- 1Department of Vascular Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
Objective: To explore the results of hypertension improvement and renal function preservation after renal artery aneurysm (RAA) repair.
Methods: This study retrospectively analyzed the change in blood pressure (BP) and renal outcomes of 59 RAA patients throughout either open or endovascular operations and follow-up at a large center. Patients were grouped according to the difference in their BP at the last follow-up vs. their baseline value. Logistic regression was conducted to explore risk factors for perioperative BP relief and long-term hypertension reonset. Previous studies of RAA with records of BP, blood creatinine level, or GFR/eGFR results are reviewed.
Results: Hypertension was observed in 62.7% (37/59) of the patients included. Postoperative BP declined from 132.20 ± 16.46/79.92 ± 9.64 mmHg to 122.41 ± 11.17/71.10 ± 9.82 mmHg, while eGFR changed from 108.17 ± 24.73 to 98.92 ± 23.87 ml/min/1.73 m2. The median follow-up was 854 [IQR: 1,405] days. Both open and endovascular techniques significantly relieved hypertension and did not impair renal function much. Lower preoperative systolic BP (SBP) was significantly associated with hypertension relief (OR = 0.83, 95% CI: 0.70–0.99). Among patients with normal BP after the operation, higher postoperative SBP was significantly associated with new-onset hypertension (OR = 1.14, 95% CI: 1.01–1.29). Literature review indicated that renal function usually remained normal at follow-up, whereas relief of hypertension varied.
Conclusion: Patients with lower preoperative SBP were likely to benefit more from the operation, while higher postoperative SBP indicated a higher chance of hypertension reonset. Creatinine level and eGFR generally remained stable regardless of operation type.
Introduction
Renal artery aneurysm (RAA) is a relatively rare condition that entails high risk if ruptured (1). Around two-thirds of RAA patients have concomitant hypertension, and refractory high blood pressure (BP) is also an indication for surgical intervention (2). The safety and efficacy of both open surgery and endovascular approaches have been summarized at various centers (3–7). Although relief of high BP following repair has been reported, hypertension reonset is common (4, 8). The prevalence of resistant and true resistant hypertension (9) for RAA is not known, and the mechanism underlying BP changes has not been fully elucidated (5). Despite the generally good baseline conditions of most RAA patients, alteration of creatinine or other indicators for renal function has not been thoroughly discussed (2).
In this study, we retrospectively analyzed the changes in BP and renal outcomes in 59 RAA patients during either open or endovascular operation and throughout follow-up at our institution. Previous studies that had recorded the detailed values of BP, blood creatinine, or glomerular filtration rate (GFR) in patients undergoing RAA open or endovascular operation are reviewed. Multivariable logistic regression was conducted to identify the predictive factors of perioperative BP relief and long-term hypertension reonset.
Methods
Study population and design
From 2009 to 2021, patients diagnosed with RAA who underwent therapy at the Department of Vascular Surgery, Peking Union Medical College Hospital (PUMCH) were consecutively included in the study. Patients without a detailed record of BP levels were excluded. Computed tomography angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA) was utilized for RAA diagnosis and evaluation. All participating subjects provided written informed consent, and the study was approved by the institutional review board.
Baseline characteristics, perioperative results, and follow-up information were retrospectively collected and analyzed. Indications for intervention included aneurysm diameter >2 cm and symptomatic RAA (rupture, hematuria, refractory hypertension not controlled by ≥3 antihypertensive agents of different classes, and flank/abdominal/chest pain excluded of other reasons). Both endovascular and open techniques were applied for treatment.
Regarding treatment strategies, coil embolization, as a minimally invasive endovascular procedure with good efficacy and safety, was the first-line treatment if suitable. Stent-assisted coiling (SAC) or simple stent implantation was applied for complicated lesions. For those who were unsuitable for endovascular therapy or too young, open surgery including aneurysmoplasty, resection and primary anastomosis, tailoring, ex vivo repair, and resection and graft reconstruction with either the saphenous vein or a prosthetic graft, was selectively conducted. Detailed procedures can be found in previous literature and in supplemental Table 1 (1, 3, 10–12).
Hypertension and renal function evaluation
Hypertension was defined as arterial systolic (SBP) or diastolic BP (DBP) levels of three or more clinical measurements above 140/90 mmHg or taking antihypertensive drugs on admission (13). BP levels were collected upon admission, on the next day after operation, and at follow-up. Patients were grouped into 6 groups according to the difference in their BP results at the last follow-up vs. baseline value:
(1) Always normal patients had normal BP throughout the study.
(2) Complete relief of hypertension was defined as SBP and DBP at three or more clinical measurements below 140/90 mmHg without anti-hypertensive drugs.
(3) Partial relief was defined as SBP and/or DBP below 140/90 mmHg while taking the same number or reduced number of medications, or a reduction in DBP by at least 15-mm Hg while on the same or reduced number of medications.
(4) No relief was defined as no change or inability to meet the above criteria.
(5) New onset referred to patients with newly-diagnosed hypertension at follow-up.
(6) Relief and new onset was defined as BP relief at discharge but had new-onset hypertension at follow-up.
Chronic kidney disease was graded according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative classification (14). Estimated GFR (eGFR) was calculated with the Cockcroft-Gault equation, impaired renal function being defined as a 30% reduction in eGFR.
Follow up
Thirty-day and long-term follow-up data were recorded and explored. Technical primary success was defined as the successful introduction and deployment of the device in the absence of surgical conversion or any complication. Color Doppler, CTA, or angiography was applied to evaluate arterial patency, which was defined as evidence of blood flow within the target renal artery or bypass and perfusion in the respective renal parenchyma. Laboratory tests, including creatinine (µmol/L), white blood cell count (WBC, 109/L), hemoglobin (HGB, g/L), platelet count (PLT 109/L), and D-dimer (mg/L), as well as ultrasounds of the kidney or renal artery, were done at least once preoperatively, postoperatively before discharge, and at three months, six months, and one year or yearly after discharge.
Logistic regression for perioperative BP relief and long-term hypertension re-onset
Logistic regression of hypertension relief was done between group 2, group 3, group 4, and group 5 patients, who had hypertension before the operation. Patients were divided into complete relief (group 2 and group 5) and incomplete relief (group 3 and group 4). Ordinal regression was first performed between the complete relief group and the incomplete relief group to identify factors with significant associations, which were then incorporated into the multivariate logistic regression model.
Logistic regression of new-onset hypertension was run between group 1, group 2, group 5, and group 6 patients who had normal BP after the operation. Patients were divided into new-onset (groups 5 and 6) and no onset (groups 1 and 2) groups. Similar factors as from the ordinal regression model were incorporated.
Literature research and review
Literature searches in English and Chinese were performed in PubMed, EMBASE and Scopus using the keyword “renal artery aneurysm”. Studies that reported the detailed preoperative, postoperative, follow-up, or changed values of BP, blood creatinine level, or GFR/eGFR level in patients who underwent open or endovascular operation for RAA were included. Single-case reports were excluded. Two authors (SL and FL) reviewed the original articles and identified 10 papers on BP and 16 papers on renal function published from 2001 to 2022.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software (IBM Corp, Armonk, NY). Continuous variables were checked for normality and are reported as median and interquartile range (IQR) or mean ± standard deviation. Categorical variables are presented as frequencies and were compared by the χ2 test or Fisher's exact test, as appropriate. The Wilcoxon test or the Kruskal‒Wallis and Friedman tests were employed for cross- or within-group comparisons, respectively. p < 0.05 was considered significant.
Results
Patient demographics
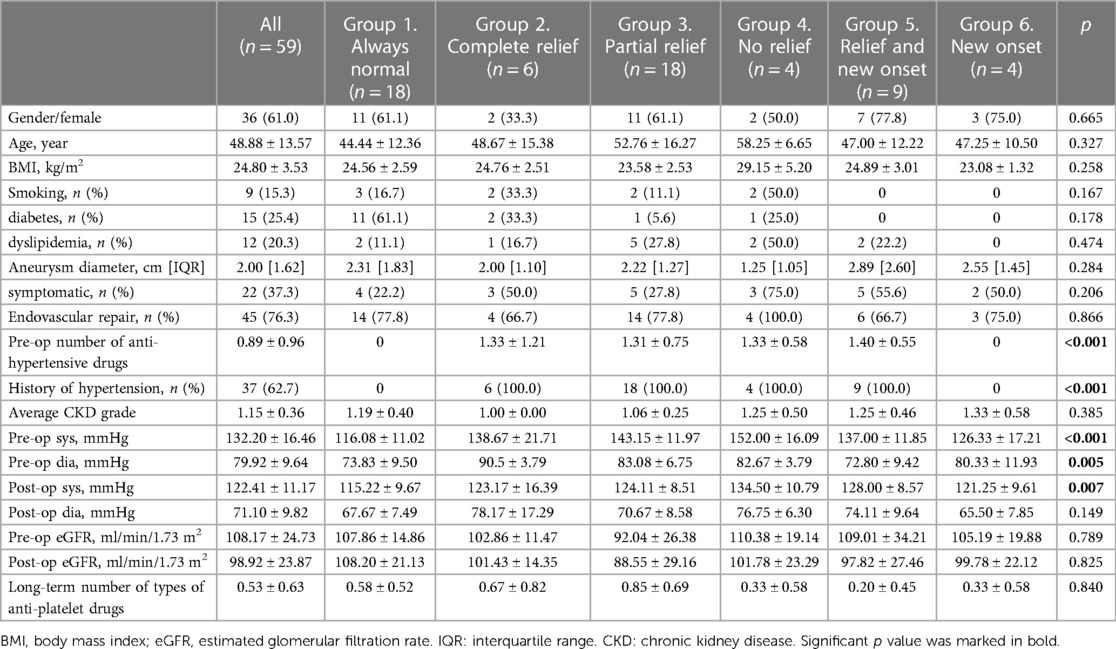
Table 1 showed the basic characteristics of all patients and for each group with different BP conditions. Of the 59 subjects included, 61.0% (36) were female. The average age was 48.88 ± 13.57 years. Smoking, diabetes, and dyslipidemia were present in 15.3% (9), 25.4% (15), and 20.3% (12) of all patients, respectively. The median diameter of the aneurysm was 2.00 [IQR 1.62] cm. Regarding aneurysm position, most of the patients had aneurysms located at the first bifurcation (25, 42.4%), followed by 23 (22.0%) at the first-order branch, 13 (12.0%) at the main renal artery, 5 (8.50%) at both the first-order branch and the main renal artery, and 3 (5.1%) at intralobular branches. Hypertension was observed in 62.7% (37) of all patients, and 37.3% (22) complained of pain. One patient had concomitant renal artery stenosis. The average chronic kidney disease grade was 1.15 ± 0.36. Endovascular repair was performed in 45 (76.3%) patients. Postoperatively, average BP declined from 132.20 ± 16.46/79.92 ± 9.64 mmHg to 122.41 ± 11.17/71.10 ± 9.82 mmHg, while eGFR changed from 108.17 ± 24.73 to 98.92 ± 23.87 ml/min/1.73 m2.
Follow-up results
The median follow-up was 64.5 [IQR: 65.2] and 25.1 [IQR: 33.1] months for patients receiving endovascular repair and open surgery, respectively (Supplementary Table S2). No significant difference in primary technical success, postoperative complications, reocclusion, or reintervention rate for open and endovascular repair was observed. Two patients in each group had occluded renal arteries but were treated conservatively because of preserved renal function. Five (11.5%) of the patients receiving endovascular repair underwent a second endovascular operation because of unsatisfactory aneurysm embolization. One patient received coiling 9 months after undergoing the tailoring technique due to unsatisfactory resolution of the aneurysm, and 1 received ballooning and stent implantation for anastomotic stenosis after ex vivo repairs and autotransplantation.
An average number of types of 0.53 ± 0.63 antiplatelet drugs (aspirin or clopidogrel) were prescribed for patients at the last follow-up (Table 1). Laboratory results at different time points after RAA repair are illustrated in Supplementary Figure S1. WBC, HGB, PLT, and D-dimer fluctuated briefly after the operation but returned to baseline at both the short-term and long-term examinations (Supplementary Table S1). No significant difference was observed in the above values between the open and endovascular groups in the long run (Supplementary Table S2).
BP and renal function results
All patients were divided into 6 groups according to their BP pattern (Table 1). Eighteen patients had constant normal BP (group 1), 6 complete relief of hypertension (group 2), 18 partial relief (group 3), 4 no relief (group 4), 9 hypertension relief but long-term new-onset (group 5), and 4 new-onset hypertension only at the follow-up test (group 6). Among patients with partial relief, 4 (22.2%) reduced their number of medications. Patients with no relief took the same number or more medications. Kruskal-Wallis test among all subgroups suggested no significant difference in comorbidity between patients with different BP statuses, but significant differences were observed in the number of antihypertensive medicines (p < 0.001), prevalence of hypertension history (p < 0.001), preoperative SBP and DBP values (p < 0.001 and =0.005), and postoperative SBP (p = 0.007).
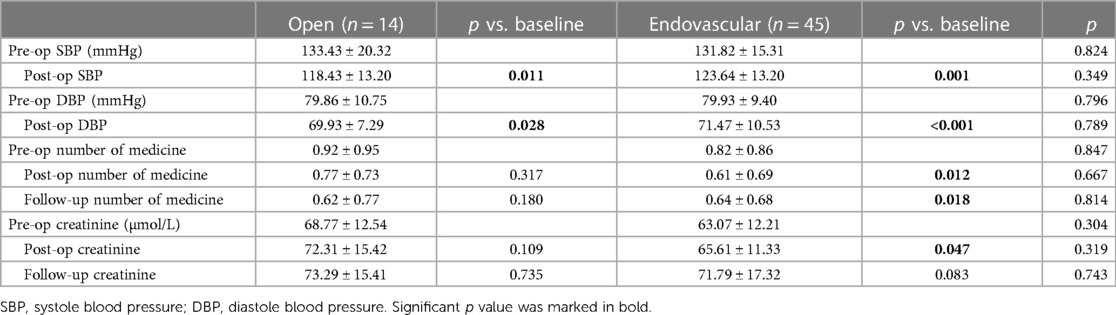
BP and renal function alterations were also explored and compared between patients who underwent open or endovascular repair (Table 2). There was no significant difference between the two approaches perioperatively or in the long run. Both SBP and DBP decreased significantly after the operation. Patients receiving endovascular repair took significantly fewer antihypertensive medicines. Regarding renal outcomes, the endovascular group had a transient increase in creatinine (p = 0.047), but no significant difference was observed in the long-term results.
Risk factors for perioperative BP relief and long-term hypertension reonset
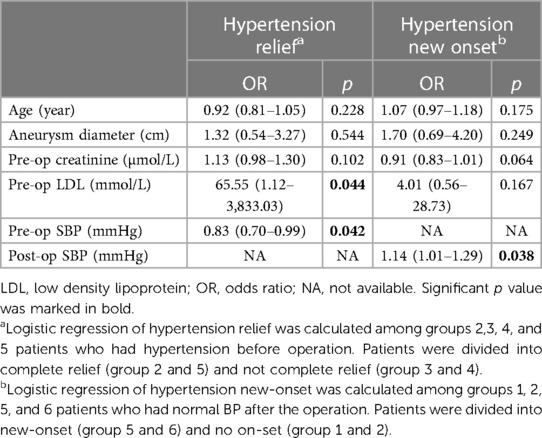
To further investigate the risk factors for perioperative BP relief and long-term hypertension reonset, logistic regression was conducted in two different sets of groups (Table 3). Higher preoperative low-density lipoprotein (LDL) level was significantly associated with hypertension relief after the operation (OR = 65.55, 95% CI: 1.12–3,833.03). Lower preoperative SBP was significantly associated with hypertension relief (OR = 0.83, 95% CI: 0.70–0.99). Among patients who had normal BP values after the operation, higher postoperative SBP was significantly associated with new-onset hypertension (OR = 1.14, 95% CI: 1.01–1.29).
Discussion
The present study demonstrated good results from our twelve-year experience with RAA repair. No significant difference was found in the perioperative laboratory parameters between the open and endovascular groups. Patients receiving open surgery had a transient increase in D-dimer and PLT levels, but their long-term laboratory tests returned to normal. A significantly higher rate of reintervention was observed among patients with new-onset hypertension than for those with other BP statuses. Since RAA patients were generally younger and healthier than other patients in the vascular surgery ward, hypertension improvement and renal function preservation were crucial to reduce morbidity and minimize any potential risk for cardiovascular events in the long run.
The mechanism of hypertension in RAA remains unclear. Concurrent RAS or compression might play a part, while microembolization in the distal renal parenchyma and hemodynamic changes from turbulent blood flow within the aneurysm could also contribute (2). The resolution of these conditions by operation may improve the hypertension (15). Our study suggested that despite an overall significant decrease in both SBP and DBP as well as in the number of anti-hypertension medicines taken postoperatively, it must be noted that baseline BP conditions, the extent of hypertension relief, and hypertension occurrence varied between individuals. Some studies have suggested distinct results between patients with renovascular hypertension and those without evidence of stenosis, but this difference was not observed in our study (4, 16, 17).
Concerning renal function, most RAA patients in this study had normal baseline levels of creatinine and eGFR. During operation, the introduction of nephrotoxic iodinated contrast medium, embolization of a part of the renal parenchyma, failure of artery reconstruction, and clamping of blood supply may all increase the risk of renal impairment (18). Nevertheless, our study showed that creatinine and eGFR remained stable over the long-term follow-up regardless of operation type.
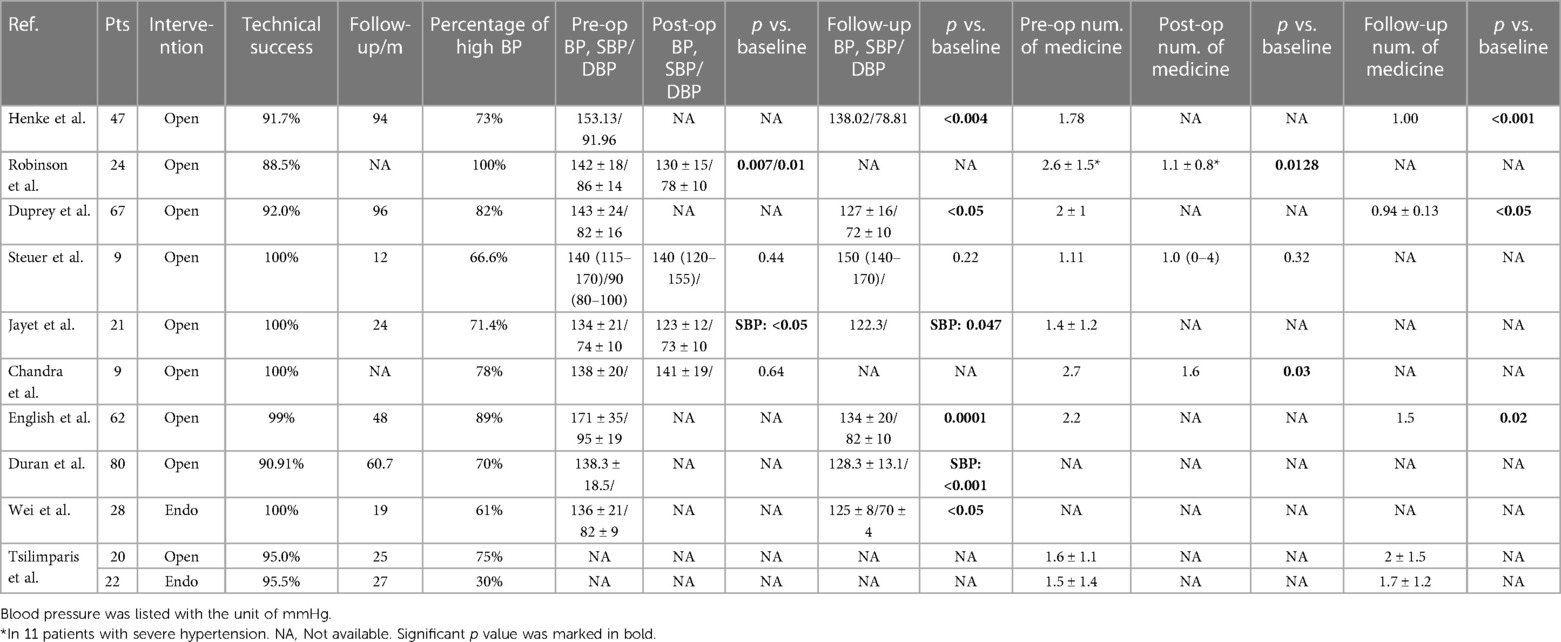
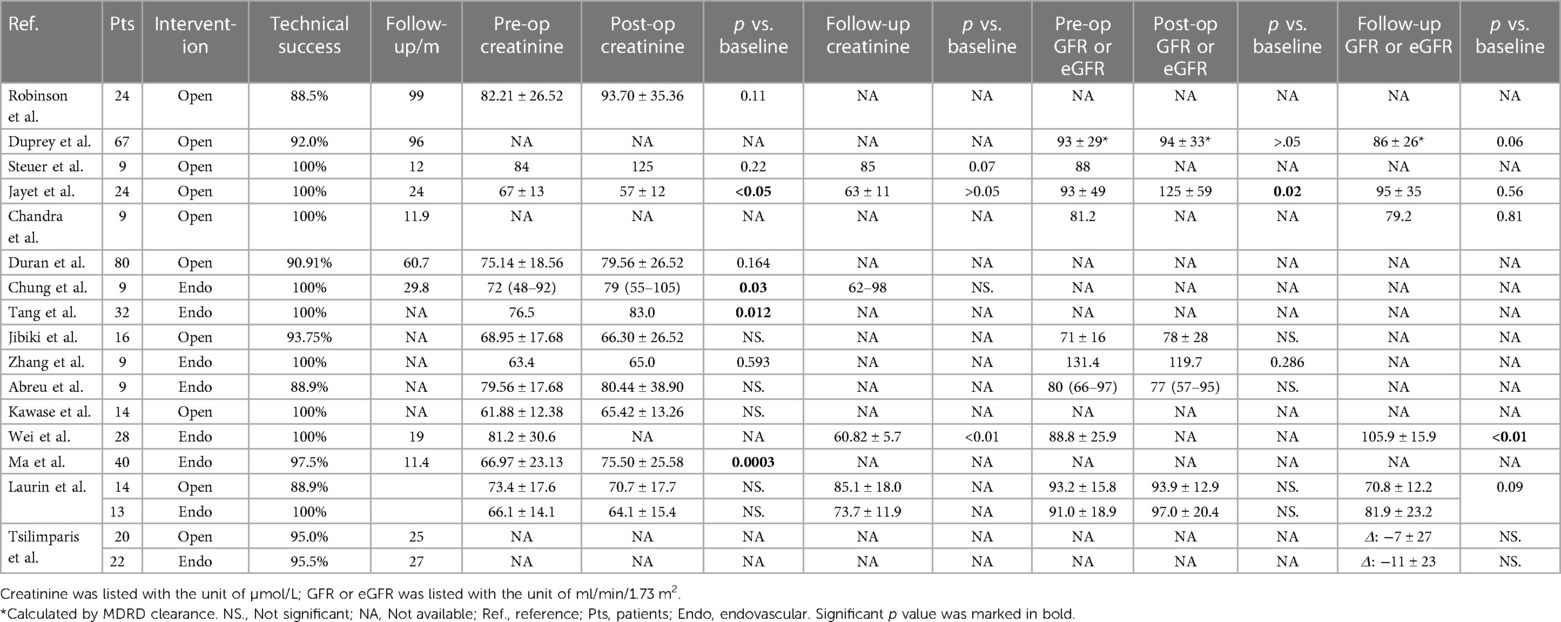
To further explore the problem of RAA and BP or renal function, we reviewed the results of BP and indicators of renal function reported in previous studies. Although expertise in RAA repair has accumulated rapidly over the last decade, few studies have reported on the exact values of these parameters. Ten papers that reported the value of BP for RAA were included (Table 4) (3, 7, 8, 10, 19–23). Of all 367 patients included, 345 received open therapy, while 50 underwent endovascular repair. The preoperative average SBP value was 146.90 mmHg. Two of 4 studies that recorded both preoperative and postoperative BP found a significant reduction, and 6 of 7 studies that recorded preoperative and follow-up results found a significant reduction. Regarding the number of antihypertensive medicines, follow-up results from 3 studies all reported a decrease. Sixteen papers concerning renal function were included (Table 5) (7, 8, 10, 18–21, 23–29). In total, 277 and 162 patients received open or endovascular repair, respectively. Twelve studies recorded both preoperative and postoperative creatinine levels, of which 2 suggested a significant increase and 1 a significant decrease. One of the 5 studies reporting preoperative and follow-up results observed a significant increase in creatinine. Only 1 study showed a significant increase in GFR transiently after the operation, but not at follow-up. In general, in line with our study, no significant deterioration of renal function was observed except for transient fluctuation. A significant decrease in SBP or DBP or antihypertensive drug number has been reported in both the short term and long term in some conditions. In some other cases, hypertension was not fully relieved after the operation, and medicines were required for BP control. It should also be noted that most of these patients received open surgical repair for RAA, which may not represent the whole picture given the rise in popularity of the endovascular approach.
Since controversial results exist, we sought to explore the underlying factors that may contribute to BP alteration using logistic regression models. As for hypertension relief after the operation, we found that patients with lower SBP and higher blood LDL levels preoperatively achieve better outcomes, which could provide predictive value for BP alteration after the operation. Several previous studies concerning BP response after operation for RAS have identified the level of SBP or DBP as a positive predictor of hypertension relief (30, 31). However, as many of these patients have renovascular hypertension, renal artery stenting could resolve this condition and directly relieve the hypertension. Only one patient in our study had a clear concomitant RAS on imaging. Hypertension in RAA patients, on the other hand, may have more complicated origins and cannot be easily cured by renal artery revascularization. As for LDL levels, little evidence of their effect has been found. On account of the wide CI range of this factor in our patients, we assumed that our LDL findings could be a false positive resulting from extreme individual values. Additionally, we observed a trend (though not significant) that greater aneurysm diameter, greater preoperative creatinine, and earlier age were positively associated with the extent of BP relief, which suggested that a combination of more underlying factors may contribute to hypertension in RAA.
Patients with higher postoperative SBP, though within the normal range, might still be at higher risk of new-onset hypertension. For these patients, renal aneurysm may not be the primary factor causing the hypertension. Thus, the operation could not fully stop the progression of hypertension. A previous study also suggested that a longer duration of hypertension may bring an unfavorable outcome (32). Since hypertension was associated with a higher chance of reintervention, postoperative BP monitoring would be more valuable for these patients.
This study has some limitations. Besides blood creatinine and eGFR, a more thorough evaluation of renal function, such as urine tests and CTA, was not normally conducted at follow-up, and ambulatory BP monitoring was not done. Subgroup analysis was hampered by the small subject numbers, making it difficult to reach robust conclusions. The activity of the renin-angiotensin system (RAAS) was not routinely tested, so the underlying pathophysiology and etiology of BP change might not be fully explained. More evidence is required to set the optimal RAA diameter threshold for surgical intervention. Nevertheless, through a literature review, we discussed the nature of BP and renal function alteration after RAA operation, which could assist in prognostic prediction for these patients. Larger multicenter studies are necessary to validate our findings.
Conclusion
Both open and endovascular techniques for RAA repair demonstrated significant relief of hypertension and little impairment of renal function, yet baseline BP conditions and the extent of hypertension relief or long-term occurrence varied. Patients with lower preoperative SBP were likely to benefit more from the operation, while higher postoperative SBP indicated a higher chance of long-term hypertension reonset. Creatinine level and eGFR generally remained stable regardless of operation type.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by The Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SL and FL contributed to the study design. SL contributed to the data analysis. SL, FL, and ZL contributed to the manuscript writing and revision. SL, FL, and RZ contribute to electronic image analysis. ZL, RZ, WY, JS, and YZ contribute to operation performance and clinical data collection. YZ supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Natural Science Foundation of China (grant number 51890894, 82070492, and 82100519), the CAMS Innovation fund for Medical Science (CIFMS, Grant No.2021-I2M-C&T-A-006 and 2021-I2M-1-016), and the National High Level Hospital Clinical Research Funding (Grant No.2022-PUMCH-B-100 and 2022-PUMCH-C-062). The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We thank Changwei Liu, Bao Liu, Yuexin Chen, Lei Wang, WY, Xiaojun Song, Leng Ni, Xiaolong Liu for their expertise in clinical assessment and operation performance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1127154/full#supplementary-material.
References
1. Coleman DM, Stanley JC. Renal artery aneurysms. J Vasc Surg. (2015) 62(3):779–85. doi: 10.1016/j.jvs.2015.05.034
2. Chaer RA, Abularrage CJ, Coleman DM, Eslami MH, Kashyap VS, Rockman C, et al. The society for vascular surgery clinical practice guidelines on the management of visceral aneurysms. J Vasc Surg. (2020) 72(1s):3s–39. doi: 10.1016/j.jvs.2020.01.039
3. Henke PK, Cardneau JD, Welling TH 3rd, Upchurch GR Jr, Wakefield TW, Jacobs LA, et al. Renal artery aneurysms: a 35-year clinical experience with 252 aneurysms in 168 patients. Ann Surg. (2001) 234(4):454–62; discussion 462–3. doi: 10.1097/00000658-200110000-00005
4. Pfeiffer T, Reiher L, Grabitz K, Grünhage B, Häfele S, Voiculescu A, et al. Reconstruction for renal artery aneurysm: operative techniques and long-term results. J Vasc Surg. (2003) 37(2):293–300. doi: 10.1067/mva.2003.117
5. González J, Esteban M, Andrés G, Linares E, Martínez-Salamanca JI. Renal artery aneurysms. Curr Urol Rep. (2014) 15(1):376. doi: 10.1007/s11934-013-0376-z
6. Buck DB, Curran T, McCallum JC, Darling J, Mamtani R, van Herwaarden JA, et al. Management and outcomes of isolated renal artery aneurysms in the endovascular era. J Vasc Surg. (2016) 63(1):77–81. doi: 10.1016/j.jvs.2015.07.094
7. Duran M, Hausmann DF, Grabitz K, Schelzig H, Simon F, Sagban TA. Reconstruction for renal artery aneurysms using the tailoring technique. J Vasc Surg. (2017) 65(2):438–43. doi: 10.1016/j.jvs.2016.07.113
8. Steuer J, Bergqvist D, Björck M. Surgical renovascular reconstruction for renal artery stenosis and aneurysm: long-term durability and survival. Eur J Vasc Endovasc Surg. (2019) 57(4):562–8. doi: 10.1016/j.ejvs.2018.09.014
9. Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence and outcomes. J Hum Hypertens. (2014) 28(8):463–8. doi: 10.1038/jhh.2013.140
10. Jayet J, Davaine JM, Tresson P, Verscheure D, Lawton J, Kashi M, et al. Direct distal renal artery aneurysm repair. Eur J Vasc Endovasc Surg. (2020) 60(2):211–8. doi: 10.1016/j.ejvs.2020.04.016
11. Li FD, Ji ZG, Liu CW, Shao J, Xie Y, Zheng YH. Orthotopic renal autotransplantation for young-onset and medical treatment-requiring complex renovascular hypertension. J Renin Angiotensin Aldosterone Syst. (2018) 19(3):1470320318789861. doi: 10.1177/1470320318789861
12. Spiotta AM, Wheeler AM, Smithason S, Hui F, Moskowitz S. Comparison of techniques for stent assisted coil embolization of aneurysms. J Neurointerv Surg. (2012) 4(5):339–44. doi: 10.1136/neurintsurg-2011-010055
13. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. (2018) 36(10):1953–2041. doi: 10.1097/HJH.0000000000001940
14. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. (2017) 7(1):1–59. doi: 10.1016/j.kisu.2017.04.001
15. Tang S, Niu G, Fang D, Yan Z, Zhang B, Li X, et al. The diagnosis and endovascular therapy of renal artery aneurysm: a 32-patient case report. Medicine (Baltimore). (2017) 96(47):e8615. doi: 10.1097/MD.0000000000008615
16. Martin RS 3rd, Meacham PW, Ditesheim JA, Mulherin JL Jr, Edwards WH. Renal artery aneurysm: selective treatment for hypertension and prevention of rupture. J Vasc Surg. (1989) 9(1):26–34. doi: 10.1016/0741-5214(89)90216-4
17. Bastounis E, Pikoulis E, Georgopoulos S, Alexiou D, Leppäniemi A, Boulafendis D. Surgery for renal artery aneurysms: a combined series of two large centers. Eur Urol. (1998) 33(1):22–7. doi: 10.1159/000019530
18. Tang H, Tang X, Fu W, Luo J, Shi Z, Wang L, et al. Coil embolization of renal artery bifurcation and branch aneurysms with flow preservation. J Vasc Surg. (2018) 68(2):451–458.e2. doi: 10.1016/j.jvs.2017.12.032
19. Robinson WP 3rd, Bafford R, Belkin M, Menard MT. Favorable outcomes with in situ techniques for surgical repair of complex renal artery aneurysms. J Vasc Surg. (2011) 53(3):684–91. doi: 10.1016/j.jvs.2010.10.050
20. Duprey A, Chavent B, Meyer-Bisch V, Varin T, Albertini JN, Favre JP, et al. Editor's choice—ex vivo renal artery repair with kidney autotransplantation for renal artery branch aneurysms: long-term results of sixty-seven procedures. Eur J Vasc Endovasc Surg. (2016) 51(6):872–9. doi: 10.1016/j.ejvs.2016.02.017
21. Chandra A, O'Connell JB, Quinones-Baldrich WJ, Lawrence PF, Moore WS, Gelabert HA, et al. Aneurysmectomy with arterial reconstruction of renal artery aneurysms in the endovascular era: a safe, effective treatment for both aneurysm and associated hypertension. Ann Vasc Surg. (2010) 24(4):503–10. doi: 10.1016/j.avsg.2009.07.030
22. Wei X, Sun Y, Wu Y, Li Z, Zhu J, Zhao Z, et al. Management of wide-based renal artery aneurysms using noncovered stent-assisted coil embolization. J Vasc Surg. (2017) 66(3):850–7. doi: 10.1016/j.jvs.2017.04.035
23. Tsilimparis N, Reeves JG, Dayama A, Perez SD, Debus ES, Ricotta JJ 2nd. Endovascular vs open repair of renal artery aneurysms: outcomes of repair and long-term renal function. J Am Coll Surg. (2013) 217(2):263–9. doi: 10.1016/j.jamcollsurg.2013.03.021
24. Chung R, Touska P, Morgan R, Belli AM. Endovascular management of true renal arterial aneurysms: results from a single centre. Cardiovasc Intervent Radiol. (2016) 39(1):36–43. doi: 10.1007/s00270-015-1135-y
25. Jibiki M, Inoue Y, Kudo T, Toyofuku T. Surgical procedures for renal artery aneurysms. Ann Vasc Dis. (2012) 5(2):157–60. doi: 10.3400/avd.oa.11.00055
26. Zhang Z, Yang M, Song L, Tong X, Zou Y. Endovascular treatment of renal artery aneurysms and renal arteriovenous fistulas. J Vasc Surg. (2013) 57(3):765–70. doi: 10.1016/j.jvs.2012.09.042
27. Abreu AL, Medina LG, Chopra S, Gill K, Cacciamani GE, Azhar RA, et al. Robotic renal artery aneurysm repair. Eur Urol. (2020) 78(1):87–96. doi: 10.1016/j.eururo.2019.06.003
28. Kawase T, Inoue Y, Matsuo J, Omura A, Seike Y, Uehara K, et al. Results of surgical repair of hilar renal artery aneurysm to preserve renal blood flow. Ann Vasc Dis. (2020) 13(3):281–5. doi: 10.3400/avd.oa.20-00020
29. Wei HB, Qi XL, Liu F, Wang J, Ni XF, Zhang Q, et al. Robot-assisted laparoscopic reconstructed management of multiple aneurysms in renal artery primary bifurcations: a case report and literature review. BMC Urol. (2017) 17(1):96. doi: 10.1186/s12894-017-0265-8
30. Modrall JG, Zhu H, Weaver FA. Clinical predictors of blood pressure response after renal artery stenting. J Vasc Surg. (2020) 72(4):1269–75. doi: 10.1016/j.jvs.2019.12.041
31. Kabłak-Ziembicka A, Rosławiecka A, Badacz R, Sokołowski A, Rzeźnik D, Trystuła M, et al. Simple clinical scores to predict blood pressure and renal function response to renal artery stenting for atherosclerotic renal artery stenosis. Pol Arch Intern Med. (2020) 130(11):953–9. doi: 10.20452/pamw.15646
32. Alhadad A, Mattiasson I, Ivancev K, Gottsäter A, Lindblad B. Revascularisation of renal artery stenosis caused by fibromuscular dysplasia: effects on blood pressure during 7-year follow-up are influenced by duration of hypertension and branch artery stenosis. J Hum Hypertens. (2005) 19(10):761–7. doi: 10.1038/sj.jhh.1001893
Keywords: renal artery aneurysm, blood pressure, renal outcome, endovascular repair, open surgery
Citation: Li S, Li F, Liu Z, Zeng R, Ye W, Shao J and Zheng Y (2023) Blood pressure and renal outcomes after renal artery aneurysm intervention: Single-center experience and review of literature. Front. Cardiovasc. Med. 10:1127154. doi: 10.3389/fcvm.2023.1127154
Received: 19 December 2022; Accepted: 31 March 2023;
Published: 21 April 2023.
Edited by:
Elise Peery Gomez-Sanchez, University of Mississippi Medical Center, United StatesReviewed by:
Edwin Takahashi, Mayo Clinic, United StatesJiaxuan Feng, Second Military Medical University, China
© 2023 Li, Li, Liu, Zeng, Ye, Shao and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuehong Zheng emhlbmd5dWVob25nMjAyMkBvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Hypertension, a section of the journal Frontiers in Cardiovascular Medicine
 Siting Li
Siting Li Fangda Li1,2,†
Fangda Li1,2,† Wei Ye
Wei Ye Yuehong Zheng
Yuehong Zheng