
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 24 February 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1127716
This article is part of the Research TopicApproaches to Personalized Medicine in Acute Heart FailureView all 5 articles
 Mohammad A. Al-Ani1*
Mohammad A. Al-Ani1* Chen Bai2
Chen Bai2 Amal Hashky3
Amal Hashky3 Alex M. Parker1
Alex M. Parker1 Juan R. Vilaro1
Juan R. Vilaro1 Juan M. Aranda Jr.1
Juan M. Aranda Jr.1 Benjamin Shickel4,5
Benjamin Shickel4,5 Parisa Rashidi5,6
Parisa Rashidi5,6 Azra Bihorac4,5
Azra Bihorac4,5 Mustafa M. Ahmed1
Mustafa M. Ahmed1 Mamoun T. Mardini2
Mamoun T. Mardini2Introduction: Artificial intelligence can recognize complex patterns in large datasets. It is a promising technology to advance heart failure practice, as many decisions rely on expert opinions in the absence of high-quality data-driven evidence.
Methods: We searched Embase, Web of Science, and PubMed databases for articles containing “artificial intelligence,” “machine learning,” or “deep learning” and any of the phrases “heart transplantation,” “ventricular assist device,” or “cardiogenic shock” from inception until August 2022. We only included original research addressing post heart transplantation (HTx) or mechanical circulatory support (MCS) clinical care. Review and data extraction were performed in accordance with PRISMA-Scr guidelines.
Results: Of 584 unique publications detected, 31 met the inclusion criteria. The majority focused on outcome prediction post HTx (n = 13) and post durable MCS (n = 7), as well as post HTx and MCS management (n = 7, n = 3, respectively). One study addressed temporary mechanical circulatory support. Most studies advocated for rapid integration of AI into clinical practice, acknowledging potential improvements in management guidance and reliability of outcomes prediction. There was a notable paucity of external data validation and integration of multiple data modalities.
Conclusion: Our review showed mounting innovation in AI application in management of MCS and HTx, with the largest evidence showing improved mortality outcome prediction.
Advanced heart failure therapies are complex interventions, including mechanical circulatory support (MCS) and heart transplantation (HTx). These treatments can be highly rewarding, restoring quality of life and longevity, however, they are associated with relatively high adverse risk profile. Additionally, the target population is heterogeneous in hemodynamic requirements and risk profile for pre- and post-intervention complications. Ethically such patients are difficult to randomize to therapies when common practice suggests a standard of care. Also, the time lag between innovation, scholarly investigation, and clinical practice significantly limits evidence to guide patient management. Artificial intelligence (AI) has the power and resilience to integrate patient data from several domains and help clinicians navigate the care of the advanced heart failure therapy patient.
As the fields of AI and heart failure therapy both evolve exponentially and in parallel, it remains unclear how AI can integrate in clinical practice and whether these methods are mature enough for clinical application. This scoping review aims to systematically summarize and appraise the literature available in this arena, under the following research question: can AI guide clinicians in personalizing the practice of HTx and MCS to optimize longevity, quality of life, and resource utilization?
The protocol was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyzes extension for Scoping Reviews (PRISMA-ScR) guidelines (1). We searched Embase, Web of Science, and PubMed databases for published articles containing any of the phrases “artificial intelligence,” “machine learning,” or “deep learning” and any of the phrases “heart transplantation,” “ventricular assist device,” or “cardiogenic shock.” The latter term was included to target the group on temporary mechanical circulatory support. Search criteria included the above terms anywhere in the title, abstract, or keywords without any filters. We excluded review articles, meta-analyzes, conference abstracts, non-English language, animal and ex-vivo studies, non-AI methods, and those whose primary outcome is in the pre-HTx or MCS phase of care. Methodology was considered “AI” based if it fell under the main categories of supervised learning, unsupervised learning, or reinforcement learning (2). The search was not restricted by the year of publication. However, the number of publications related to AI in medicine has increased exponentially since 2008 (3).
Full text review and data extraction of each article were performed by at least one HF and one AI specialist. Conflicts were resolved by a HF specialist (MA). Search results were exported to EndNote (version 20.4.1), where duplicates were automatically identified and removed. The Covidence platform was used for title and abstract screening, full text screening, and data extraction. As this is a scoping review with most studies being first of kind or proof of concept, we have not excluded studies based on quality. Also, the group is heterogenous in methodologies, making objective head–head quality assessment unfeasible. The strength of recommending the AI algorithm for clinical use was categorized based on the message conveyed to the reviewer by the article discussion and conclusion sections.
Our search resulted in 584 publications, of which 17.5% were included in PubMed as many were published via biomedical informatics outlets that are not usually indexed in PubMed. Figure 1 summarizes study screening and exclusion reasons. A total of 31 manuscripts were included in our review, of which data were extracted and summarized from both clinical and informatics perspectives.
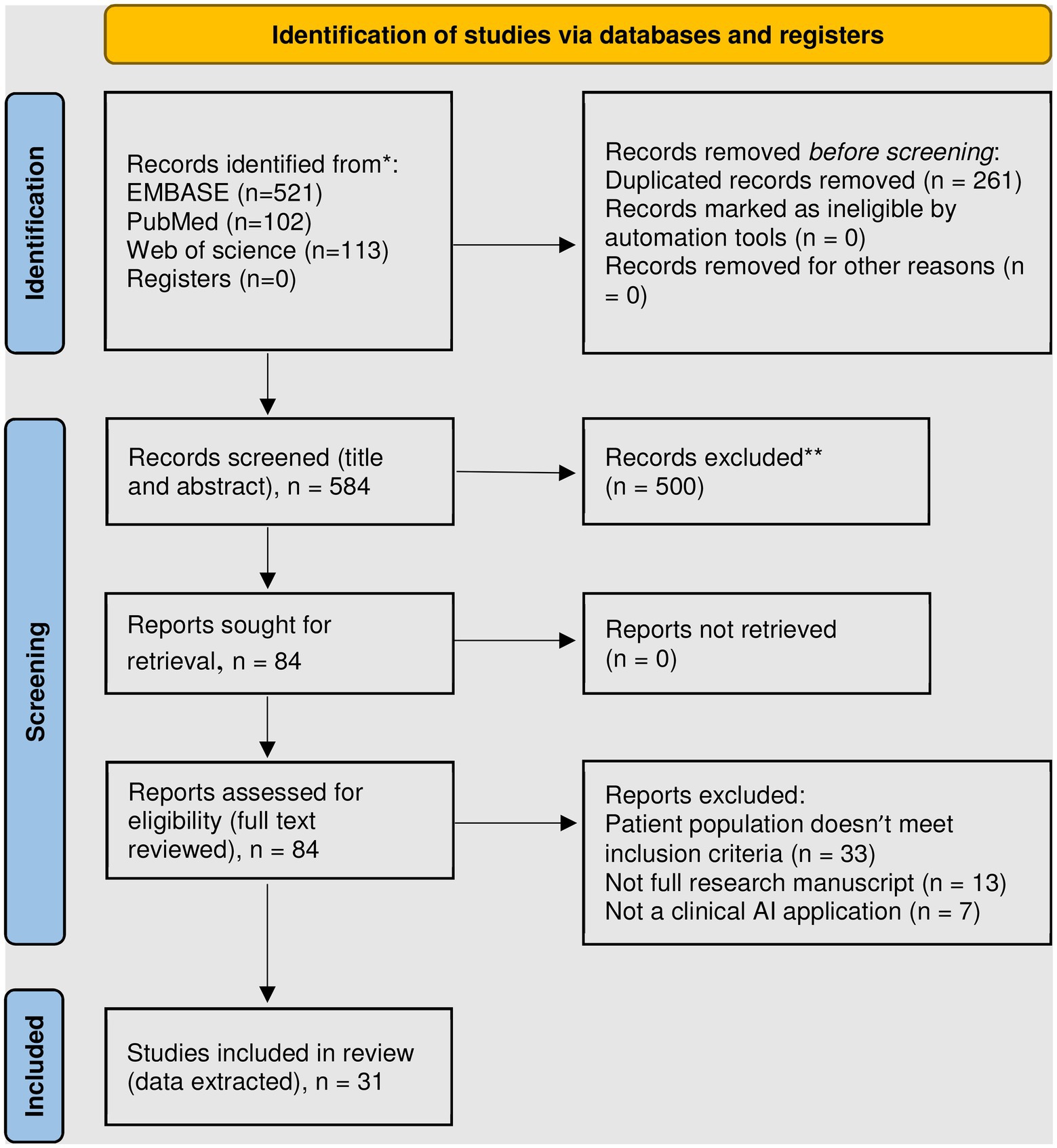
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only (4). *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases). **Exclusion criteria included: review articles, meta-analyzes, conference abstracts, non-English language, animal and ex-vivo studies, non-AI methods, and those whose primary outcome is in the phase of care prior to transplantation or mechanical circulatory support.
We found 13 studies that used AI to predict post-HTx outcomes (Table 1). The most common data sources used for development, training, and validation of AI algorithms are the United for Organ Sharing (UNOS) and the International Society of Heart and Lung Transplantation (ISHLT) registry. Both data souces include massive numbers of HTx recipients and donors over four decades with a wide range of relevant donor and recipient variables of relatively high accuracy. While both data sources overcome limitations of generalizability of single center data, special challenges emerged when applying AI algorithms. The main challenge with UNOS data is the high number of missing values, requiring variable elimination and complex data imputation methods (5–7). The ISHLT registry, on the other hand, includes the UNOS database plus data from other centers worldwide – recently over 350 entities contributing (8). The ISHLT registry does not include wait list duration or mortality (9, 10). In addition, the data reporting varied between regions, centers, and eras. This could introduce systematic difference between training, testing, and validation datasets, thus confounding algorithm development (11). Algorithms applied to local datasets showed much higher performance upon validation, likely due to better data homogeneity (12, 13).
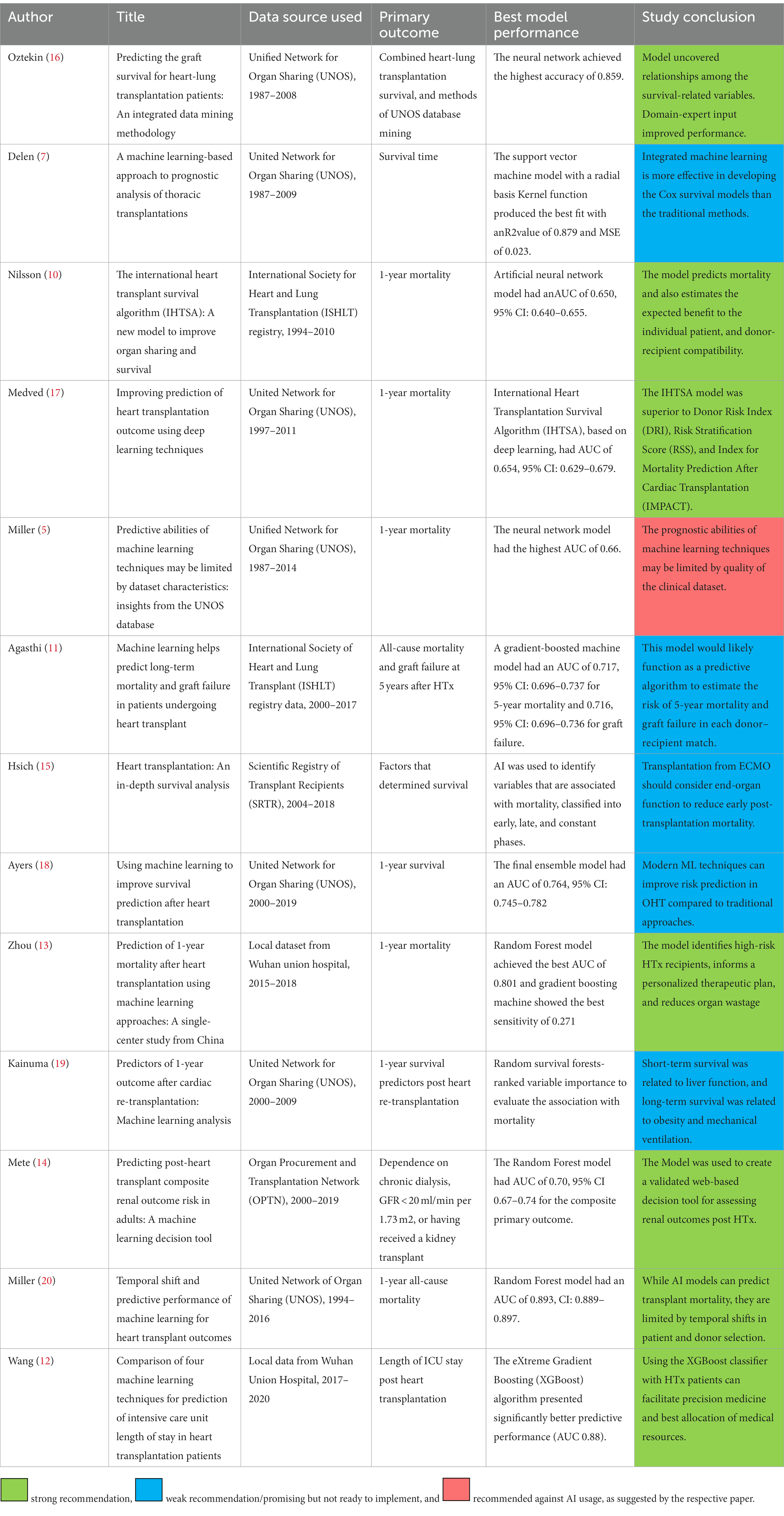
Table 1. Summary of publications describing artificial intelligence application in predicting heart transplant outcomes.
Most studies focused on transplantation survival (n = 10 studies), excluding re-transplantation and multiorgan transplantations (n = 9 studies). This is in recognition that these subgroups inherently have low frequency, significant patient heterogeneity, and variable management practices. More recent algorithms addressed specific post HTx complications, such as renal dysfunction and ICU stay (12, 14). These tools are key as they provide actionable knowledge that can guide multiorgan transplantation, pre-HTx rehabilitation, and perioperative practices to optimize outcomes (15).
Seven studies were identified utilizing machine learning or AI and management of heart transplant patients (Table 2). The clinical questions targeted were detection of rejection, cardiac allograft vasculopathy, and guidance of immunosuppression dosing. Models either attempted to automate the steps normally performed by human experts or leverage detailed molecular data to improve sensitivity for early rejection. Two groups described promising AI models for automatic endomyocardial biopsy interpretation; the CRANE model developed by Lipkova and the CACHE-Grader model by Peyster et al., where both reported performance similar to human experts with less variability (21, 22). The CRANE model offers comprehensive biopsy interpretation (rejection type and grade), and it is adaptable to various populations and camera systems allowing for utilization of multicenter data for research and quality control. The CACHE-grader model, on the other hand, applies transcriptome mapping to identify graft rejection earlier than histopathology. Combining automated histopathologic and transcriptomic data will likely advance the accuracy and efficiency of allograft rejection surveillance to a new level (23).
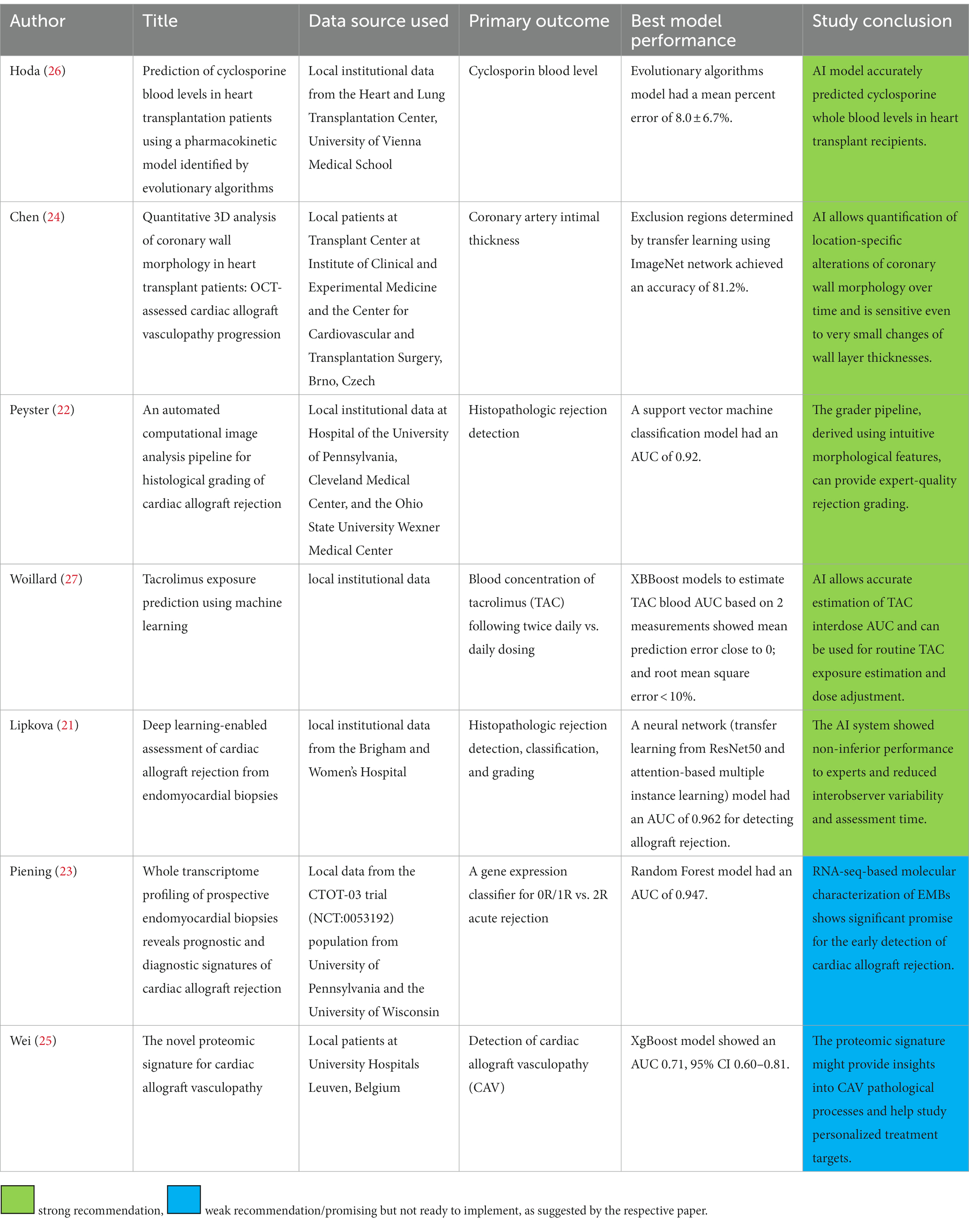
Table 2. Summary of publications describing artificial intelligence application in practicing post heart transplant care.
The study by Chen et al. offered a deep learning algorithm to analyze high resolution coronary optical tomography images looking for vasculopathic changes (24). Their work offers automatic segmentation of all vessel layers, and it can efficiently detect small changes in coronary architecture on serial measurements. It is novel as it detects vasculopathy early, at a stage where preventative measures might be more effective at avoiding frank graft dysfunction. AI application also allows translation application of molecular markers of graft vasculopathy in the urine, with outcomes nuanced enough to differentiate myocardial injury secondary to rejection vs. vasculopathy (25).
As for medical therapy guidance, two studies developed models to predict cyclosporin and tacrolimus levels (26, 27). Both models used medication history, hepatic and renal functions, infectious status and risk factors, and patient demographics. AI allows plotting drug pharmacokinetics beyond mere trough level, potentially offering more accurate dosing recommendations. While systems demonstrated good performance, they faced the challenges of inability to determine which factors contributed to the outcome, were overfitted, and missed the opportunity to incorporate genomic and transcriptomic variables.
A total of 8 studies utilizing AI and MCS outcomes were identified (Table 3). Of these, one examined VA-ECMO, while all others focused on durable MCS with left ventricular assist devices (LVADs) (28). All studies evaluated survival or adverse events. Two studies utilized AI to identify adverse event profiles, time sensitive analyzes of adverse events, and phenomapping of patient profiles as it relates to the former in the LVAD population (29, 30). Grouping of patients facilitates streamlining evaluation and perioperative care pathways that are closely tailored to the patient’s particular risk profile.
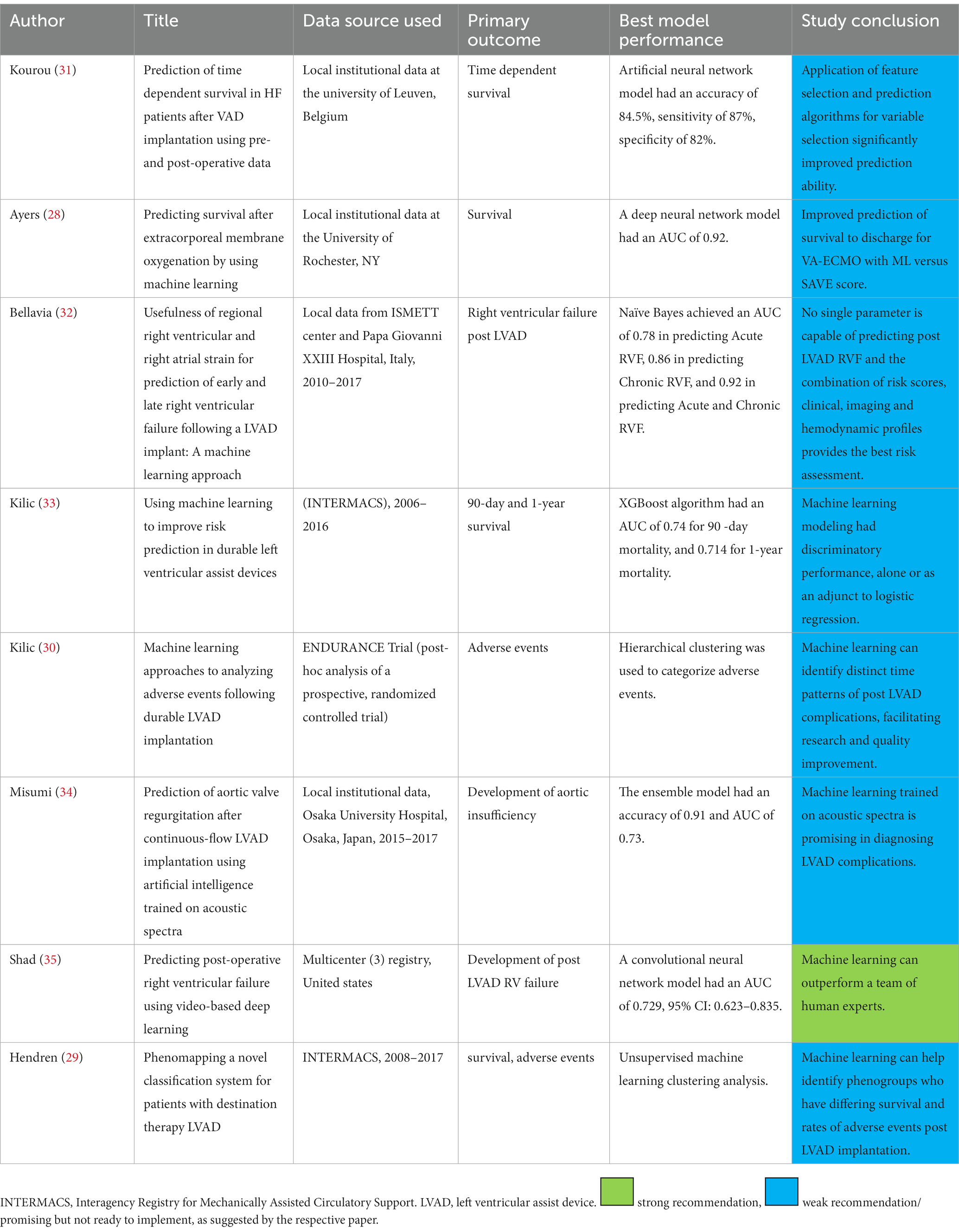
Table 3. Summary of publications describing artificial intelligence application in predicting mechanical circulatory support outcomes.
The remaining five LVAD studies all evaluated various methods of predicting survival and adverse events post implantation. Consistently, these were determined to have better discriminatory power than human experts given the same task, or conventional risk scoring systems. Collectively, these data suggest that AI techniques can allow for better understanding of patient profiles, timing of MCS related adverse events and can be additive to presently available methods of estimating the risk of post implant mortality. AI also opens new horizons for innovation in device development and surgical techniques, as we can now systematically homogenize study populations to assess the efficacy of each support platform. Ideally, this can then assist in preimplant patient selection as well as post implant monitoring and management to optimize MCS outcomes.
Guidance of post MCS care has been targeted by only three algorithms (Table 4). The InDetector project successfully implemented deep learning to segment driveline pictures for objective detection and grading of driveline infection (36). This can also be used to follow up response to therapy in the outpatient setting. The algorithm by Maw et al. utilized LVAD log data to diagnose suction events with high success, despite the model overfitting (see below in AI methods) (37). Such physiologic control systems are likely to become more common in the LVAD world, akin to the case of pacemakers, as the large amount of data generated by these devices facilitate AI model training.
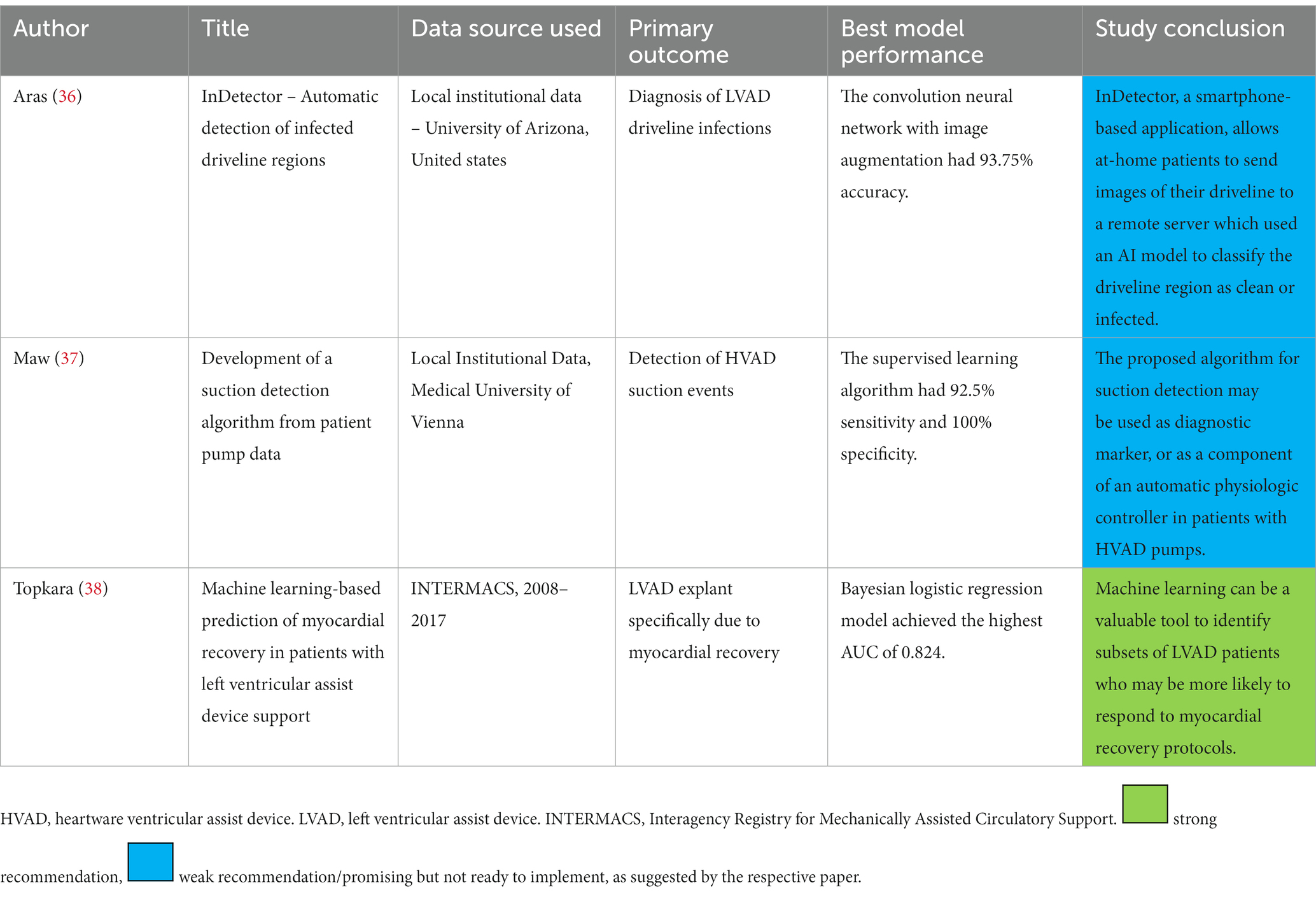
Table 4. Summary of publications describing artificial intelligence application in guiding mechanical circulatory support practice.
One study used patient clinical data to guide post-LVAD medical therapy aiming for myocardial recovery (38). The paucity of similar studies is likely due to the lack of large databanks suitable for AI model development, that follows post MCS management practices along with outcomes. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) lacks enough granularity on post LVAD care that would be needed for reliable training of AI models to guide medical therapy.
We noticed a prevalence of utilizing supervised machine learning techniques over unsupervised learning (Supplementary Table 1). A major difference between supervised and unsupervised learning is the availability of labels. Only three studies applied unsupervised ML techniques, while the remaining used supervised ML techniques (7, 29, 30). Each one of these learning techniques encompasses a set of ML algorithms. The choice of the algorithm is governed mostly by the type of data [structured (e.g., medical history), images (e.g., pathology images), longitudinal (e.g., repeated lab measurements), and clinical notes]. The common ML models used to analyze structured data in the reviewed papers were logistic regression, random forest, and eXtreme gradient boosting (XGBoost), likely due to their superior clinical interpretability (see below) (39). There is a notable underutilization of the treasure trove of clinical notes; none of the reviewed papers analyzed clinical notes. Also, we have not yet seen multidomain data integration, such as combining histopathology, echocardiography, and proteomics to diagnose rejection. These models are expected to emerge in the near future via transferring AI methods being used in other fields into heart failure cardiology. Of note, the clinical natural language processing methods have been increasingly recognized and matured in healthcare over the past years. Utilizing these methods in heart transplant research may provide insightful information beyond the structured electronic health records.
Deep learning models are more common with unstructured data types such as images and videos, due to their superior abilities in automatically extracting important features from raw data that can help in predicting the outcome. Four of the reviewed papers used transfer learning with convolutional neural networks (CNN). For longitudinal data (e.g., lab measurements collected over time and snapshots of pump data), all the reviewed studies manually extracted fixed-dimensional summary statistics (e.g., minimum, maximum, and standard deviation of the laboratory values in each time frame) from the temporal time series before building the ML model.
Despite the intuitive need for interpretable AI (explanation of why the decision was made) in medical applications, it is relatively underexplored. Only 15 manuscripts described model interpretability. Two of these papers (Shad et al., Lipkova et al.) used saliency maps to highlight the most contributing region of an image to the predicted outcomes (21, 35, 40). Zhou et al. and Wang et al. used Shapely Additive exPlanations (SHAP), which quantifies the contribution of each feature (variable) to the predicted outcome related to a specific instance (12, 13). The rest of the papers used feature importance to explain the outcome of their ML models. Feature importance derivation is done by calculating the model’s performance following the permutation of that feature. If the model performance decreases, then the permutated feature is important. While feature importance and SHAP might look similar, the main difference is that feature importance is centered around the decrease in model performance. In contrast, SHAP confers the magnitude contribution of the feature toward the predicted outcome.
The area under the receiver operating characteristic curve (AUC) was the primary performance metric used for model evaluation. Accuracy, sensitivity, and specificity were reported inconsistently between studies. Root mean square error (RMSE), mean percent error, and R2 were commonly reported when evaluating regression models (e.g., continuous outcome). Overall, moderate to high performance was achieved in the studies for survival prediction after heart transplant, likely due to the availability of large training datasets (UNOS and ISHLT registry). That said, biases (e.g., racial and gender bias) in clinical ML is a key constraint and must be addressed to ensure fairness (41–43). However, only the study conducted by Nilsson et al. investigated the potential bias of the developed model (10). The other studies did not have any bias assessment of the developed AI models.
Model validation enhances confidence in model generalizability and scalability to other medical systems. K-fold cross-validation was used to evaluate and enhance model performance, in which the dataset is split into K subsets (folds) and the model is trained on K-1 folds and tested on the remaining validation fold. The process is repeated until the algorithm is tested on all folds, and the average performance across all test folds is reported (44). Three studies, in which sample size was less than 60, used leave-one-out cross-validation in evaluating the model’s performance; evaluating the model on one instance / case and training the model using the rest of the cases, iteratively (32, 34, 37). External validation was only used in the study conducted by Lipkova et al. (21). ML predictive performance varies across settings, populations, regions, time, potential biases, and practice patterns, and therefore, there is a need of validating on external sources (45).
In this scoping review, we identified 31 studies addressing the implementation of artificial intelligence in the clinical practice of MCS and heart transplantation published between 2005 and 2022. Most publications focused on outcome prediction using large existing databases. However, there is a rising wave of innovation in AI methods to tackle challenging care aspects that currently consume most post-intervention resources. We found the most mature AI applications in this field: the prediction of survival and significant complications, as well as HTx rejection identification. Moreover, early work is being conducted to further leverage AI power by introducing practical concepts (the art of medicine) into AI systems and integrating multiple biodomains (laboratory data, ultrasound, histopathology) into model conclusions. An important area of active investigation is post HTx graft vasculopathy detection, a highly morbid complication. The capabilities of AI methods demonstrated in the current review have the potential to incorporate medical literature into predictive algorithms, providing personalized guidance to medical management and complication surveillance of HTx and MCS (Figure 2).
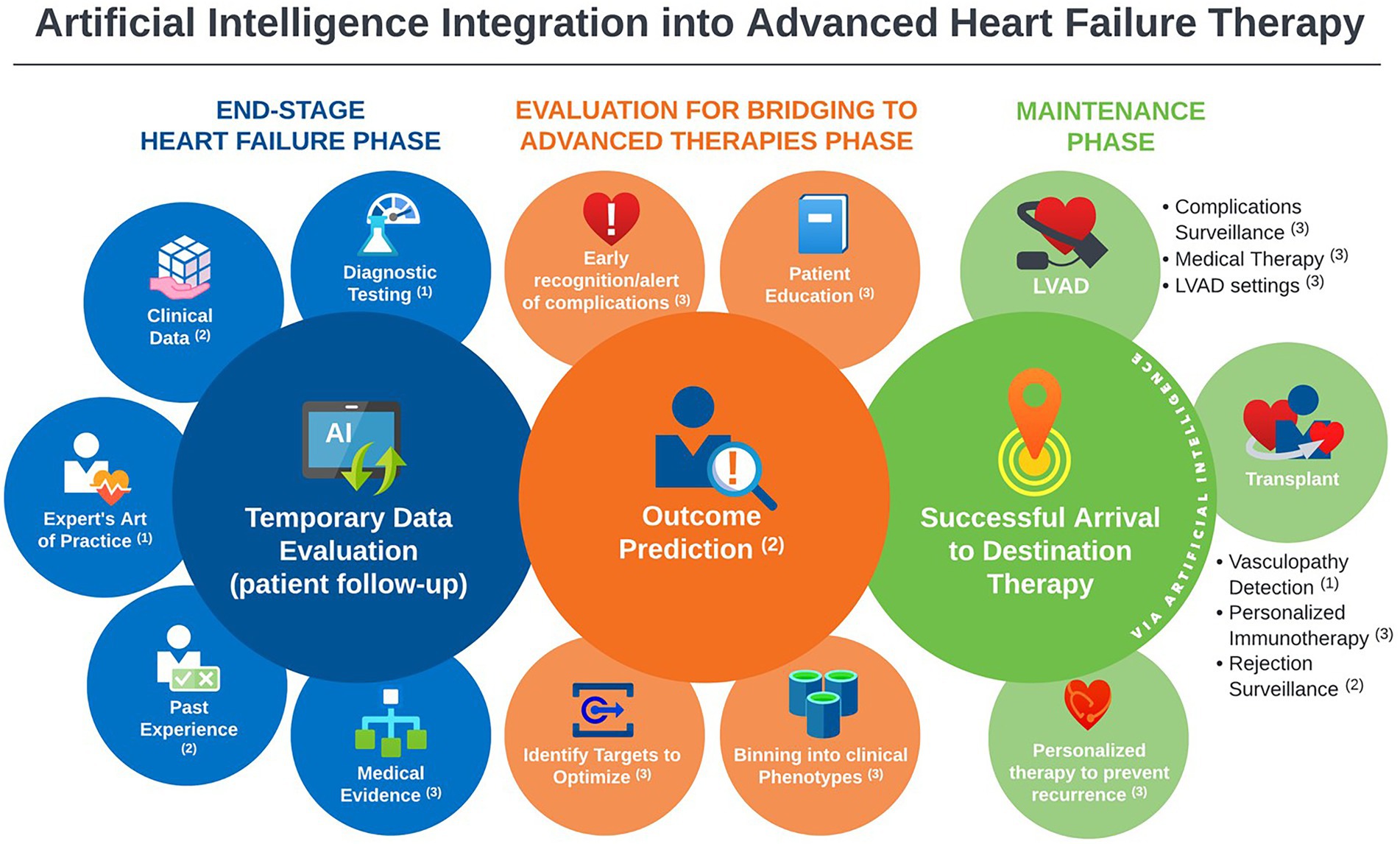
Figure 2. Landscape overview of artificial intelligence applications in advanced heart failure practice, with annotations indicative the level of maturity of the available literature of each application; 1Promising, but not yet mature for clinical use. 2Good support, ready for prospective testing. 3Theoretical potential, but no/negligible support.
Electronic health records contain rich sources of historical and current information that span multiple domains (procedures, diagnoses, medication, and demographics). When harnessed appropriately, it is expected to reveal hidden insights that traditional methods are unable to discover (46). Machine learning (ML) offers flexibility and scalability in assimilating and evaluating large amounts of complex healthcare data. Unlike the traditional statistical methods that focus on inference, ML methods concentrate on prediction by finding patterns in rich and unwieldy data (46). This is evident in complex data formats such as images, time-resolved data series (e.g., LVAD data logs) or wide data matrices (e.g., genomic array). Even though ML can demonstrate superior capabilities in predict patients’ clinical outcomes and risk-stratifying patients according to their clinical and physiological data, it is challenged by the (1) non-explainability of complex algorithms; (2) lack of randomized controlled trials (RCTs) of AI systems, which may not always be feasible; (3) robust evaluation, validation and generalization to various healthcare systems; and (4) identification of biases and unfairness in algorithms. All these factors can hinder the implementation of AI systems in the clinical practice (47, 48).
The domain of Explainable AI (XAI) has emerged as a natural progression to the recent AI developments to increase users’ trust and understanding of the ML black-box systems (49, 50). While some ML models like decision trees, linear models, and attention models are intrinsically explainable, they have lower model accuracies compared to more complex ML models like neural network models (51). However, complex ML models require creating another model to construct explanations, such as using SHAP. The trade-off between intrinsic models and post-hoc models lies between model accuracy and explanation fidelity. Deploying ML in the medical practice requires researchers to put more effort into investigating and evaluating these different explanation techniques to identify which one can best serve health care providers to assess risks and make better decisions.
We cannot overlook the demand to improve the trust and transparency of AI systems used in advanced heart failure, as these decisions affect patients’ quality of life and longevity. Requiring ML systems to (1) justify their decisions/output, (2) enable healthcare providers to take control to identify errors and correct them, and (3) integrate human expert knowledge into models, can contribute to achieving these demands (16). In this scoping review, we found several models that, if validated and implemented, can address vital clinical needs. However, validation was limited by the database availability. The UNOS, INTERMACS, and the ISHLT registry databases are the largest databases available. There is a critical need for data sharing infrastructure that is inclusive of multiple biodomains (imaging, clinical text, electronic heart care system entries, and vital outcomes) to enable generation of accurate ML models that can be validated, meet user’s expectations, and continuously updated to remain current with the clinical practice (52).
As individual systems emerge and become publicly available, pragmatic evaluation for accuracy, gender and ethnic bias and fairness, and safety for medical application becomes challenging. AI programs are recognized as medical devices by the food and drug administration (FDA), with ongoing efforts to govern their clinical application (53). As experts specializing in each particular AI method and application are scarce, unbiased external oversight becomes challenging (54). We have noticed that only one study has external validation. The latter process assures that AI model remain accurate in various settings and are not specifically fitting the population used in the model derivation. “Model waste” can occur where excellent AI models are not clinically applied due to lack of validation (55). Also, there is possibly a publication bias as there is only one manuscript that suggested limited AI benefit (5).
Our scoping review has some limitations. Our search included the 3 major medical databases for feasibility, however, there are many studies published in engineering and bioinformatics journals that may not be indexed in the searched databases. Our results are only up to date as of August 15th, 2022. The search criteria may have missed related studies focusing on cardiogenic shock, cardiac imaging, or heart failure patients not on MCS or post HTx, however, with models transferrable to such populations. Second, the strength of recommending the AI algorithm for clinical use was categorized based on the message conveyed to the reviewer by the article discussion and conclusion, which can be subjective. Despite that papers were reviewed by a multidisciplinary team; a more refined approach could be adopted in the future. Lastly, the outcomes of ML algorithms are subject to systematic errors such as biases. Data sources, mathematical approaches, and results interpretation could introduce these biases into the ML pipeline (56). Given that the nature of this review is to highlight the utilization of AI in the field of heart transplantation, the publication bias assessment was not feasible. However, researchers who aim to implement AI applications in the medical field are warranted to assess these biases.
Our scoping review showed mounting innovation in AI application in MCS and HTx, with largest evidence being for mortality outcome prediction. The past 2 years have witnessed promising models that can guide heart failure cardiologists in HTx donor-recipient matching, allograft surveillance, immunosuppression dosing, and MCS complication screening. While still in infancy, the rate of development and motivation in the community will likely bring AI into heart failure practice in the upcoming 3–5 years.
MA-A and MM: study design. MA-A, CB, AP, JV, and MM: literature review and data collection. MA-A and BS: tables and figures. All authors have participated meaningfully in the study and approve the final manuscript, interpreted the data, and developed and edited the manuscript.
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida and Florida State University Clinical and Translational Science Award UL1TR001427 (MA-A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1127716/full#supplementary-material
1.Tricco, AC, Lillie, E, Zarin, W, O'Brien, KK, Colquhoun, H, Levac, D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
2.Jovel, J, and Greiner, R. An introduction to machine learning approaches for biomedical research. Front Med. (2021) 8:771607. doi: 10.3389/fmed.2021.771607
3.Tran, BX, Vu, GT, Ha, GH, Vuong, QH, Ho, MT, Vuong, TT, et al. Global evolution of research in artificial intelligence in health and medicine: a bibliometric study. J Clin Med. (2019) 8:360. doi: 10.3390/jcm8030360
4.Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
5.Miller, PE, Pawar, S, Vaccaro, B, McCullough, M, Rao, P, Ghosh, R, et al. Predictive abilities of machine learning techniques may be limited by dataset characteristics: insights from the UNOS database. J Card Fail. (2019) 25:479–83. doi: 10.1016/j.cardfail.2019.01.018
6.Godown, J, Gaies, M, and Wilkinson, JD. Leveraging big data to advance knowledge in pediatric heart failure and heart transplantation. Transl Pediatr. (2019) 8:342–8. doi: 10.21037/tp.2019.07.09
7.Delen, D, Oztekin, A, and Kong, Z. A machine learning-based approach to prognostic analysis of thoracic transplantations. Artif Intell Med. (2010) 49:33–42. doi: 10.1016/j.artmed.2010.01.002
8.Puri, K, Schweiger, M, and Rossano, JW. The fate of the failing Fontan circulation-no two are alike. J Heart Lung Transplant. (2021) 40:1682–4. doi: 10.1016/j.healun.2021.08.014
9.Weiss, ES, Allen, JG, Arnaoutakis, GJ, George, TJ, Russell, SD, Shah, AS, et al. Creation of a quantitative recipient risk index for mortality prediction after cardiac transplantation (IMPACT). Ann Thorac Surg. (2011) 92:914–21. doi: 10.1016/j.athoracsur.2011.04.030
10.Nilsson, J, Ohlsson, M, Höglund, P, Ekmehag, B, Koul, B, and Andersson, B. The international heart transplant survival algorithm (IHTSA): a new model to improve organ sharing and survival. PLoS One. (2015) 10:e0118644. doi: 10.1371/journal.pone.0118644
11.Agasthi, P, Buras, MR, Smith, SD, Golafshar, MA, Mookadam, F, Anand, S, et al. Machine learning helps predict long-term mortality and graft failure in patients undergoing heart transplant. Gen Thorac Cardiovasc Surg. (2020) 68:1369–76. doi: 10.1007/s11748-020-01375-6
12.Wang, K, Yan, LZ, Li, WZ, Jiang, C, Wang, NN, Zheng, Q, et al. Comparison of four machine learning techniques for prediction of intensive care unit length of stay in heart transplantation patients. Front Cardiovasc Med. (2022) 9:863642. doi: 10.3389/fcvm.2022.863642
13.Zhou, Y, Chen, S, Rao, ZQ, Yang, D, Liu, X, Dong, NG, et al. Prediction of 1-year mortality after heart transplantation using machine learning approaches: a single-center study from China. Int J Cardiol. (2021) 339:21–7. doi: 10.1016/j.ijcard.2021.07.024
14.Mete, M, Ayvaci, MUS, Ariyamuthu, VK, Amin, A, Peltz, M, Thibodeau, JT, et al. Predicting post-heart transplant composite renal outcome risk in adults: a machine learning decision tool. Kidney Int Rep. (2022) 7:1410–5. doi: 10.1016/j.ekir.2022.04.004
15.Hsich, EM, Blackstone, EH, Thuita, LW, McNamara, DM, Rogers, JG, Yancy, CW, et al. Heart transplantation: an in-depth survival analysis. JACC Heart Fail. (2020) 8:557–68. doi: 10.1016/j.jchf.2020.03.014
16.Oztekin, A, Delen, D, and Kong, ZY. Predicting the graft survival for heart-lung transplantation patients: an integrated data mining methodology. Int J Med Inform. (2009) 78:E84–96. doi: 10.1016/j.ijmedinf.2009.04.007
17.Medved, D, Ohlsson, M, Hoglund, P, Andersson, B, Nugues, P, and Nilsson, J. Improving prediction of heart transplantation outcome using deep learning techniques. Sci Rep. (2018) 8:9. doi: 10.1038/s41598-018-21417-7
18.Ayers, B, Sandhold, T, Gosev, I, Prasad, S, and Kilic, A. Using machine learning to improve survival prediction after heart transplantation. J Card Surg. (2021) 36:4113–20. doi: 10.1111/jocs.15917
19.Kainuma, A, Ning, YM, Kurlansky, PA, Wang, AS, Latif, F, Sayer, GT, et al. Predictors of 1-year outcome after cardiac re-transplantation: machine learning analysis. Clin Transpl. (2022) 36:e14761. doi: 10.1111/ctr.14761
20.Miller, RJH, Sabovcik, F, Cauwenberghs, N, Vens, C, Khush, KK, Heidenreich, PA, et al. Temporal shift and predictive performance of machine learning for heart transplant outcomes. J Heart Lung Transplant. (2022) 41:928–36. doi: 10.1016/j.healun.2022.03.019
21.Lipkova, J, Chen, TY, Lu, MY, Chen, RJ, Shady, M, Williams, M, et al. Deep learning-enabled assessment of cardiac allograft rejection from endomyocardial biopsies. Nat Med. (2022) 28:575–82. doi: 10.1038/s41591-022-01709-2
22.Peyster, EG, Arabyarmohammadi, S, Janowczyk, A, Azarianpour-Esfahani, S, Sekulic, M, Cassol, C, et al. An automated computational image analysis pipeline for histological grading of cardiac allograft rejection. Eur Heart J. (2021) 42:2356–69. doi: 10.1093/eurheartj/ehab241
23.Piening, BD, Dowdell, AK, Zhang, M, Loza, BL, Walls, D, Gao, H, et al. Whole transcriptome profiling of prospective endomyocardial biopsies reveals prognostic and diagnostic signatures of cardiac allograft rejection. J Heart Lung Transplant. (2022) 41:840–8. doi: 10.1016/j.healun.2022.01.1377
24.Chen, Z, Pazdernik, M, Zhang, H, Wahle, A, Guo, Z, Bedanova, H, et al. Quantitative 3D analysis of coronary wall morphology in heart transplant patients: OCT-assessed cardiac allograft vasculopathy progression. Med Image Anal. (2018) 50:95–105. doi: 10.1016/j.media.2018.09.003
25.Wei, D, Trenson, S, Van Keer, JM, Melgarejo, J, Cutsforth, E, Thijs, L, et al. The novel proteomic signature for cardiac allograft vasculopathy. ESC Heart Fail. (2022) 9:1216–27. doi: 10.1002/ehf2.13796
26.Hoda, MR, Grimm, M, and Laufer, G. Prediction of cyclosporine blood levels in heart transplantation patients using a pharmacokinetic model identified by evolutionary algorithms. J Heart Lung Transplant. (2005) 24:1855–62. doi: 10.1016/j.healun.2005.02.021
27.Woillard, JB, Labriffe, M, Debord, J, and Marquet, P. Tacrolimus exposure prediction using machine learning. Clin Pharmacol Ther. (2021) 110:361–9. doi: 10.1002/cpt.2123
28.Ayers, B, Wood, K, Gosev, I, and Prasad, S. Predicting survival after extracorporeal membrane oxygenation by using machine learning. Ann Thorac Surg. (2020) 110:1193–200. doi: 10.1016/j.athoracsur.2020.03.128
29.Hendren, NS, Segar, MW, Zhong, L, Michelis, KC, Drazner, MH, Young, JB, et al. Phenomapping a novel classification system for patients with destination therapy left ventricular assist devices. Am J Cardiol. (2022) 164:93–9. doi: 10.1016/j.amjcard.2021.10.028
30.Kilic, A, Macickova, J, Duan, L, Movahedi, F, Seese, L, Zhang, Y, et al. Machine learning approaches to analyzing adverse events following durable LVAD implantation. Ann Thorac Surg. (2021) 112:770–7. doi: 10.1016/j.athoracsur.2020.09.040
31.Kourou, K, Rigas, G, Exarchos, KP, Goletsis, Y, Exarchos, TP, Jacobs, S, et al. Prediction of time dependent survival in HF patients after VAD implantation using pre- and post-operative data. Comput Biol Med. (2016) 70:99–105. doi: 10.1016/j.compbiomed.2016.01.005
32.Bellavia, D, Iacovoni, A, Agnese, V, Falletta, C, Coronnello, C, Pasta, S, et al. Usefulness of regional right ventricular and right atrial strain for prediction of early and late right ventricular failure following a left ventricular assist device implant: a machine learning approach. Int J Artif Organs. (2020) 43:297–314. doi: 10.1177/0391398819884941
33.Kilic, A, Dochtermann, D, Padman, R, Miller, JK, and Dubrawski, A. Using machine learning to improve risk prediction in durable left ventricular assist devices. PLoS One. (2021) 16:e0247866. doi: 10.1371/journal.pone.0247866
34.Misumi, Y, Miyagawa, S, Yoshioka, D, Kainuma, S, Kawamura, T, Kawamura, A, et al. Prediction of aortic valve regurgitation after continuous-flow left ventricular assist device implantation using artificial intelligence trained on acoustic spectra. J Artif Organs. (2021) 24:164–72. doi: 10.1007/s10047-020-01243-3
35.Shad, R, Quach, N, Fong, R, Kasinpila, P, Bowles, C, Castro, M, et al. Predicting post-operative right ventricular failure using video-based deep learning. Nat Commun. (2021) 12:5192. doi: 10.1038/s41467-021-25503-9
36.Aras, S, Johnson, T, Gniady, C, Skaria, R, and Khalpey, Z. InDetector – automatic detection of infected driveline regions. Smart Health. (2018) 9-10:170–8. doi: 10.1016/j.smhl.2018.07.016
37.Maw, M, Gross, C, Schima, H, and Moscato, F. Development of a suction detection algorithm from patient pump data. Artif Organs. (2017) 41:A50. doi: 10.1016/j.bspc.2021.102910
38.Topkara, VK, Elias, P, Jain, R, Sayer, G, Burkhoff, D, and Uriel, N. Machine learning-based prediction of myocardial recovery in patients with left ventricular assist device support. Circ Heart Fail. (2022) 15:e008711. doi: 10.1161/CIRCHEARTFAILURE.121.008711
39.Abdullah, TAA, Zahid, MSM, and Ali, W. A review of interpretable ML in healthcare: taxonomy, applications, challenges, and future directions. Symmetry. (2021) 13:2439. doi: 10.3390/sym13122439
40.Simonyan, K, Vedaldi, A, and Zisserman, A. Deep inside convolutional networks: visualising image classification models and saliency maps. CoRR (2014). doi: 10.48550/arXiv.1312.6034
41.Ricci Lara, MA, Echeveste, R, and Ferrante, E. Addressing fairness in artificial intelligence for medical imaging. Nat Commun. (2022) 13:4581. doi: 10.1038/s41467-022-32186-3
42.Huang, J, Galal, G, Etemadi, M, and Vaidyanathan, M. Evaluation and mitigation of racial bias in clinical machine learning models: scoping review. JMIR Med Inform. (2022) 10:e36388. doi: 10.2196/36388
43.Starke, G, De Clercq, E, and Elger, BS. Towards a pragmatist dealing with algorithmic bias in medical machine learning. Med Health Care Philos. (2021) 24:341–9. doi: 10.1007/s11019-021-10008-5
44.Hastie, T, Tibshirani, R, and Friedman, J. The Eements of Statistical Learning. New York, NY: Springer series in statistics (2001).
45.Rahimian, F, Salimi-Khorshidi, G, Payberah, AH, Tran, J, Ayala Solares, R, Raimondi, F, et al. Predicting the risk of emergency admission with machine learning: development and validation using linked electronic health records. PLoS Med. (2018) 15:e1002695. doi: 10.1371/journal.pmed.1002695
46.Bzdok, D, Altman, N, and Krzywinski, M. Statistics versus machine learning. Nat Methods. (2018) 15:233–4. doi: 10.1038/nmeth.4642
47.Choudhury, A, Renjilian, E, and Asan, O. Use of machine learning in geriatric clinical care for chronic diseases: a systematic literature review. JAMIA Open. (2020) 3:459–71. doi: 10.1093/jamiaopen/ooaa034
48.Kelly, CJ, Karthikesalingam, A, Suleyman, M, Corrado, G, and King, D. Key challenges for delivering clinical impact with artificial intelligence. BMC Med. (2019) 17:195. doi: 10.1186/s12916-019-1426-2
49.Amann, J, Blasimme, A, Vayena, E, Frey, D, and Madai, VI, Precise4Q Consortium. Explainability for artificial intelligence in healthcare: a multidisciplinary perspective. BMC Med Inform Decis Mak. (2020) 20:310. doi: 10.1186/s12911-020-01332-6
50.Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat MacH Intell. (2019) 1:206–15. doi: 10.1038/s42256-019-0048-x
51.Du, M, Liu, N, and Hu, X. Techniques for interpretable machine learning. Commun ACM. (2019) 63:68–77. doi: 10.1145/3359786
52.Kumar, K, Kumar, P, Deb, D, Unguresan, ML, and Muresan, V. Artificial intelligence and machine learning based intervention in medical infrastructure: a review and future trends. Healthcare. (2023) 11:207. doi: 10.3390/healthcare11020207
53.Hwang, TJ, Kesselheim, AS, and Vokinger, KN. Lifecycle regulation of artificial intelligence– and machine learning–based software devices in medicine. JAMA. (2019) 322:2285–6. doi: 10.1001/jama.2019.16842
54.Smith, JA, Abhari, RE, Hussain, Z, Heneghan, C, Collins, GS, and Carr, AJ. Industry ties and evidence in public comments on the FDA framework for modifications to artificial intelligence/machine learning-based medical devices: a cross sectional study. BMJ Open. (2020) 10:e039969. doi: 10.1136/bmjopen-2020-039969
55.Collins, GS, de Groot, JA, Dutton, S, Omar, O, Shanyinde, M, Tajar, A, et al. External validation of multivariable prediction models: a systematic review of methodological conduct and reporting. BMC Med Res Methodol. (2014) 14:40. doi: 10.1186/1471-2288-14-40
Keywords: artificial intelligence, machine learning, deep learning, heart transplantation, mechanical circulatory support, LVAD
Citation: Al-Ani MA, Bai C, Hashky A, Parker AM, Vilaro JR, Aranda Jr. JM, Shickel B, Rashidi P, Bihorac A, Ahmed MM and Mardini MT (2023) Artificial intelligence guidance of advanced heart failure therapies: A systematic scoping review. Front. Cardiovasc. Med. 10:1127716. doi: 10.3389/fcvm.2023.1127716
Received: 19 December 2022; Accepted: 07 February 2023;
Published: 24 February 2023.
Edited by:
Benedikt Schrage, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Benedikt Beer, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2023 Al-Ani, Bai, Hashky, Parker, Vilaro, Aranda, Shickel, Rashidi, Bihorac, Ahmed and Mardini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad A. Al-Ani, ✉ bW9oYW1tYWRhejIwMzhAbGl2ZS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.