
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 10 May 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1130237
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2022 View all 39 articles
Unicentric Castleman disease (UCD) is a rare, benign lymphoproliferative disorder. Mediastinal UCD has tumors with no clear boundaries that are highly vascularized. Resection surgery results in bleeding, leading to further challenges. Mixed-type UCD is rare. We report the case of a 38-year-old asymptomatic patient with mixed-type UCD; the tumor measured 7.8 cm in size and had unclear boundaries. The tumor was successfully resected by performing a cardiopulmonary bypass on the beating heart; the patient recovered uneventfully.
Castleman disease (CD), first reported by Castleman and Towne in 1954, is a rare, benign lymphoproliferative disorder (1). CD can be divided into two categories: unicentric Castleman disease (UCD) and multicentric Castleman disease (MCD). Mediastinal UCD is a common type of UCD that is accompanied by prominent feeding vessels. Owing to the lack of typical clinical signs, CD is difficult to diagnose. Herein, we report the case of a 38-year-old asymptomatic patient with mixed-type UCD; the tumor measured 7.8 cm in size and had unclear boundaries. The tumor was successfully resected by performing a cardiopulmonary bypass (CPB) on the beating heart.
A 38-year-old man with no obvious symptoms was found to have a mediastinal tumor during a physical examination. The patient had no significant medical history except having diabetes mellitus for a year. He was referred to our hospital for medical treatment. No pathologic signs were found through other physical examinations. His laboratory test results were all within the normal limits, except for the lymphocyte count (13.1%), which was lower than the normal range. The levels of tumor markers alpha-fetoprotein, human chorionic gonadotropin, and lactate dehydrogenase were all normal. The thoracic computed tomography (CT) scan revealed a huge soft tissue density shadow of 72 × 48 mm in the mediastinum. The tumor was compressed near the main pulmonary artery bifurcation and extended along the right pulmonary artery and did not have clear boundaries (Figure 1). His cardiac ultrasonography revealed a huge pericardial effusion, approximately 28 mm. Considering the location of the tumor and the risk of bleeding, we did not schedule a biopsy for him. The tumor was planned for resection via median sternotomy.
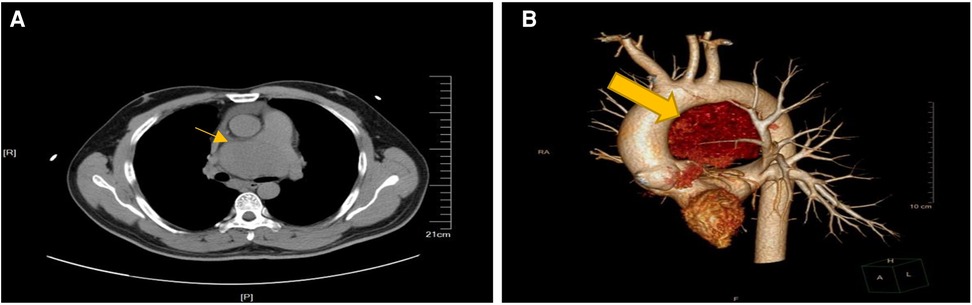
Figure 1. Preparative computed tomography scan of the chest showing in the anterior mediastinum. (A) The mass close to the main pulmonary artery bifurcation and extended along the right pulmonary artery. (B) Lateral view of 3-D computerized tomography showing the mass between aortic arch and pulmonary artery.
After sternotomy, the tumor was observed to occupy the area within the aorta-pulmonary window, which also severely compressed the cardiac left atrium. The tumor was vascularized and thus was highly susceptible to bleeding. Disassociation of the pulmonary arteries and the left atrium was essential for successful and complete resection of the tumor. The tumor was huge, and dissection of the tumor was attempted from the pulmonary artery and the left atrium, but the patient was hemodynamically unstable. To guarantee the safety of the patient, the surgical team decided to complete the surgery by performing CPB on the beating heart as an adjunct surgical support. CPB was performed via cannulation of the femoral artery and superior and inferior vena cava. The pressure in the pulmonary artery and heart was reduced through CPB. The aorta and pulmonary artery were pulled away to expose the tumor completely, and then, the tumor was meticulously dissected along the margins from the surrounding tissues. Before dissociation, the area of strong adhesion was gently peeled off and the borders of the blood vessels and the surrounding tissue were carefully confirmed. Electrocoagulation was used to control bleeding from tiny bleeding spots, whereas ligation was done when bigger blood vessel branches were found. The tumor was completely resected after a CPB duration of 165 min. After recovery of stable hemodynamics, CPB was discontinued. The remainder of the surgery was smooth, including hemostasis and chest closure.
On gross inspection, the resected tumor measured 7.8 cm in size (Figure 2). Microscopic examination demonstrated preserved lymph node architecture with a capsule, surrounded by peripheral lymphocytes in concentric circles, interfollicular vascular proliferation with perivascular hyalinization, plasma cells, and macrophage infiltration (Figure 3). Immunohistochemical staining showed the following: CD20 (+), CD3 (+), PAX5 (+), CD5 (+), CyclinD1 (−), CD10 (−), Bcl6 (−), CD21 (FDC +), Kappa (+), Lambda (+), IgD (weak +), Ki67 (positive rate 15%), CD23 (FDC +), CD38 (+), CD30 (scattered +), MUMI (partial +), CD34 (vascular +), HHVV8 (−), and CD123 (−). Based on the pathology slides, the final diagnosis of this patient was mixed-type UCD.
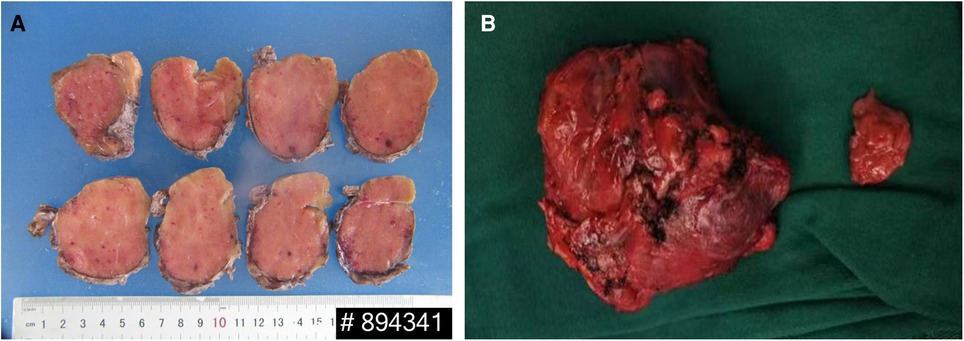
Figure 2. (A) The cut surface showing a solid, homogeneous, and gray mass. (B) Gross appearance of the mass.
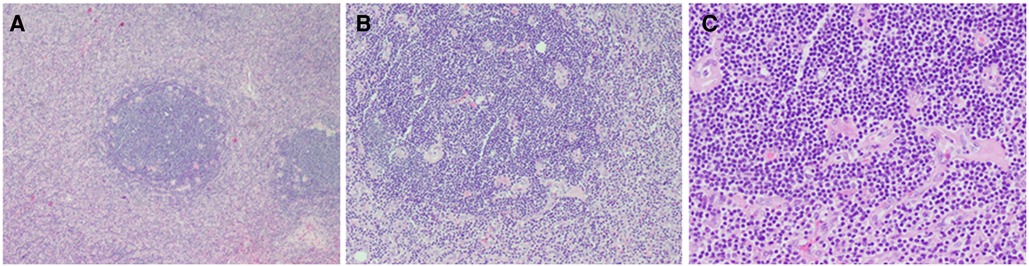
Figure 3. Histopathological examination showed (A) preserved lymph node architecture with a capsule, (B) surrounded by peripheral lymphocytes in concentric circles, (C) interfollicular vascular proliferation with perivascular hyalinization and plasma cells and histiocytes infiltration.
The patient had an uneventful postoperative course and was discharged on postoperative day 21. The patient was continuously followed up; he was doing well 1 year postoperatively. The 1-year CT scan showed no residual tumor and no recurrence of the tumor (Figure 4). Regular annual CT imaging follow-up was advised for the patient.
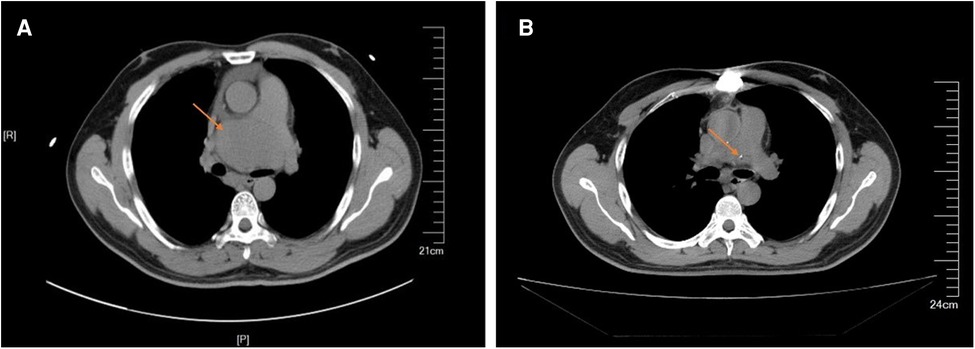
Figure 4. (A) Preparative computed tomography scan showed that the mass close to the main pulmonary artery bifurcation and extended along the right pulmonary artery. (B) Postoperative computed tomography scan showed no residual and no recurrence of the tumor.
CD is a rare, benign lymphoproliferative disorder of unknown etiology and is also called follicular lymph node hyperplasia. Pathologically, it can be divided into hyaline vascular type, plasma cell type, or mixed type. Clinically, single lymph node region enlargement is defined as UCD and multiple lymph node region enlargement as MCD. UCD is mostly hyaline vascular type, MCD is mostly plasma cell type and mixed type, and mixed-type UCD is rare (2). Our patient had mixed-type UCD, which is rare.
UCD is mostly asymptomatic, and chest tightness, dyspnea, cough, and dysphagia can occur when the tumor increases in size and starts oppressing the surrounding tissues and organs. UCD often occurs in the mediastinum and needs to be differentiated from thymoma, lymphoma, metastatic tumor, and neurogenic tumor. Pericardial effusion is associated with CD (3, 4), possibly due to the generalized inflammatory syndrome related to plasma cell histology. The pathological type of our patient was categorized as mixed type, and pericardial effusion is not a common clinical manifestation of mixed type. To the best of our knowledge, this is the first case of mixed-type UCD presenting with pericardial effusion as a clinical manifestation.
Surgical excision of the tumor is both a diagnostic and therapeutic procedure. The gold standard of diagnosis is pathological examination (5). Although ultrasound and CT can be used as diagnostic tools, ultrasound-guided penetration has the risk of causing hemorrhage because UCD is densely packed with blood vessels (6). Our team believes that preoperative puncture has a high risk of bleeding; thus, biopsy during surgery was considered to be safer. Therefore, in our case, we did not perform penetration before surgery.
Mediastinal UCD is treated with surgical resection, which includes open surgery and video-assisted thoracoscopy. A previous study has shown video-assisted thoracoscopy to be not useful (7). However, there have been cases of effective thoracoscopic resection in recent years (8, 9). Video-assisted thoracoscopy is a good option for UCD that is located in the anterior mediastinum or upper mediastinum and has distinct boundaries and does not oppress the heart or blood vessels. The tumor in our case was near the pulmonary arteries and left atrium with no clear boundaries. Therefore, VATS was not used in our case; we believed that median sternotomy was safer for the patient.
Surgical resection of UCD is complicated by excessive blood loss because CD is highly vascularized. Preoperative embolization can be used as an adjunct to avoid intraoperative bleeding (10). In our case, we used CPB to guarantee complete resection of the tumor and the safety of the patient. The application of CPB is of great contribution to intraoperative dynamic stability and meticulous surgical bleeding control. Moreover, to reduce the chances of rupture during operation, the pressure on the pulmonary and cardiac arteries can be relieved by CPB. In our case, the patient did not require any blood transfusion. Thus, our experience supports the application of CPB as a safe and effective technique for the successful surgical treatment of UCD.
CD is a rather uncommon condition. Pathological diagnosis is the gold standard, as radiographic examination is not specific. For UCD, surgical resection is the treatment of choice. The application of CPB is a useful and effective technique for successful surgical treatment.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the ethics Committee of the second hospital of jilin university. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
WR, CL, and LX: contributed to the conception of the manuscript. WR and PL: organized the data collection. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Castleman B, Iverson L, Menendez VP. Localized mediastinal lymph-node hyperplasia resembling thymoma. Cancer. (1956) 9(4):822–30. doi: 10.1002/1097-0142(195607/08)9:4%3C822::AID-CNCR2820090430%3E3.0.CO;2-4
2. Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer. (1972) 29(3):670. doi: 10.1002/1097-0142(197203)29:3%3C670::AID-CNCR2820290321%3E3.0.CO;2
3. Mihăilă M, Herlea V, Dobrea C. An unusual presentation of plasma cells—castleman disease: a case report. Rom J Intern Med. (2016) 54(2):129–33. doi: 10.1515/rjim-2016-0014
4. Nicolosi AC, Almassi GH, Komorowski R. Cardiac tamponade secondary to giant lymph node hyperplasia: castleman's Disease. Chest. (1995) 105(2):637–9. doi: 10.1378/chest.105.2.637
5. Arlet J-B, Hermine O, Darnige L, Ostland V, Westerman M, Badoual C, et al. Iron-deficiency anemia in castleman disease: implication of the interleukin 6/hepcidin pathway. Pediatrics. (2010) 126(6):e1608. doi: 10.1542/peds.2010-1123
6. Madan R, Chen JH, Trotman-Dickenson B, Jacobson F, Hunsaker A. The spectrum of Castleman's disease: mimics, radiologic pathologic correlation and role of imaging in patient management. Eur J Radiol. (2012) 81(1):123–31. doi: 10.1016/j.ejrad.2010.06.018
7. Talat N, Belgaumkar AP, Schulte KM. Surgery in Castleman's disease: a systematic review of 404 published cases. Ann Surg. (2012) 255:677–84. doi: 10.1097/SLA.0b013e318249dcdc
8. Wang YQ, Li SQ, Guo F. Video-assisted thoracoscopic surgery is a safe and effective method to treat intrathoracic unicentric Castleman's disease. BMC Surg. (2020) 20:127. doi: 10.1186/s12893-020-00789-6
9. Amano Y, Takai D, Ohishi N, Shinozaki-Ushiku A, Fukayama M, Akahane M, et al. Successful treatment of mediastinal unicentric Castleman's disease using video-assisted thoracoscopic surgery with preoperative embolization. Case Rep Med. (2013) 2013:354507. doi: 10.1155/2013/354507
Keywords: castleman's disease, middle mediastinal tumor, mixed pathology, cardiopulmonary bypass, surgery
Citation: Ran W, Cuilin Z, Hulin P and Kexiang L (2023) Case report: Successful treatment of mediastinal unicentric castleman disease using cardiopulmonary bypass. Front. Cardiovasc. Med. 10:1130237. doi: 10.3389/fcvm.2023.1130237
Received: 23 December 2022; Accepted: 21 April 2023;
Published: 10 May 2023.
Edited by:
Xiang Wei, Huazhong University of Science and Technology, ChinaReviewed by:
JunMing Zhu, Capital Medical University, China© 2023 Ran, Cuilin, Hulin and Kexiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Kexiang a3hsaXU2NEBob3RtYWlsLmNvbQ==">email@uni.edua3hsaXU2NEBob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.