle-tracking echocardiography enables to assess myocardial function and cardiac chamber deformation. In patients with AF, LA strain can predict successful electrical cardioversion (11) or catheter ablation (12). Although several studies have attempted to identify the direct relationship between LA strain and extent of fibrosis, these studies were conducted in patients with mitral valve disease (13) or undergoing heart transplantation (14), not those with AF. The aim of this study was to evaluate the association of LA strain with LA fibrosis and outcomes in patients with AF undergoing thoracoscopic ablation.
Between February 2012 and March 2015, patients undergoing totally thoracoscopic ablation (TTA) were enrolled in a prospective registry in Samsung Medical Center, Republic of Korea. Patients with AF refractory to at least 1 antiarrhythmic drug or electrical cardioversion were candidates for TTA. The indications for TTA also included refractory symptom, history of stroke, or intolerance for anticoagulant therapy. TTA was contraindicated in patients with LA thrombi or who were intolerant of one-lung ventilation (15, 16).
Among a total of 150 consecutive patients who underwent TTA during the study period, 22 patients were excluded from the present study as follows: (1) those who did not undergo adequate surgical procedure due to huge LA or severe pericardial adhesion (3 patients), or who underwent left atrial appendage (LAA) resection alone for stroke prevention (1 patient); (2) those who did not undergo LAA resection during the surgery (17 patients); (3) who did not have adequate echocardiographic images for analysis. Finally, 128 patients were eligible for final analysis (Figure 1). Eligible patients were divided into 3 groups according to the interquartile range of degree of LAA fibrosis as mild (1st quartile), moderate (2nd and 3rd quartile), or severe (4th quartile).
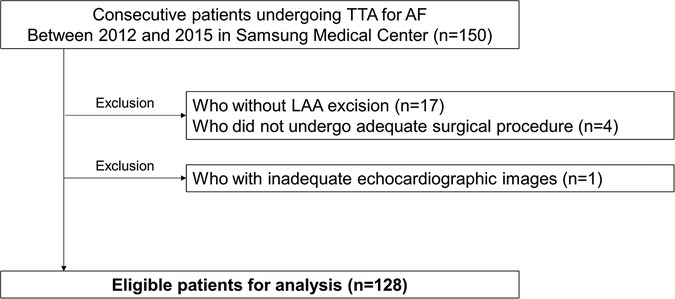
Figure 1. Study population. AF, atrial fibrillation; LAA, left atrial appendage; TTA, totally thoracoscopic ablation.
The Samsung Medical Center Institutional Review Bord approved the present study (2020-05-146-003).
TTA was a video-assisted thoracoscopic surgical technique without thoracotomy and cardiopulmonary bypass. A bilateral approach was required, and the detailed techniques of TTA were described in our previous report (17). Ablation lines for pulmonary vein isolation were created using an AtriCure Isolator Transpolar Clamp (Atricure, Inc., Cincinnati, Ohio) and the left atrial roof and floor lesions connecting both pulmonary veins were created with a linear pen device (Atricure, Inc., Cincinnati, Ohio). After creating pulmonary vein isolation and box lesions, exit and entrance block test using an AtriCure Cooltip pen (Atricure, Inc., Cincinnati, Ohio) are performed. Next, the ganglionated plexuses were checked and ablated. The ligament of Marshall, which might be a source of adrenergic atrial tachycardia, was always divided and ablated (18). LAA was removed by stapling with an Echelon Flex 60 articulating endoscopic linear stapler (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA).
Patients were monitored in intensive care unit for the first 24 h. Heparin was administered after 2 h postoperatively and conversion to either warfarin or novel oral anticoagulant as soon as possible. Oral amiodarone was prescribed if the heart rate was >80 beats/min with AF rhythm at rest. Patients were followed up at 3, 6, and 12 months, and annually thereafter with 24 h Holter monitoring. Antiarrhythmic drugs were discontinued after 3 months or up to 6 months based on the results of 24 h Holter monitoring. Anticoagulants were also discontinued after 3 months considering the risk of stroke for each patient.
Clinical outcome was recurrent AF or atrial flutter (AFL) detected on electrocardiogram or lasting more than 30 s in 24 h Holter monitoring during the 5 years after TTA. The median follow-up duration of study subjects was 5.0 years.
We analyzed the pathology of LAA after resection. Portion of each LA tissue (about 2 × 4 mm to 4 × 10 mm) was fixed in 10% buffered formalin and embedded in paraffin (Figure 2). To quantify the extent of atrial fibrosis, sections (5 µm) stained with Picro Sirius red were scanned (×400) and the percentage of fibrosis area was automatically measured using the Image Pro Plus program (Media Cybernetics, Inc., Bethesda, MD). Because automatic measure of myocardial fibrosis tends to overestimate the real pathologic sclerotic tissue, we excluded non-pathologic fibrosis areas such as peri-adventitial, adipose tissue, and tangentially cut subendocardial portions. Average values of 5 random fields from each slide were calculated (19).
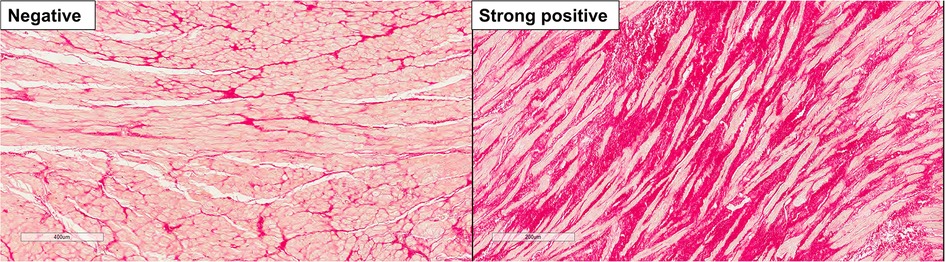
Figure 2. Representative histologic images of left atrial appendage fibrosis. Left panel, negative staining for fibrosis; right panel, strong positive staining for fibrosis.
Preoperative comprehensive transthoracic echocardiography was performed with commercially available equipment (Vivid 7, GE Medical Systems, Milwaukee, WI, USA; Acuson 512, Siemens Medical Solution, Mountain View, CA, USA, or; Sonos 5500, Philips Medical System, Andover, MA, USA) according to the practice guideline (20). Left ventricular end-diastolic and end-systolic diameter, and LA diameter were calculated from parasternal long-axis view. Left ventricular ejection fraction were calculated from 2-dimensional recordings using the modified biplane Simpson's method. LA volume was assessed by the modified biplane area-length method and was indexed to body surface area (LA volume index, LAVI). Early diastolic mitral inflow velocity (E) was measured using the pulsed wave Doppler method, by placing the sample volume at the level of the mitral valve leaflet tips. The tissue Doppler-derived early diastolic mitral annular velocity (e′) was measured from the septal corner of the mitral annulus in the apical 4-chamber view. For patients with AF rhythm, the average of 5 consecutive doppler signals was used.
Peak longitudinal LA strain (reservoir strain) was calculated using vender-independent dedicated software (2D cardiac Performance Analysis 1.4, Tomtec Imaging System) according to the current guideline (Supplementary Figure S1) (21). LA endocardial border was automatically traced by the software then was manually adjusted in both apical four- and two-chamber views. Pulmonary veins and LAA orifices were carefully excluded. The regions of interest encompassed endocardial border of the mitral annulus, and the thickness of regions of interest was adjusted to the thinnest part to adapt to the atria. The data were analyzed by two independent echocardiologists blinded to clinical status. All strain values were calculated from cardiac cycle with heart rate less than 110 per minute to avoid inadequate LA emptying or filling. LA stiffness index was defined as E/e′ divided by peak longitudinal LA strain.
Continuous variables were presented as mean ± standard deviation or median [interquartile range]. Categorical variables were compared using the chi-square test. Continuous variables were compared using the Student's t test or Mann–Whitney U test as appropriate. Distribution of patients with mild, moderate, and severe LAA fibrosis was assessed according to the median value of LA strain, LA diameter and LAVI, paroxysmal AF, or LA stiffness index in baseline characteristics of the overall population. The incidence of recurrent AF or AFL at 5 years after TTA were estimated using the Kaplan-Meier method. Survival curves were compared with the log-rank test. The best cutoff value of extent of LAA fibrosis to maximize the difference in 5-year recurrence rate was estimated by plotting the standardized log-rank statistic. Association of LAA fibrosis extent with clinical or echocardiographic parameters was analyzed using logistic regression. In multivariable logistic regression analysis, strain-derived indices (LA strain and stiffness index) were not adjusted simultaneously because of multicollinearity. Receiver-operating characteristic analysis was used to find the best cutoff value of echocardiographic parameters for predicting mild LAA fibrosis.
Since the rhythm status during echocardiography may affect LA strain measurements, we conducted a subgroup analysis that included only patients with non-paroxysmal AF. All tests were two-sided, and a p value <0.05 was considered statistically significant. Statistical analysis was performed using R 3.6.2 (R Foundation for Statistical Computing).
Of 128 study subjects, mean age was 54.3 ± 8.8 years, 95.3% were male, and 18.8% had paroxysmal AF (Table 1). The median LA diameter and LAVI was 45.0 mm (40.0‒50.0) and 45.2 ml/m2 [36.4‒54.8], respectively. A median value of LA strain was 15.3% [12.1‒19.2] and a median value of LAA fibrosis extent was 38.6% [33.1‒44.7].
Patients were divided into 3 groups according to the interquartile range of degree of LAA fibrosis as mild (1st quartile), moderate (2nd and 3rd quartile), or severe (4th quartile). Peak LA strain and LA stiffness index were significantly different among the 3 groups, but other echocardiographic parameters including LA diameter and LAVI were not. Clinical variables were not significantly different among the 3 groups except for gender.
During the 5-year follow-up period, the risk of recurrent AF or AFL was significantly different according the 3 groups (log rank p = 0.035, Figure 3). The risk of 5-year AF or AFL recurrence was significantly lower in patients with mild LAA fibrosis (23.3%) compared with those with moderate (51.4%, hazard ratio [HR] 2.69; 95% confidence interval [CI] 1.19‒6.12; p = 0.018) and those with severe (53.2%; HR 2.84; 95% CI 1.16‒6.97; p = 0.023) LAA fibrosis.
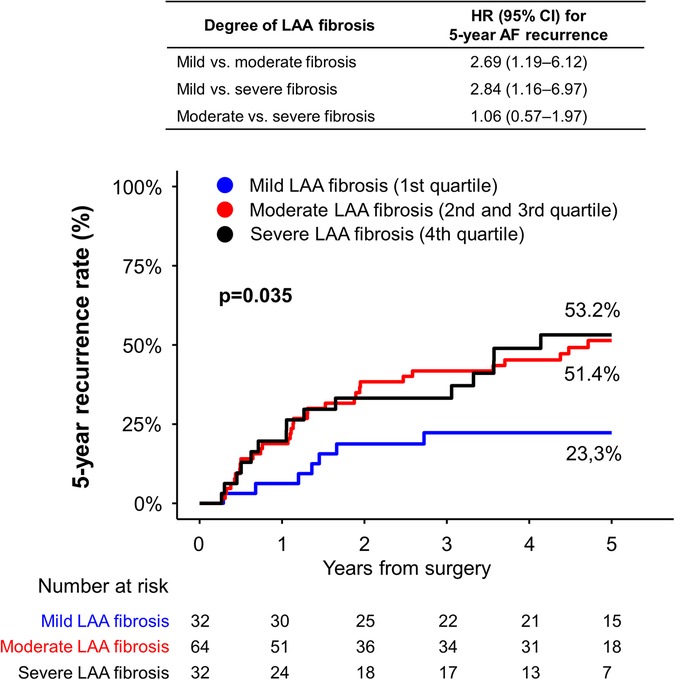
Figure 3. Five-year incidence of recurrent AF or AFL after thoracoscopic ablation according to degree of fibrosis. The degree of fibrosis was defined as mild (the first quartile), moderate (the second and third quartile), or severe (the fourth quartile). There was a significant difference in the risk of recurrent AF or AFL among the fibrosis group. AF, atrial fibrillation; AFL, atrial flutter; CI, confidence interval; HR, hazard ratio; LAA, left atrial appendage.
The best cutoff value of degree of LAA fibrosis for the risk of 5-year AF or AFL recurrence was 33.6%, which was similar to the definition of mild LAA fibrosis (Supplementary Figure S2).
Figure 4 shows distributions of the 3 fibrosis groups according to the median value of baseline variables. In patients with LA strain ≥15.3%, 40.6% of patients had mild LAA fibrosis, whereas only 9.4% had mild fibrosis in those with LA strain <15.3% (p < 0.001). The proportion of mild LAA fibrosis was not significantly different between LA diameter <45 vs. ≥45 mm, LAVI <45.2 vs. ≥45.2 ml/m2, paroxysmal AF vs. non-paroxysmal AF, or LA stiffness index <0.5%−1 vs. ≥0.5%−1.
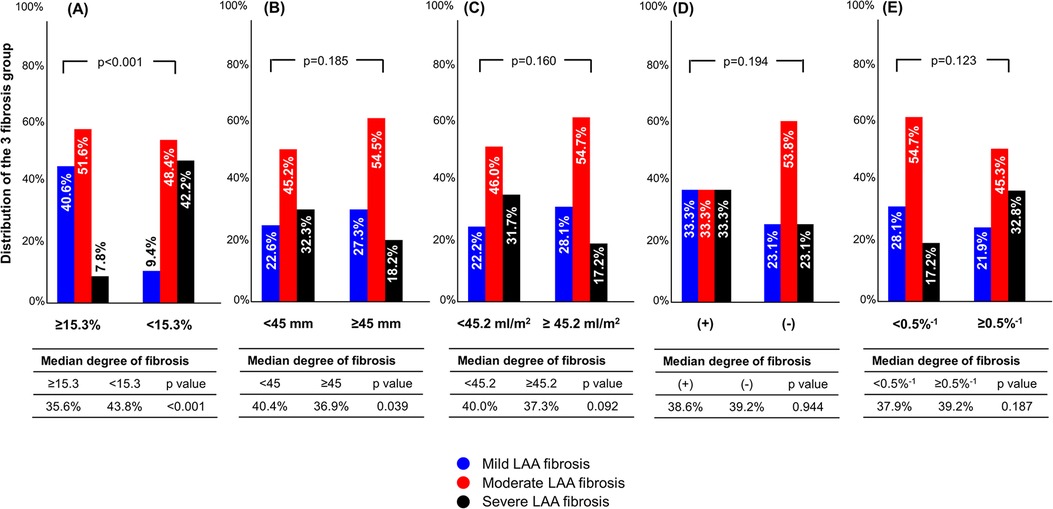
Figure 4. Distribution of fibrosis group according to preoperative parameters. The cutoff values were determined as the median of baseline parameters in overall population. (A) LA strain; (B), LA diameter, (C), LA volume index; (D), paroxysmal AF; (E), LA stiffness index. AF, atrial fibrillation; LA, left atrium.
Lower LA strain was the only parameter significantly associated with mild LAA fibrosis in univariable and multivariable analysis (Table 2).
The best cutoff value of LA strain for predicting mild LAA fibrosis was 14.7% with area under the curve of 0.732 (Figure 5). In patients with LA strain ≥14.7%, 40% of patients had mild LAA fibrosis, whereas only 6.9% had mild LAA fibrosis in those with LA strain >14.7% (p = 0.001).
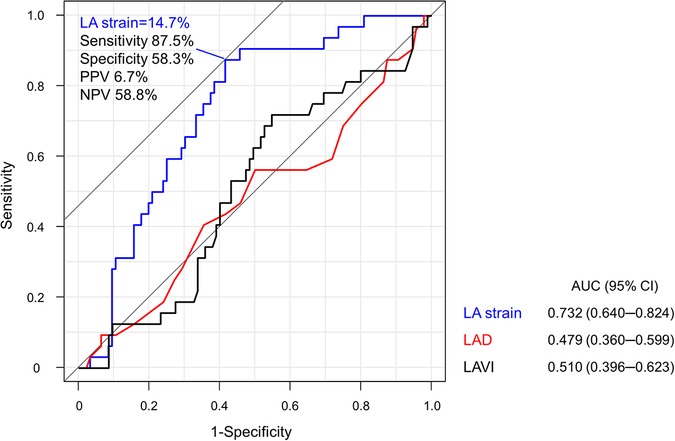
Figure 5. Receiver-operating characteristic analysis for the optimal cutoff value to predict mild LAA fibrosis. LA strain of 14.7% was the best cutoff value for predicting mild LAA fibrosis. LA, left atrium; LAD, left atrial diameter; LAVI, left atrial volume index; NPV, negative predictive value; PPV, positive predictive value.
A total of 104 patients with non-paroxysmal AF were included in a subgroup analysis, comprising 24 (23.1%) with mild LAA fibrosis, 56 (53.8%) with moderate LAA fibrosis, and 24 (23.1%) with severe LAA fibrosis. Significant differences were observed in LA strain and LA stiffness index among the three groups (Supplementary Table S1). Of the clinical and echocardiographic variables, only LA strain was significantly associated with mild LAA fibrosis (Supplementary Table S2).
The main findings of this study were as follows. First, mild LAA fibrosis was associated with a lower risk of recurrent AF or AFL at 5 years after TTA for AF. However, the risk of recurrent AF or AFL was not significantly different between the moderate and severe LAA fibrosis. Second, LA strain was the only parameter for predicting mild LAA fibrosis invasively assessed, whereas the conventional echocardiographic parameters such as LA diameter and LAVI were not.
Atrial fibrosis is a main pathophysiology that disrupts electrical activity and results in conduction abnormalities (3, 5, 22). In patients with AF, attempts to predict outcomes by quantifying atrial fibrosis have been largely based on MRI. Degree of fibrosis assessed by delayed enhancement MRI is known to be independently associated with arrhythmia recurrence after catheter ablation (9, 10, 23, 24). However, data on the prognosis of rhythm control management in patients with AF based on histologic evaluation are limited. In observational studies analyzing surgical specimens, advanced LA fibrosis was related with an unsuccessful maze operation (7) or a reduced recovery of atrial mechanical contraction (19). Recently, TTA has been highlighted with minimal invasiveness and favorable outcomes (25–27) because traditional maze procedure is highly invasive in patients not planned for cardiac surgery. Ma et al. reported that the degree of LAA fibrosis was an independent predictor of 5-year recurrence rate after TTA (28). In our study, only mild LAA fibrosis was significantly associated with a lower recurrence rate compared with severe fibrosis, but moderate fibrosis was not. The different findings between the 2 TTA studies may be due to patients' characteristics. Our study patients had more advanced AF, such as larger LA diameter, higher prevalence of persistent AF and history of previous catheter ablation, and higher recurrence rate compared with the study by Ma et al. Our finding suggests that more complex or enhanced therapeutic strategies may be needed to prevent AF recurrence in patients with advanced atrial fibrosis (10).
Limited data are available on the relationship between echocardiographic parameters and degree of fibrosis determined by histopathology in patients with AF. In this issue, LA strain has emerged as a potential parameter representing atrial function and remodeling. In patients undergoing mitral valve surgery, LA strain was independently correlated with the degree of LA fibrosis (13, 29). In these studies, however, all patients had either severe mitral stenosis or mitral regurgitation resulting in chronic pressure or volume overload on LA, and a large number of patients had no AF. A recent study reported that LA strain had a good correlation with degree of LA fibrosis, but the results were derived from patients with advanced heart failure undergoing heart transplantation (14). In our study, LA strain was an independent predictor of mild LAA fibrosis that was related with favorable outcome after TTA. Patients with LA strain ≥15.3%, comped with those with LA strain <15.3%, had a 4-fold higher prevalence of mild LAA fibrosis. Whereas classic indices such as LA diameter, LAVI, E/e′, or paroxysmal AF were not associated with the degree of LAA fibrosis. In line with previous studies, our study suggests that in patients with lone AF, LA fibrosis cannot be predicted by classic morphologic parameters, while LA strain predicts the atrial fibrosis and provides prognostic information. However, it should be noted that these findings were derived from patients undergoing TTA Therefore, it is necessary to verify the clinical implications of LA strain in a wider population of individuals with AF. The absence of a significant difference in fibrosis degree between patients with lower and higher LA stiffness index may be attributed to the lack of a significant difference in E/e′ values among the mild, moderate, and severe fibrosis groups. This could have resulted in a dilution of the difference in LA strain, leading to our observed findings.
Several factors may influence LA strain measurements, such as the patient's rhythm status during measurements or prior radiofrequency catheter ablation. However, we observed a consistent association between higher LA strain and less LAA fibrosis after excluding patients with paroxysmal AF. Given that one of the indications of thoracoscopic surgical ablation is for patients who have failed percutaneous AF ablation (30), a history of prior radiofrequency ablation is relatively common in TTA candidates. In a meta-analysis of TTA, 52.9% of patients had a history of one or more failed catheter ablation (31). Therefore, the prevalence of prior radiofrequency catheter ablation of 15.6% in our study is not particularly high for use of LA strain in this population. In fact, out of 108 patients without previous RFCA, those with mild, moderate, and severe LAA fibrosis had median LA strain values of 18.7% [15.8–20.9], 15.6% [13.1–19.2], and 10.7% [9.6–13.8], respectively (p < 0.001).
Whether the LAA fibrosis is representative of LA fibrosis is a separate issue. A previous autopsy study found similar degrees of fibrosis in different locations of the LA in patients with AF (32). Moreover, a study using high-density bipolar voltage mapping suggested that voltage reduction in the LA is a diffuse process associated with fibrosis, indicating that AF-related structural remodeling is widespread (33). Our study revealed a significant association between mild LAA fibrosis and a reduced risk of AF recurrence after TTA, which could be predicted by LA strain, a surrogate for LA remodeling. These findings suggest that LAA fibrosis may serve as a marker for whole LA remodeling in patients with AF, which is consistent with previous studies.
Our study had several limitations. First, this was a single-center retrospective study. Characteristics of patients and experience with TTA might be different by centers. Second, the best cutoff value or predictive power of LA strain might be different by image quality of echocardiography, experience for delineation of region of interests, or speckle-tracking software (21). Third, the present findings were derived from limited population who underwent TTA. However, the relationship between preoperative LA strain and degree of fibrosis would not be influenced by treatment modality. In addition, although data are limited, studies on Maze procedure also have suggested the degree of atrial fibrosis as a prognostic factor after AF ablation. Last, as this study was observational, it may not have had sufficient statistical power to evaluate the predictive value of several echocardiographic variables, such as LA diameter and LA volume index, on LAA fibrosis. However, our findings suggest that patients with LA enlargement did not have more fibrosis than those without LA enlargement, either numerically or statistically.
The strength of our study is that it directly compared noninvasive parameters with histologically confirmed atrial fibrosis in patients with AF and provides prognostic implications of atrial fibrosis and LA strain in rhythm control management. Future large-scale studies are needed to confirm the current findings.
In patients with AF, mild LAA fibrosis was associated with a lower risk of 5-year AF recurrence after TTA. LA strain was the only predictor of mild LAA fibrosis that reflects a lower risk of 5-year AF recurrence.
The raw data supporting the conclusions of this article will be made available from the corresponding author [DSJ], upon reasonable request.
The studies involving human participants were reviewed and approved by The Samsung Medical Center Institutional Review Bord (2020-05-146-003). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JK, S-JP, and DJ conceived of the presented idea and designed the study. S-JP encouraged JK to investigate a statistical analysis and supervised the findings of this work. All authors discussed the results and contributed to the final manuscript. JK wrote the manuscript with support from S-JP and DJ. SC, KJ, MB, DK, EKK, S-AC, and SWP contributed to the interpretation of the results. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1130372/full#supplementary-material.
AF, atrial fibrillation; AFL, atrial flutter; LA, left atrium; LAA, left atrium appendage; LAVI, left atrium volume index; MRI, magnetic resonance imaging; TTA, totally thoracoscopic ablation.
1. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. (2021) 16(2):217–21. doi: 10.1177/1747493019897870
2. Tan TC, Koutsogeorgis ID, Grapsa J, Papadopoulos C, Katsivas A, Nihoyannopoulos P. Left atrium and the imaging of atrial fibrosis: catch it if you can!. Eur J Clin Invest. (2014) 44(9):872–81. doi: 10.1111/eci.12305
3. Kostin S, Klein G, Szalay Z, Hein S, Bauer EP, Schaper J. Structural correlate of atrial fibrillation in human patients. Cardiovasc Res. (2002) 54(2):361–79. doi: 10.1016/s0008-6363(02)00273-0
4. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. (1997) 96(4):1180–4. doi: 10.1161/01.cir.96.4.1180
5. Goette A, Juenemann G, Peters B, Klein HU, Roessner A, Huth C, et al. Determinants and consequences of atrial fibrosis in patients undergoing open heart surgery. Cardiovasc Res. (2002) 54(2):390–6. doi: 10.1016/s0008-6363(02)00251-1
6. Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. (2004) 109(3):363–8. doi: 10.1161/01.Cir.0000109495.02213.52
7. Kainuma S, Masai T, Yoshitatsu M, Miyagawa S, Yamauchi T, Takeda K, et al. Advanced left-atrial fibrosis is associated with unsuccessful maze operation for valvular atrial fibrillation. Eur J Cardiothorac Surg. (2011) 40(1):61–9. doi: 10.1016/j.ejcts.2010.11.008
8. Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. (2004) 43(11):2044–53. doi: 10.1016/j.jacc.2003.12.054
9. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. (2009) 119(13):1758–67. doi: 10.1161/circulationaha.108.811877
10. Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally S, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J Cardiovasc Electrophysiol. (2011) 22(1):16–22. doi: 10.1111/j.1540-8167.2010.01876.x
11. Costa C, González-Alujas T, Valente F, Aranda C, Rodríguez-Palomares J, Gutierrez L, et al. Left atrial strain: a new predictor of thrombotic risk and successful electrical cardioversion. Echo Res Pract. (2016) 3(2):45–52. doi: 10.1530/erp-16-0009
12. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. (2011) 57(3):324–31. doi: 10.1016/j.jacc.2010.05.063
13. Her AY, Choi EY, Shim CY, Song BW, Lee S, Ha JW, et al. Prediction of left atrial fibrosis with speckle tracking echocardiography in mitral valve disease: a comparative study with histopathology. Korean Circ J. (2012) 42(5):311–8. doi: 10.4070/kcj.2012.42.5.311
14. Lisi M, Mandoli GE, Cameli M, Pastore MC, Righini FM, Benfari G, et al. Left atrial strain by speckle tracking predicts atrial fibrosis in patients undergoing heart transplantation. Eur Heart J Cardiovasc Imaging. (2022) 23(6):829–35. doi: 10.1093/ehjci/jeab106
15. Kwon H-J, Jeong DS, Park S-J, Park K-M, Kim JS, On YK. Long-term outcome of totally thoracoscopic surgical ablation in atrial fibrillation: a single-center experience. IJC Heart & Vasculature. (2021) 36:100861. doi: 10.1016/j.ijcha.2021.100861
16. Lim SK, Kim JY, On YK, Jeong DS. Mid-term results of totally thoracoscopic ablation in patients with recurrent atrial fibrillation after catheter ablation. J Chest Surg. (2020) 53(5):270–6. doi: 10.5090/kjtcs.19.059
17. On YK, Park KM, Jeong DS, Park PW, Lee YT, Park SJ, et al. Electrophysiologic results after thoracoscopic ablation for chronic atrial fibrillation. Ann Thorac Surg. (2015) 100(5):1595–602; discussion 602–3. doi: 10.1016/j.athoracsur.2015.04.127
18. Wolf RK, Schneeberger EW, Osterday R, Miller D, Merrill W, Flege JB Jr, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg. (2005) 130(3):797–802. doi: 10.1016/j.jtcvs.2005.03.041
19. Park SJ, On YK, Kim JS, Choi JO, Ju ES, Jeong DS, et al. Transforming growth factor β1-mediated atrial fibrotic activity and the recovery of atrial mechanical contraction after surgical maze procedure. Int J Cardiol. (2013) 164(2):232–7. doi: 10.1016/j.ijcard.2013.01.066
20. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
21. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19(6):591–600. doi: 10.1093/ehjci/jey042
22. Krul SP, Berger WR, Smit NW, van Amersfoorth SC, Driessen AH, van Boven WJ, et al. Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythm Electrophysiol. (2015) 8(2):288–95. doi: 10.1161/circep.114.001752
23. Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. Jama. (2014) 311(5):498–506. doi: 10.1001/jama.2014.3
24. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. (2014) 7(1):23–30. doi: 10.1161/circep.113.000689
25. Vos LM, Bentala M, Geuzebroek GS, Molhoek SG, van Putte BP. Long-term outcome after totally thoracoscopic ablation for atrial fibrillation. J Cardiovasc Electrophysiol. (2020) 31(1):40–5. doi: 10.1111/jce.14267
26. Castellá M, Kotecha D, van Laar C, Wintgens L, Castillo Y, Kelder J, et al. Thoracoscopic vs. catheter ablation for atrial fibrillation: long-term follow-up of the FAST randomized trial. EP Europace. (2019) 21(5):746–53. doi: 10.1093/europace/euy325
27. Kim HJ, Kim JS, Kim TS. Epicardial thoracoscopic ablation versus endocardial catheter ablation for management of atrial fibrillation: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. (2016) 22(6):729–37. doi: 10.1093/icvts/ivw027
28. Ma N, Lu R, Zhao D, Jiang Z, Tang M, Bao C, et al. Left atrial appendage fibrosis and 3-year clinical outcomes in atrial fibrillation after endoscopic ablation: a histologic analysis. Ann Thorac Surg. (2020) 109(1):69–76. doi: 10.1016/j.athoracsur.2019.05.055
29. Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. (2013) 111(4):595–601. doi: 10.1016/j.amjcard.2012.10.049
30. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2020) 00:1–125. doi: 10.1093/eurheartj/ehaa612
31. Vos LM, Kotecha D, Geuzebroek GSC, Hofman FN, van Boven WJP, Kelder J, et al. Totally thoracoscopic ablation for atrial fibrillation: a systematic safety analysis. Europace. (2018) 20(11):1790–7. doi: 10.1093/europace/eux385
32. Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. (2011) 58(21):2225–32. doi: 10.1016/j.jacc.2011.05.061
33. Yamaguchi T, Otsubo T, Takahashi Y, Nakashima K, Fukui A, Hirota K, et al. Atrial structural remodeling in patients with atrial fibrillation is a diffuse fibrotic process: evidence from high-density voltage mapping and atrial biopsy. J Am Heart Assoc. (2022) 11(6):e024521. doi: 10.1161/JAHA.121.024521
Keywords: atrial fibrillation, ablation, fibrosis, strain, prognosis
Citation: Kim J, Park S-J, Jeong DS, Chung S, Jeon K, Bak M, Kim D, Kim EK, Chang Sung-A, Lee S-C and Park SW (2023) Left atrial strain predicts fibrosis of left atrial appendage in patients with atrial fibrillation undergoing totally thoracoscopic ablation. Front. Cardiovasc. Med. 10:1130372. doi: 10.3389/fcvm.2023.1130372
Received: 23 December 2022; Accepted: 24 April 2023;
Published: 17 May 2023.
Edited by:
Teresa Strisciuglio, University of Naples Federico II, ItalyReviewed by:
Albert Sinusas, Yale University, United States© 2023 Kim, Park, Jeong, Chung, Jeon, Bak, Kim, Kim, Chang, Lee and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung-Ji Park dHljaGUucGFya0BnbWFpbC5jb20= Dong Seop Jeong b3BoZWFydDFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.