
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 April 2023
Sec. Cardiovascular Epidemiology and Prevention
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1126110
This article is part of the Research Topic Prevention and Treatment of Cardiovascular Disease with Integrated Traditional Chinese and Western Medicine View all 21 articles
Background: This study aimed to evaluate the efficacy of Chinese patent medicines (CPMs) combined with dexrazoxane (DEX) against anthracycline-induced cardiotoxicity (AIC) and further explore their pharmacological mechanism by integrating the network meta-analysis (NMA) and network pharmacology approach.
Methods: We searched for clinical trials on the efficacy of DEX + CPMs for AIC until March 10, 2023 (Database: PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure, China Science and Technology Journal and China Online Journals). The evaluating outcomes were cardiac troponin I (cTnI) level, creatine kinase MB (CK-MB) level, left ventricular ejection fraction (LVEF) value, and electrocardiogram (ECG) abnormal rate. Subsequently, the results of NMA were further analyzed in combination with network pharmacology.
Results: We included 14 randomized controlled trials (RCTs) and 1 retrospective cohort study (n = 1,214), containing six CPMs: Wenxinkeli (WXKL), Cinobufotalin injection (CI), Shenqifuzheng injection (SQFZ), Shenmai injection (SM), Astragalus injection (AI) and AI + CI. The NMA was implemented in Stata (16.0) using the mvmeta package. Compared with using DEX only, DEX + SM displayed the best effective for lowering cTnI level (MD = −0.44, 95%CI [−0.56, −0.33], SUCRA 93.4%) and improving LVEF value (MD = 14.64, 95%CI [9.36, 19.91], SUCRA 98.4%). DEX + SQFZ showed the most effectiveness for lowering CK-MB level (MD = −11.57, 95%CI [−15.79, −7.35], SUCRA 97.3%). And DEX + AI + CI has the highest effectiveness for alleviating ECG abnormalities (MD = −2.51, 95%CI [−4.06, −0.96], SUCRA 96.8%). So that we recommended SM + DEX, SQFZ + DEX, and DEX + AI + CI as the top three effective interventions against AIC. Then, we explored their pharmacological mechanism respectively. The CPMs' active components and AIC-related targets were screened to construct the component-target network. The potential pathways related to CPMs against AIC were determined by KEGG. For SM, we identified 118 co-targeted genes of active components and AIC, which were significantly enriched in pathways of cancer pathways, EGFR tyrosine kinase inhibitor resistance and AGE-RAGE signaling pathway in diabetic complications. For SQFZ, 41 co-targeted genes involving pathways of microRNAs in cancer, Rap1 signaling pathway, MAPK signaling pathway, and lipid and atherosclerosis. As for AI + CI, 224 co-targeted genes were obtained, and KEGG analysis showed that the calcium signaling pathway plays an important role except for the consistent pathways of SM and SQFZ in anti-AIC.
Conclusions: DEX + CPMs might be positive efficacious interventions from which patients with AIC will derive benefits. DEX + SM, DEX + SQFZ, and DEX + AI + CI might be the preferred intervention for improving LVEF value, CK-MB level, and ECG abnormalities, respectively. And these CPMs play different advantages in alleviating AIC by targeting multiple biological processes.
Anthracyclines, representative drugs such as doxorubicin, daunorubicin, and epirubicin, are the cornerstone in treating various cancers. However, dose-dependent cardiotoxicity always significantly limited the clinical application of anthracyclines (1). Research reported that cumulative doxorubicin doses of 400, 550, and 700 mg/m2 could increase the incidence of congestive heart failure by 4.7%, 26%, and 48% (2). The cumulative toxic effect on the heart will affect oncology patients' prognosis of seriously (3). Thus, the treatment and prevention of AIC is a significant challenge and cannot be ignored. So far, dexrazoxane (DEX) remains the only drug that works as a cardioprotective agent against AIC, which has been approved by the U.S. Food and Drug Administration (4, 5). But DEX inevitably induces adverse effects, such as myelosuppression and secondary malignancies (6). Hence, optimizing the clinical treatment of AIC to improve patient's quality of life deserves further exploration.
Several scholars have tried to explore some profitable ways against AIC from the perspective of the combination of traditional Chinese medicine (TCM) and western medicine. The concept of integrated TCM and western medicine originated in the 17th century (7). Long-term historical experiences show that the cooperation of TCM and western medicine may be more efficient for the cure and prevention of disease than each of them separately. Numerous studies reported the potential benefits of combined TCM and western medicine in improving tumor prognosis, reducing adverse reactions, and enhancing life quality (8–12). Recent clinical evidence indicates that the cooperation of Chinese patent medicines (CPMs) and DEX had a good effect on lowing cardiotoxicity and improving the anti-tumor efficiency of anthracyclines (13). There are kinds of CPMs commonly selected, such as Wenxinkeli (WXKL), Cinobufotalin injection (CI), Shenqifuzheng injection (SQFZ), Shenmai injection (SM), Astragalus injection (AI) and so on. However, the comparative efficacy of these CPMs in treating AIC remains unknown. And their clinical improvement characteristics, active components, and potential targets still need to be well clarified.
Therefore, for comprehensive information for clinicians to determine the optimal combination of CPMs and DEX for patients with AIC, a network meta-analysis was performed in the present study to evaluate the efficacy of different CPMs and rank their effectiveness. All of these CPMs are authorized by the China State Food and Drug Administration. At the same time, network pharmacology will further analyze the more effective CMPs with a high evidence grade to explore their anti-AIC mechanism.
This study protocol has been registered in the international prospective register of systematic reviews (PROSPERO) (https://www.crd.york.ac.uk/PROSPERO) (14). The registration number is CRD42022297523. We searched PubMed, Embase, Cochrane Library, Chinese National Knowledge Infrastructure (CNKI), China Science and Technology Journal (CSTJ) and China Online Journals (COJ) from inception to March 10, 2023. The initial search items were “anthracycline”, “cardiotoxicity” (title/abstract) and “randomized controlled trial” (title/abstract). The search was conducted using a combination of medical subject headings (MeSH) and free-text words, and the detailed search terms were listed in Supplementary Table S1.
The inclusion criteria were: (1) Clinical trials related to AIC. (2) Patients: All included patients were diagnosed with tumors and received anthracyclines, such as epirubicin, pirarubicin, or doxorubicin. The dose and duration of drug treatment and the gender and age of patients were unlimited. (3) Interventions: before chemotherapy, the experiment group used the combination of CPMs and DEX to counteract the AIC, and the control groups only used DEX. (4) Outcomes: cardiac troponin I (cTnI), creatine kinase MB (CK-MB) or left ventricular ejection fraction (LVEF) at baseline and after chemotherapy, and the rate of abnormal electrocardiogram (ECG). (5) The language of the literature is not limited.
The exclusion criteria were: (1) Repetitive published studies. (2) Non-clinical trials. (3) Cardiotoxicity due to non-anthracycline chemotherapy. (4) Studies with incomplete or incorrect data. (5) Animal experiments, literature review, and conference abstracts.
Two investigators assessed all trials for eligibility and extracted data by screening the titles and abstracts independently. If disagreements occurred, the third investigator judged by retrieving the full articles and discussion. Data extracted included: (1) Basic information, including title, source, author, and year. (2) Baseline characteristics of the study population, containing the number of trial participants, age, and disease type. (3) Intervention details and follow-up time. (4) Key elements of the risk of bias evaluation. (5) Data on outcome indicators and outcome measures of cnTI, CK-MB or LVEF at baseline and after chemotherapy, and the rate of abnormal ECG.
We considered the following aspects for quality assessment: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. Quality assessment was performed using Review Manager (version 5.3; The Cochrane Collaboration, London, United Kingdom). Disagreements between the two investigators were resolved by consensus.
We applied Stata 16 software (StataCorp, College Station, TX, United States) to perform the network meta-analyses. The normalized mean difference (MD) was calculated for continuous variables, the odds ratios (ORs) were used as the effect analysis statistic for dichotomous variables. All results were provided with its 95% confidence interval (95%CI), I2 statistics and chi-square tests were used to assess statistical heterogeneity. Fixed effects were used if there was no heterogeneity between studies (I2 < 50%, P > 0.1). If there was heterogeneity (I2 > 50%, P < 0.1), the source of heterogeneity was analyzed, and a meta-analysis was performed with random effects after excluding the influence of heterogeneity.
The mvmeta package was used for preprocessing data and drawing the network relationship of different intervention measures. Network meta-analysis (multiple treatments meta-analysis, mixed treatment comparisons) allows the comparison of any two treatments within the network even a direct comparison from a trial is not available.
Such as combine the information from direct evidence (pairwise comparisons) and indirect evidence (comparing B–C from comparisons of A–B and A–C). The outcome indicators for each intervention were ranked by plotting the surface under the cumulative ranking curve (SUCRA), which expressed a percentage of the efficacy or safety of every intervention relative to an imaginary intervention (15). A larger SUCRA score was considered to indicate a more effective or safer intervention. Comparison-adjusted funnel plots were used to assess publication bias and the effects of the small sample in included studies.
AIC-related targets were obtained from the Genecards database (https://www.genecards.org/) and the DisGeNET database (http://www.disgenet.org/) (16). The search phrases contained “anthracycline-induced cardiotoxicity”, “anthracycline-induced heart failure”, “anthracycline-induced cardiomyopathy”, “adriamycin-induced heart failure”, “doxorubicin-induced cardiotoxicity”, and “doxorubicin-induced cardiomyopathy”. The database of AIC-related targets was established after removing repetitions.
The basic information of the effective components and compounds of CMPs were obtained through various literature search. The related targets of CPMs components were obtained from the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP, http://tcmspw.com/tcmsp.php) and the SwissTargetPrediction database (STP, http://www.swisstargetprediction.ch/) (17, 18). The canonical SMILES information of each ingredient was obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) and was imported into the STP to predict the potential targets (19). Only the targets with probability >0.11 were retained. Then, the Uniprot Knowledgebase (http://www.uniprot.org/) was used for gene standardization (20). The overlapping part of the AIC-related targets and CPMs-related targets are the targets of this article that we researched. VENNY2.1 (https://bioinfogp.cnb.csic.es/tools/venny/) was used to obtain the Venn diagram and their intersection targets (21).
To further identify the core regulatory targets, The protein-protein interaction (PPI) network was performed by submitting the overlapping targets of active ingredients to the STRING database (https://string-db.org/) (22), which is used for searching known and predicted interactions between proteins. The species were selected as “Homo sapiens”, and score >0.4 was set as statistically significant. The obtained protein interaction relationship results were exported in TSV format, and the acquired data were imported into Cytoscape 3.8.2 (23). Then, the topological analysis was performed, and targets with a degree value greater than the median were selected as the high correlation targets for further research.
To clarify the role of core targets in gene function, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed via R packages (24). Bubbles and histograms were used to visualize the result. Pathways with adjusted P < 0.01 were considered statistically significant. The terms were selected for visualization according to the P-value.
We searched a total of 2,977 articles. After the movement of 537 duplicate references and the deletion of 2,196 irrelevant studies, the full text of the remaining 244 references was scanned. Then, we excluded 227 studies with inconsistent intervention, 1 conference abstract, and 1 literature without qualitative outcomes. Finally, the remaining 15 published studies comprised 14 RCTs and 1 retrospective cohort study, including 1,214 participants and 7 therapy regimens, were available for the network meta-analysis (25–39). The study selection flow chart is shown in Figure 1.
We selected DEX as the control to compare the effects of different DEX + CPMs interventions on AIC. Six DEX + CPMs interventions include DEX + WXKL, DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, DEX + AI + CI. Among included 15 RCTs, 12 studies reported the improvement of LVEF value, 8 studies reported a reduction in cTnI level, 7 studies reported a decrease in CK-MB level and 11 studies reported the rate of abnormal ECG in patients. Levels of cTnI and CK-MB as early predictive markers for AIC, which could significantly increase after cardiac injury and reflect the sensitive change of abnormal myocardial status (40). LVEF is an indicator for monitoring cardiac function, the LVEF value with a drop of 10% from the baseline to an absolute value of <50% is commonly used in identifying ACT (41). ECG is also widely used as an initial screening tool for AIC (42). ECG can display a variety of non-specific abnormalities, covering ST-T segment elevation, sinus tachycardia, atrioventricular block and malignant premature beat (43). The comparative efficacy of these DEX + CPMs in treating AIC was performed by network meta-analysis. Detailed characteristics of all included studies are shown in Table 1. The information of main CMPs were shown in the Supplementary Table S2.
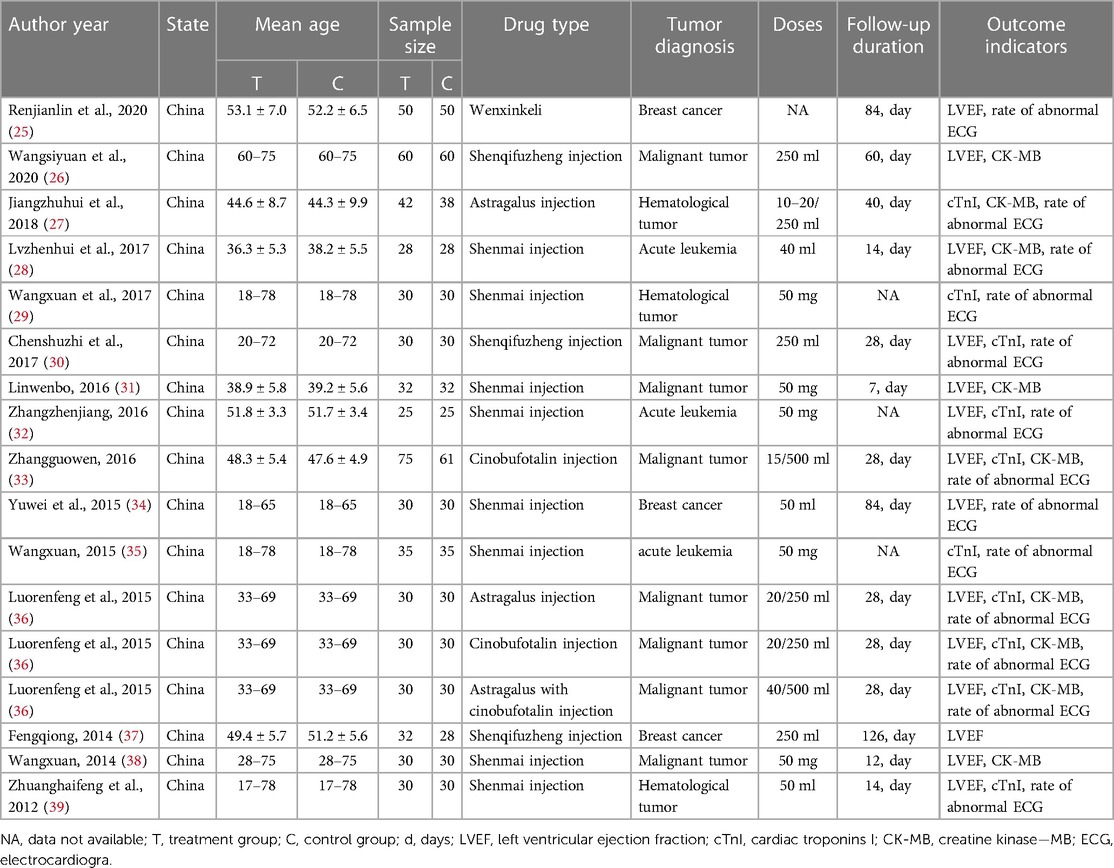
Table 1. General characteristics in a network meta-analysis of the therapy of anthracycline-induced cardiotoxicity patients.
Two investigators independently assessed the risk of bias in the included studies and cross-checked the results. Data were collated from 15 published articles comprising 14 RCTs and 1 retrospective cohort study. 14 RCTs were all assigned in random group. Concretely, 5 RCTs performed the “random number table” method, 1 RCT used the “treatment order” method, 1 RCT employed the “medication method”, and the rest studies failed to specify the randomization method. All studies did not report allocation concealment. Blinding was assessed as an unclear source of bias because of insufficient information. Regarding data completeness, selective reporting and other aspects showed a low risk of bias. The results of a detailed bias evaluation are shown in Figure 2.
As shown in Figure 3A, 7 studies containing 5 therapeutic regimens (DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, DEX + AI + CI) reported the cTnI level. The network meta-analysis results in the cTnI level are displayed in Figure 4A. The cTnI level in different DEX + CPMs treatments were significantly decreased compared with DEX treatment. The detailed comparative statistics were DEX + CI vs. DEX (MD = −0.22, 95% CI [−0.41, −0.03]), DEX + SQFZ vs. DEX (MD = −0.16, 95% CI [−0.38, 0.06]), DEX + SM vs. DEX (MD = −0.44, 95% CI [−0.56, −0.33]), DEX + AI vs. DEX (MD = −0.09, 95% CI [−0.42, 0.24]), DEX + AI + CI vs. DEX (MD = −0.33, 95% CI [−0.68, 0.02]).
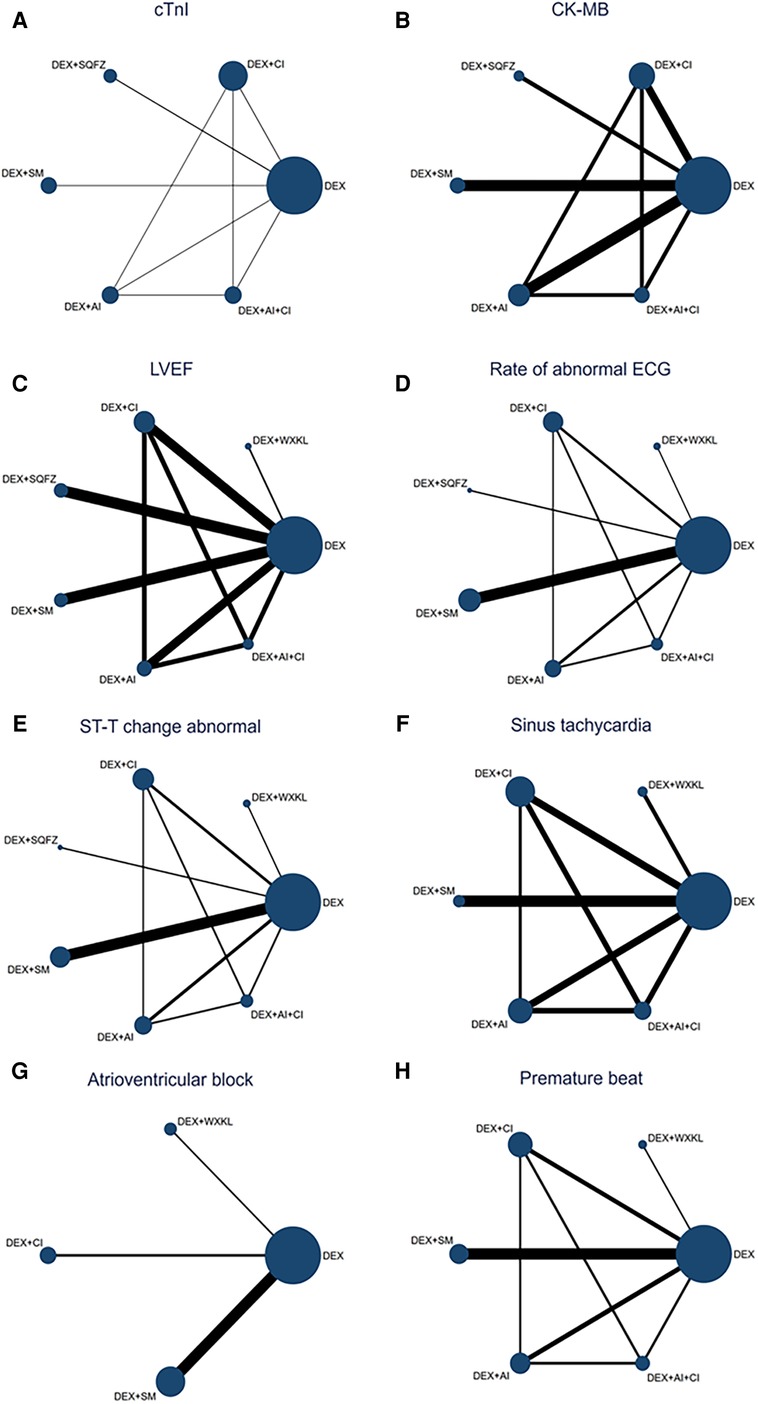
Figure 3. Network map for all outcomes. (A) cTnI. (B) CK-MB. (C) LVEF. (D) Rate of abnormal of ECG. (E) ST-T change abnormal. (F) Sinus tachycardia. (G) Atrioventricular block. (H) Premature beat. (DEX, Dexrazoxane; DEX + WXKL, Dexrazoxane combined with Wenxinkeli; DEX + CI, Dexrazoxane combined with Cinobufotalin injection; DEX + SQFZ, Dexrazoxane combined with Shenqifuzheng injection; DEX + SM, Dexrazoxane combined with Shenmai injection; DEX + AI, Dexrazoxane combined with Astragalus injection; DEX + AI + CI, Dexrazoxane combined with Astragalus with Cinobufotalin injection). The size of every node is proportional to the number of participants (sample size). Lines represent the available direct comparisons between pairs of treatments. Their width is proportional to the number of trials comparing every pair of treatments.
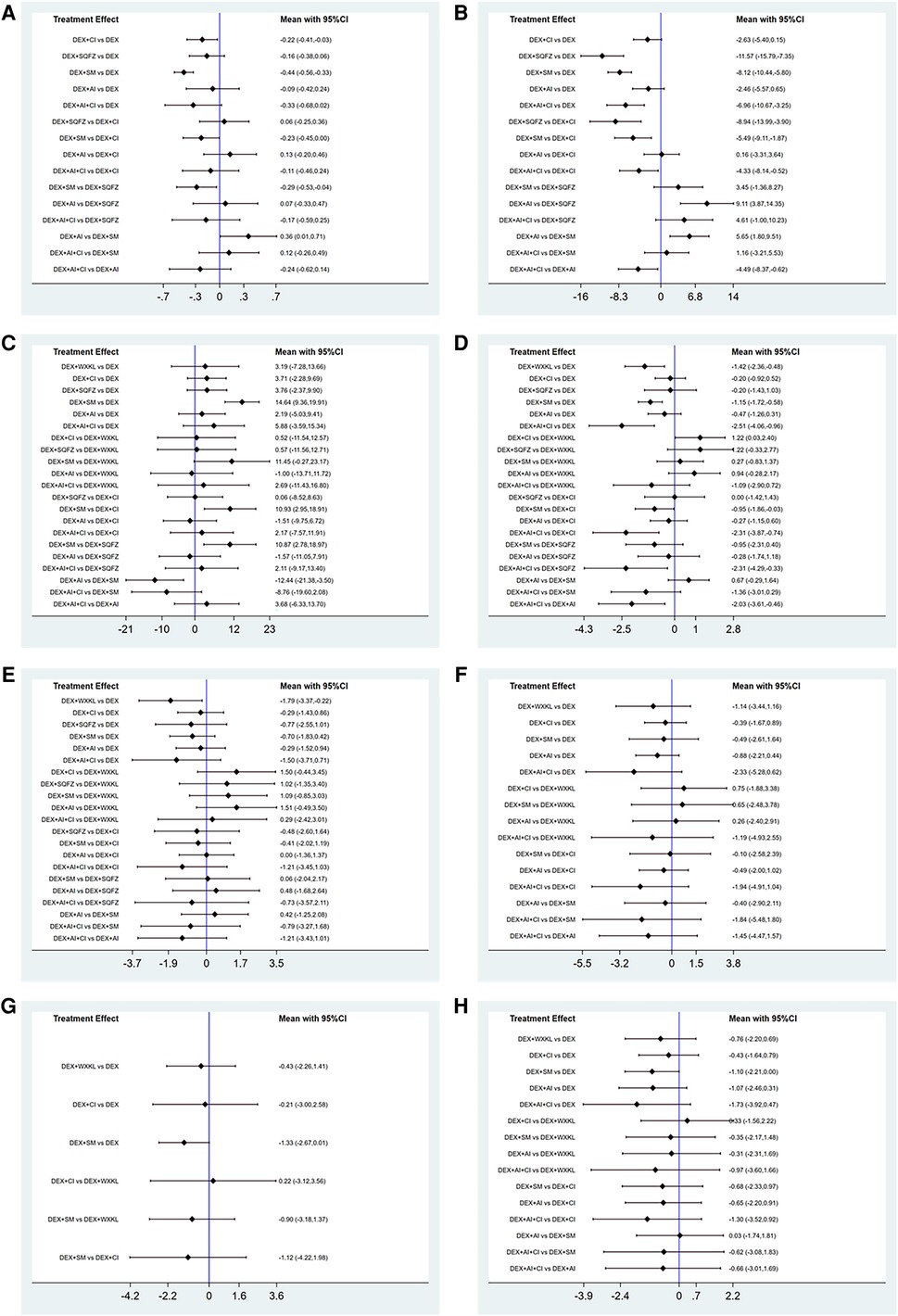
Figure 4. Forest plot of the studies included. (A) cTnI. (B) CK-MB. (C) LVEF. (D) Rate of abnormal of ECG. (E) ST-T change abnormal. (F) Sinus tachycardia. (G) Atrioventricular block. (H) Premature beat (DEX, Dexrazoxane; DEX + WXKL, Dexrazoxane combined with Wenxinkeli; DEX + CI, Dexrazoxane combined with Cinobufotalin injection; DEX + SQFZ, Dexrazoxane combined with Shenqifuzheng injection; DEX + SM, Dexrazoxane combined with Shenmai injection; DEX + AI, Dexrazoxane combined with Astragalus injection; DEX + AI + CI, Dexrazoxane combined with Astragalus with Cinobufotalin injection).
Among these 5 DEX + CPMs therapeutic regimens, the larger SUCRA indicated the better effect in reducing the cTnI level. According to Figures 5A, 6A, the rank of effective medication regimen was DEX + SM (SUCRA = 93.4%), DEX + AI + CI (SUCRA = 72.7%), DEX + CI (SUCRA = 54.8%), DEX + SQFZ (SUCRA = 42.6%), DEX + AI (SUCRA = 28.1%). These data indicate that the patients treated with DEX + SM had the highest probability of reducing the cTnI level.
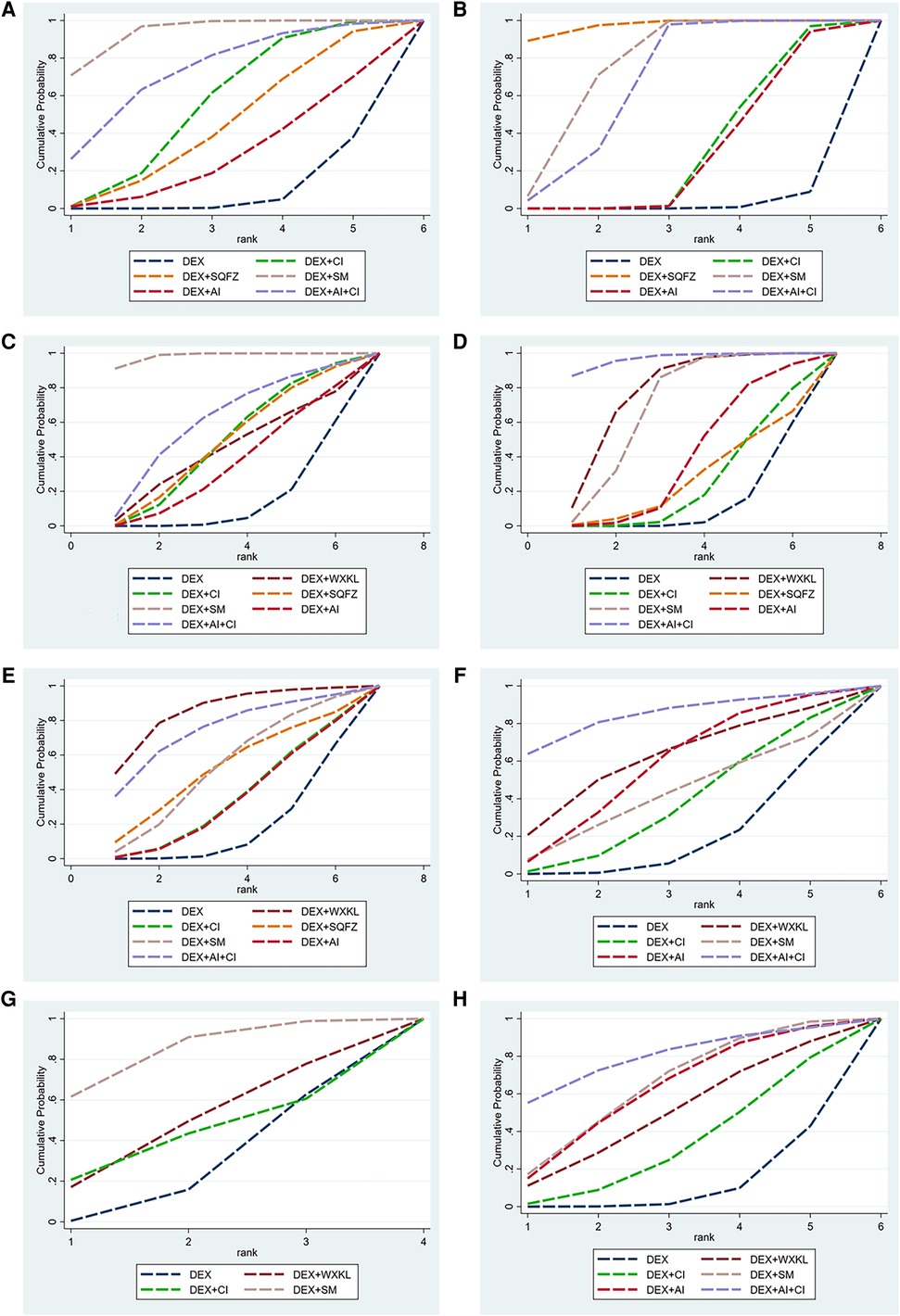
Figure 5. The surface under the cumulative ranking curve (SUCRA) is shown for each treatment. (A) cTnI. (B) CK-MB. (C) LVEF. (D) Rate of abnormal of ECG. (E) ST-T change abnormal. (F) Sinus tachycardia. (G) Atrioventricular block. (H) Premature beat (DEX, Dexrazoxane; DEX + WXKL, Dexrazoxane combined with Wenxinkeli; DEX + CI, Dexrazoxane combined with Cinobufotalin injection; DEX + SQFZ, Dexrazoxane combined with Shenqifuzheng injection; DEX + SM, Dexrazoxane combined with Shenmai injection; DEX + AI, Dexrazoxane combined with Astragalus injection; DEX + AI + CI, Dexrazoxane combined with Astragalus with Cinobufotalin injection).
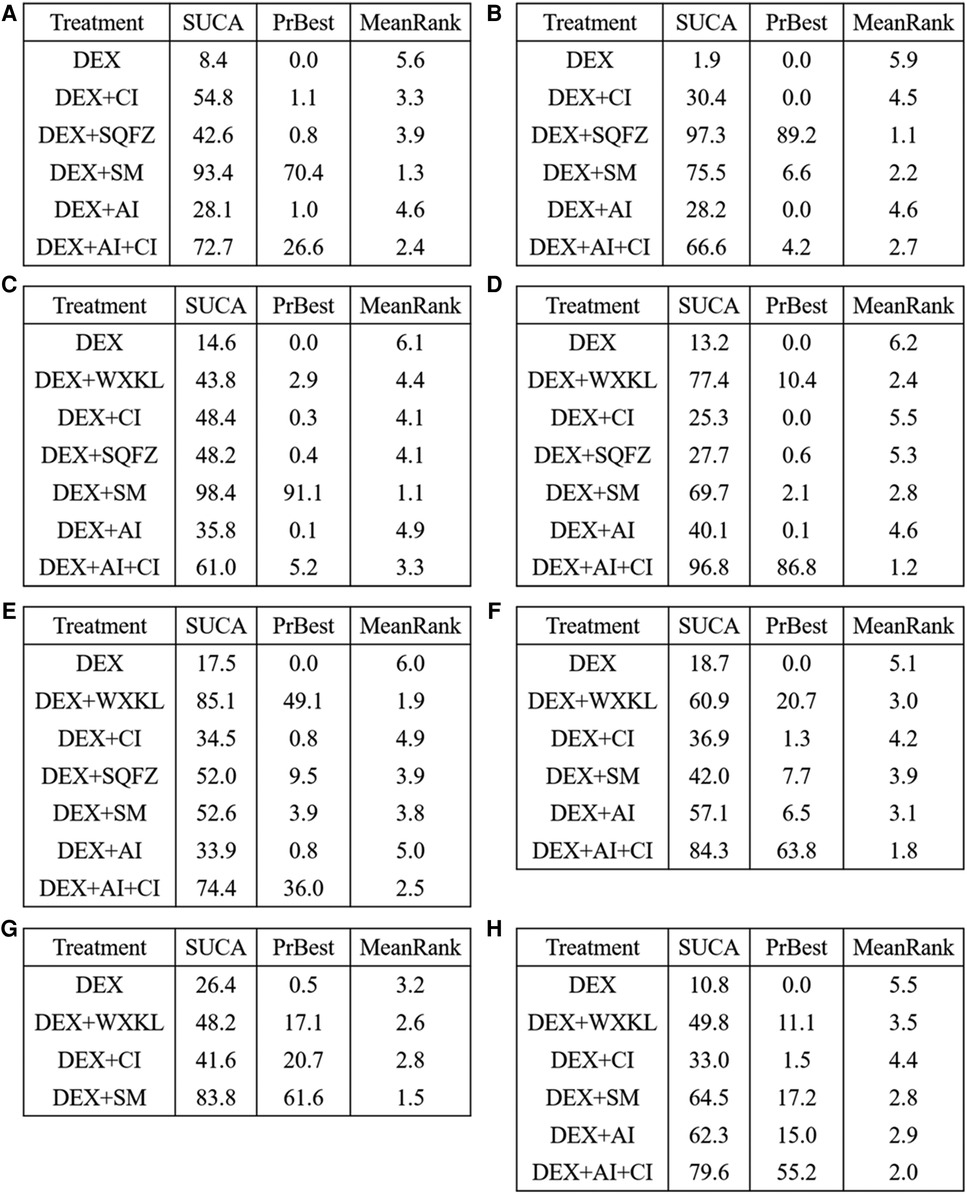
Figure 6. SUCRA rank of each intervention. (A) cTnI. (B) CK-MB. (C) LVEF. (D) Rate of abnormal of ECG. (E) ST-T change abnormal. (F) Sinus tachycardia. (G) Atrioventricular block. (H) Premature beat (DEX, Dexrazoxane; DEX + WXKL, Dexrazoxane combined with Wenxinkeli; DEX + CI, Dexrazoxane combined with Cinobufotalin injection; DEX + SQFZ, Dexrazoxane combined with Shenqifuz)heng injection; DEX + SM, Dexrazoxane combined with Shenmai injection; DEX + AI, Dexrazoxane combined with Astragalus injection; DEX + AI + CI, Dexrazoxane combined with Astragalus with Cinobufotalin injection).
From Figure 3B, 7 studies containing 5 therapeutic regimens (DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, DEX + AI + CI) reported the CK-MB level. The network meta-analysis results in Figure 4B showed that the cTnI level in different DEX + CPMs treatments were lower than in the DEX treatment. The detailed comparative statistics were DEX + CI vs. DEX (MD = −2.63, 95% CI [−5.40, 0.15]), DEX + SQFZ vs. DEX (MD = −11.57, 95% CI [−15.79, −7.35]), DEX + SM vs. DEX (MD = −8.12, 95% CI [−10.44, −5.80]), DEX + AI vs. DEX (MD = −2.46, 95% CI [−5.57, 0.65]), and DEX + AI + CI vs. DEX (MD = −6.96, 95% CI [−10.67, −3.25]).
From the Figures 5B, 6B, SQFZ + DEX was the most effective treatment in reducing the CK-MB level for its SUCRA value of 97.3%, followed by DEX + SM (75.5%), DEX + AI + CI (66.6%), DEX + CI (30.4%), DEX + AI (28.2%), and DEX (1.9%). The above results demonstrate that the patients with DEX + SQFZ treatment had the highest probability of decreasing the CK-MB level.
In Figure 3C, 12 studies covering six therapeutic regimens (DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, DEX + AI + CI, and DEX + WXKL) assessed the change in LVEF value. The network meta-analysis results in Figure 4C showed that DEX + SM administration (MD = 14.64, 95% CI [9.36, 19.91]) could significantly increase the LVEF value more than the DEX treatment. At the same time, the other five therapeutic regimens all had no significant differences in the LVEF value from the DEX treatment. And their detailed comparative statistics were DEX + WXKL vs. DEX (MD = 3.19, 95% CI [−7.28, 13.66]), DEX + CI vs. DEX (MD = 3.71, 95% CI [−2.28, 9.69]), DEX + SQFZ vs. DEX (MD = 3.76, 95% CI [−2.37, 9.90]), DEX + AI vs. DEX (MD = 2.19, 95% CI [−5.03, 9.41]), DEX + AI + CI vs. DEX (MD = 5.88, 95% CI [−3.59, 15.34]).
As shown in Figures 5C, 6C, the highest SUCRA value of DEX + SM treatment (98.4%) meant the effective improvement of LVEF, followed by DEX + AI + CI (61.0%), DEX + CI (48.4%), DEX + SQFZ (48.2%), DEX + WXKL (43.8%), DEX + AI (35.8%), and DEX (14.6%). These data indicate that the patients with DEX + SM treatment had the highest probability of improving LVEF value.
As displayed in Figure 3D, 11 studies, including 6 therapeutic regimens (DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, DEX + AI + CI, and DEX + WXKL), calculated the abnormal rate of ECG. The network meta-analysis results in Figure 4D exhibited that intervention protocols of DEX + WXKL, DEX + CI, DEX + SM, DEX + AI, and DEX + AI + CI all had significant improvement in the abnormal rate of ECG when compared with DEX treatment (MD = −1.42, 95% CI [−2.36, −0.48]; MD = −0.20, 95% CI [−0.92, 0.52]; MD = −1.15, 95% CI [−1.72, −0.58]; MD = −0.47, 95% CI [−1.26, 0.31]; MD = −2.51, 95% CI [−4.06, −0.96], respectively), whereas DEX + SQFZ administration (MD = −0.20, 95% CI [−1.43, 1.03]) showed no apparent alleviation in the abnormal rate of ECG.
According to Figures 5D, 6D, the DEX + AI + CI administration displayed the most effective reduction in the abnormal rate of ECG with a SUCRA value of 96.8%, followed by treatment of DEX + WXKL (77.4%), DEX + SM (69.7%), DEX + AI (40.1%), DEX + SQFZ (27.7%), and DEX + CI (25.3%), and DEX (13.2%). These data imply that the patients with DEX + AI + CI treatment had the highest probability of alleviating the abnormal rate of ECG.
From Figure 3E, 10 studies covering 6 therapeutic regimens (DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, DEX + AI + CI, and DEX + WXKL) compared the ECG ST-T segment alteration rates. The network meta-analysis results in Figure 4E showed that intervention protocols of DEX + WXKL, DEX + CI, DEX + SM, DEX + AI, and DEX + AI + CI all significantly decreased the ST-T segment alteration rates when compared with DEX treatment (MD = −1.79, 95% CI [−3.37, −0.22]; MD = −0.29, 95% CI [−1.43, 0.86]; MD = −0.70, 95% CI [−1.83, 0.42]; (MD = −0.29, 95% CI [−1.52, 0.94]; MD = −1.50, 95% CI [−3.71, 0.71], respectively), whereas DEX + SQFZ administration (MD = −0.77, 95% CI [−2.55, 1.01]) had no obvious effect in reducing the ST-T segment (ECG Change) alteration rates.
As shown in Figures 5E, 6E, the DEX + WXKL administration displayed the most effective reduction in the ST-T segment alteration rates with a SUCRA value of 85.1%, followed by DEX + AI + CI (74.4%), DEX + SM (52.6%), DEX + SQFZ (52.0%), DEX + CI (34.5%), DEX + AI (33.9%), and DEX (17.5%). These data suggest that the patients with DEX + WXKL treatment had the highest probability of reducing the ST-T segment alteration rates.
As shown in Figure 3F, 6 studies including 5 therapeutic regimens (DEX + CI, DEX + SM, DEX + AI, DEX + AI + CI, and DEX + WXKL), reported the improvement of sinus tachycardia. The network meta-analysis results in Figure 4F displayed that only three therapeutic regimens of CPMs and DEX showed a potent effect in improving sinus tachycardia than DEX treatment, including DEX + CI (MD = −0.39, 95% CI [−1.67, 0.89]), DEX + AI (MD = −0.88, 95% CI [−2.21, 0.44]), and DEX + AI + CI (MD = −2.33, 95% CI [−5.28, 0.62]). And there were no evident differences in sinus tachycardia improvement observed in WXKL + DEX (MD = −1.14, 95% CI [−3.44, 1.16]), DEX + SM (MD = −0.49, 95% CI [−2.61, 1.64]) when compared with DEX treatment.
The SUCRA in Figures 5F, 6F exhibited that the DEX + AI + CI administration had the most potent in improving sinus tachycardia with a SUCRA value of 84.3%, followed by DEX + WXKL (60.9%), DEX + AI (57.1%), DEX + SM (42.0%), DEX + CI (36.9%) and DEX (18.7%). These data indicate that the patients with DEX + AI + CI treatment had the highest probability of sinus tachycardia improvement.
From Figure 3G, 6 studies, involving 3 therapeutic regimens (DEX + WXKL, DEX + CI, DEX + SM), reported the improvement of the atrioventricular block. The network meta-analysis results in Figure 4G revealed that only DEX + SM administration (MD = −1.33, 95% CI [−2.67, 0.01]) could more significantly ameliorate atrioventricular block than DEX treatment. And other arms, such as DEX + WXKL (MD = −0.43, 95% CI [−2.26, 1.41]) and DEX + CI (MD = −0.21, 95% CI [−3.00, 2.58]), showed no significant differences in atrioventricular block improvement compared with DEX treatment. At the same time, the SUCRA value of DEX + SM administration (83.8%) also indicates that the patients with DEX + SM treatment had the highest probability of relieving atrioventricular block.
As shown in Figure 3H, 5 studies covering 5 therapeutic regimens (DEX + CI, DEX + SM, DEX + AI, DEX + AI + CI, and DEX + WXKL) reported the improvement of premature beat. The network meta-analysis results in Figure 4H showed that all five therapeutic regimens could improve the premature beat more than the DEX treatment, including DEX + WXKL (MD = −0.76, 95% CI [−2.20, 0.69]), DEX + CI (MD = −0.43, 95% CI [−1.64, 0.79]), DEX + SM (MD = −1.10, 95% CI [−2.21, 0.00]), DEX + AI (MD = −1.07, 95% CI [−2.46, 0.31]), DEX + AI + CI (MD = −1.73, 95% CI [−3.92, 0.47]).
Additionally, as shown in Figures 5H, 6H, the SUCRA of DEX + AI + CI (79.6%) demonstrates that patients had the highest probability of relieving premature beat with DEX + AI + CI treatment, followed by DEX + SM (64.5%), DEX + AI (62.3%), DEX + WXKL (49.8%), DEX + CI (33.0%), and DEX (10.8%).
The abnormal rate of ECG was set as an outcome indicator for publication bias which is plotted in Figure 7. From symmetry in the funnel plot, the studies are roughly symmetrically distributed on either side of the midline, indicating that a small sample effect is less likely to exist. There is insufficient evidence to support the publication bias.
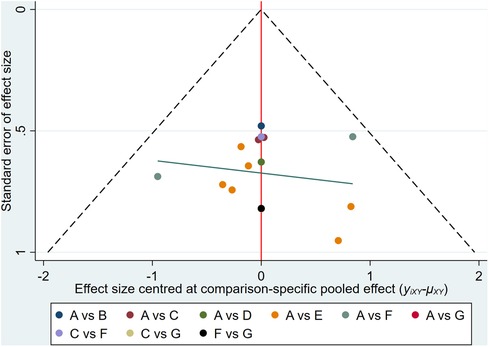
Figure 7. Comparison-correction funnel plot. (A, Dexrazoxane; B, Dexrazoxane combined with Wenxinkeli; C, Dexrazoxane combined with Cinobufotalin injection; D, Dexrazoxane combined with Shenqifuzheng injection; E, Dexrazoxane combined with Shenmai injection; F, Dexrazoxane combined with Astragalus injection; G, Dexrazoxane combined Astragalus with Cinobufotalin injection with).
We analyzed the characteristics of the 6 different DEX + CPMs interventions against AIC from the network meta-analysis. DEX + SM, DEX + SQFZ and DEX + AI + CI are the top three recommended interventions. Subsequently, the pharmacological mechanism of SM, SQFZ, and AI + CI against AIC was performed separately with the network pharmacology.
Through searching the prescribed database, we screened out 2,205 AIC-related targets. For SM, 232 herb targets were obtained from reported literature, and 118 co-targets from herb and AIC were intersected in Figure 8A. For SQFZ, 93 herb targets were acquired, and 41 co-targets from herb and AIC were intersected in Figure 9A. As for AI + CI, 521 herb targets were received, and 224 co-targets from herb and AIC were crossed in Figure 10A.
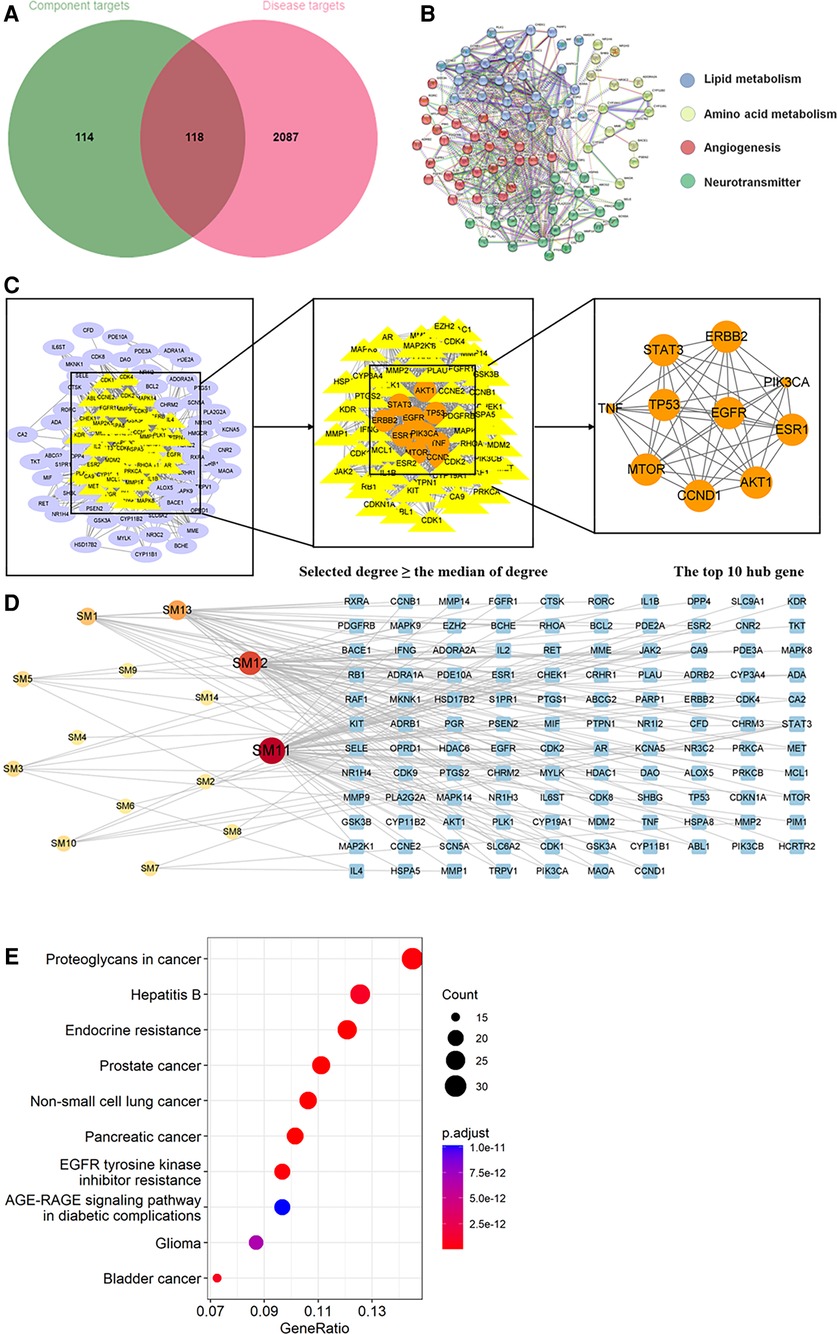
Figure 8. Analysis of target genes about shenmai injection. (A) Venn diagram of component target genes and disease target genes. The green circle stands for effective component target genes. The red circle stands for AIC target genes, and the intersection genes are shown in the middle. (B) The PPI network of the intersection genes. (C) Network topology analysis to screen the hub targets. (D) Component-Target Network of the active components and the target genes. (E) KEGG enrichment analysis of hub genes.
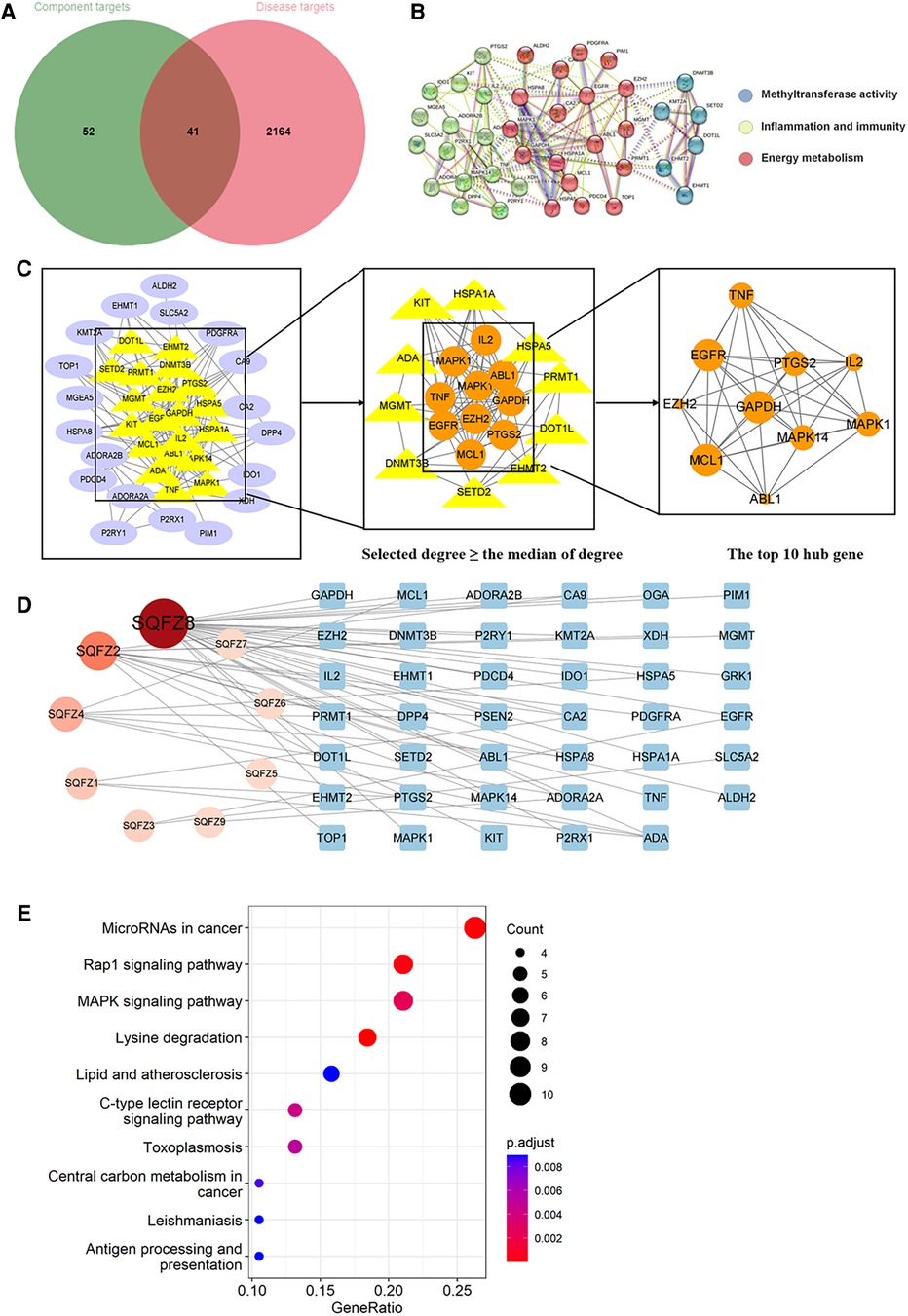
Figure 9. Analysis of target genes about shenqifuzheng injection. (A) Venn diagram of component target genes and disease target genes. The green circle stands for effective component target genes. The red circle stands for AIC target genes, and the intersection genes are shown in the middle. (B) The PPI network of the intersection genes. (C) Network topology analysis to screen the hub targets. (D) Component-Target Network of the active components and the target genes. (E) KEGG enrichment analysis of hub genes.
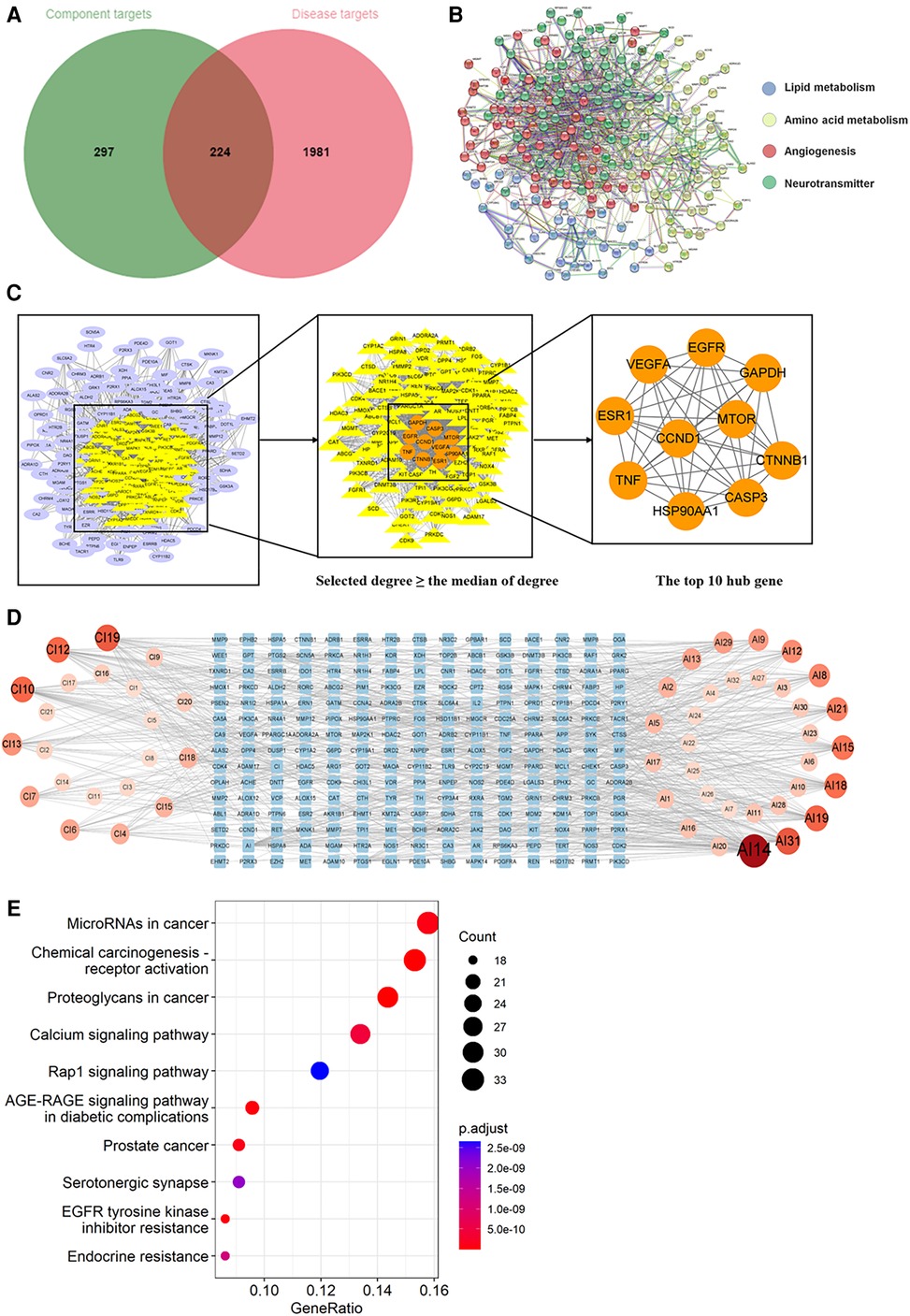
Figure 10. Analysis of target genes about Astragalus with cinobufotalin injection. (A) Venn diagram of component target genes and disease target genes. The green circle stands for effective component target genes. The red circle stands for AIC target genes, and the intersection genes are shown in the middle. (B) The PPI network of the intersection genes. (C) Network topology analysis to screen the hub targets. (D) Component-Target Network of the active components and the target genes. (E) KEGG enrichment analysis of hub genes.
As shown in Figures 8B, 9B, 10B, the co-targets of selected CPMs (SM, SQFZ, AI + CI) and AIC were respectively imported into the String platform. The PPI network of SM against AIC contains 118 nodes and 1,283 edges, and the median degree value screened out 60 core targets. Its top 10 hub genes are shown in Figure 8C: TP53, ESR1, EGFR, STAT3, AKT1, CCND1, MTOR, ERBB2, TNF, and PIK3CA. The PPI network of SQFZ against AIC contains 41 nodes and 147 edges, and the median degree value screened out 20 core targets. Its top 10 hub genes are shown in Figure 9C: GAPDH, EGFR, EZH2, MCL1, TNF, MAPK14, MAPK1, IL2, PTGS2 and ABL1. The PPI network of AI + CI contains 224 nodes and 2,908 edges, and the median degree value screened out 121 core targets. Its top 10 hub genes are shown in Figure 10C: GAPDH, EGFR, CASP3, TNF, CTNNB1, VEGFA, ESR1, HSP90AA1, MTOR and CCND1.
As shown in Figures 8D, 9D, 10D, the core ingredients and targets of selected CPMs (SM, SQFZ, AI + CI) against AIC were imported into Cytoscape 3.8.2 to establish the component-target (C-T) network. The C-T network of SM against AIC contains 14 active compounds and 176 targets, and its active components include beta-elemene, uridine, stigmasterol, ruscogenin, Parisaponin I, orchinol, ophiopogonin D, ophiopogonanone E, n-trans-feruloyltyramine, Notoginsenoside R1, guanosine, ginsenoside Rg1, ginsenoside Rf, diosgenin. The C-T network of SQFZ against AIC contains 9 active compounds and 55 targets, and its active components include uridine, ononin, lobetyolin, guanosine, cytidine, astragaloside VI, astragaloside II, adenine and adenosine. The C-T network of AI + CI against AIC contains 53 active compounds and 651 targets, and its active components mainly include isoastragaloside II, adenosine, uridine, cycloastragenol, ethenzamide, cholesterol, formononetin, glycine, calycosin and phenylalanine.
KEGG enrichment analysis was performed with the co-targets of selected CPMs (SM, SQFZ, AI + CI) and AIC respectively.
The KEGG pathway annotation indicated that 267 pathways were yielded. The top 10 KEGG pathways in Figure 8E except some abnormal transcription of cancers, were mainly including EGFR tyrosine kinase inhibitor resistance and AGE-RAGE signaling pathway in diabetic complications. And their detailed characteristics were listed in Supplementary Table S3.
The KEGG pathway annotation indicated that 205 pathways were yielded. The top 10 KEGG pathways in Figure 9E were mainly including lysine degradation, microRNAs in cancer, Rap1 signaling pathway, MAPK signaling pathway, C-type lectin receptor signaling pathway and lipid and atherosclerosis, and their characteristics were listed in Supplementary Table S4.
The KEGG pathway annotation indicated that 276 pathways were yielded. The top 10 KEGG pathways in Figure 10E were mainly including AGE-RAGE signaling pathway in diabetic complications, EGFR tyrosine kinase inhibitor resistance, MicroRNAs in cancer, calcium signaling pathway, and serotonergic synapse and Rap1 signaling pathway except some abnormal transcription of cancers, and their characteristics were listed in Supplementary Table S5.
AIC clinically encompasses any cardiac complications containing early myocardial injury that can lead to acute or chronic left ventricular dysfunction and, subsequently, irreversible heart failure (44). From a contemporary cohort of 2,625 anthracycline-treated patients, AIC incidence was up to 9% after a median time of 5.2 years (45). Several studies have reported favorable advantages of combining CPMs and western medicine treatments in alleviating AIC (46, 47). To evaluate the efficacy of CPMs combined with DEX against AIC, and further explore their effective pharmacological mechanism, we adopted a combination of network meta-analysis and network pharmacological approach.
Network meta-analysis could compare multiple treatments simultaneously and consider other potential sources of heterogeneity (48), which could better identify the best approach for against AIC. According to the network meta-analysis results, all DEX + CPMs treatments could significantly relieve the AIC when compared with the DEX alone. The leading evaluating indicators include cTnI level, CK-MB level, LVEF value, and ECG abnormal rate. cTnI and CK-MB are the representative indexes of myocardial zymograms, and always as blood biomarkers identified to detect cardiac damage (49). The elevated levels of cTnI and CK-MB could reflect the changes in myocardial injury degree in different periods (50, 51). Our results revealed that five DEX + CPMs interventions (DEX + CI, DEX + SQFZ, DEX + SM, DEX + AI, and DEX + AI + CI) could significantly decrease the levels of cTnI and CK-MB. Among those effective interventions, DEX + SM treatment has higher advantages in lowering the cTnI level, and DEX + SQFZ treatment has higher advantages in reducing the CK-MB level. LVEF is the common index in assessing left ventricular systolic function and is usually employed in the early detection of cardiotoxicity (52). It is worth noting that DEX + SM treatment also has significant advantages over other therapies in improving LVEF value. ECG could reflect various states of the subject's heart, such as the origin of the rhythm, beat frequency, myocardial ischemia or not, and electrolyte changes in the body (53). The abnormal ECG related to suspicion of cardiotoxicity includes ST-T segment change, sinus tachycardia, atrioventricular block, and premature beat. Depression or elevations of the ST-T segment was the best indicator of myocardial blood supply, premature beat could trigger sustained arrhythmias and cause cardiomyopathies (54), and atrioventricular block and sinus tachycardia were also the most common myocarditis complication (55). Our results exhibited that DEX + WXKL treatment was the most effective in improving the ST-T segment, DEX + SM treatment was the best intervention in mitigating atrioventricular block, and DEX + AI + CI treatment was the best intervention in relieving both sinus tachycardia and premature beat.
In general, DEX + SM, DEX + SQFZ, and DEX + AI + CI were the top three recommended interventions against AIC from the network meta-analysis in the present study. Subsequently, we conducted network pharmacological analysis on the top three interventions to explore their potential mechanism of anti-AIC respectively.
Network pharmacology is a promising way to understand disease mechanisms and medication action mechanisms in the context of larger biological networks (56), which is conducive to exploring the target research of DEX + CPMs against AIC. However, DEX as a western medicine with a single component, which mechanism and targets have been clearly reported. That both chelate iron and target topoisomerase 2 alpha (top2α) (57). Therefore, the multi-component and multi- target of CMPs became the core content of our exploration under the network pharmacology.
SM, a commonly used CPMs for treating cardiovascular diseases in the clinic, consists of extractions from Red Ginseng (Chinese name: Hongshen) and Radix Ophiopogonis (Chinese name: Maidong) (58, 59). It was widely used in cardiovascular diseases and has a certain synergistic effect when combined with chemotherapy drugs, which could reduce the side effects caused by chemotherapy drugs. Some studies have shown that SM can reduce AIC by regulating inflammation and restoring cardiac dysfunction (60). The network pharmacological analysis collected 14 components and 176 potential targets of SM against AIC. Several identified components in SM have been reported with therapeutic effects on cardiac diseases, which supported our results of network prediction. For example, beta-elemene could alleviate heart failure by blocking lipid-induced inflammatory pathways (61). The cardiovascular protection effects of stigmasterol have been widely documented (62–64). Saponins, including ginsenosides, ophiopogonin, and diosgenin, are regarded as effective options for anti-AIC (65–67). From target prediction and pathway analysis, EGFR tyrosine kinase inhibitor resistance may play a vital role in the pharmacological mechanism of SM against AIC. As we all know, the abnormal cardiomyocyte homeostasis induced by autophagy is essential for AIC occurrence, and EGFR tyrosine kinase inhibitor has been reported could induce autophagy in cancer cells (68, 69). Meanwhile, literature reported that EGFR tyrosine kinase inhibitor could activate mitochondrial apoptosis, which is also a crucial factor for AIC (70). All evidence implied that the anti-AIC effect of SM may relate with inhibiting autophagy and mitochondrial apoptosis through regulating the EGFR tyrosine kinase inhibitor resistance pathway. In addition, the AGE-RAGE signaling pathway in diabetic complications may be another important pathway for SM against AIC from our results. Literature showed that AGE-RAGE signaling activation could stimulate inflammation response to exaggerate inflammatory cell influx into the susceptible myocardium (71), while targeting the AGE-RAGE pathway is a potential therapeutic strategy for ameliorating myocardial injury (72). And AGE-RAGE signaling cascade could result in numerous profibrotic growth factors secretion and collagen deposition, which may contribute to the left ventricle stiffness, one of the clinical manifestations of AIC (73, 74). Thus, the AGE-RAGE signaling pathway in diabetic complications might be the critical pathway of SM in improving anthracycline-induced left ventricular dysfunction.
SQFZ is concocted from Radix Astragali (Chinese name: Huangqi) and Radix Codonopsis (Chinese name: Dangshen), and is clinically suggested as a complementary treatment of chemotherapy, and clinical practices suggest that SQFZ has therapeutic effects on cardiovascular diseases (75, 76). Intensive researches reported that SQFZ exerts protective through improving myocardial energy metabolism, inhibiting cell adhesion and inflammatory reaction, and reducing myocardial apoptosis and avoiding ventricular remodel (77). The network pharmacological analysis collected 9 components and 55 potential targets of SQFZ against AIC. Among anti-AIC components of SQFZ, several members have been recognized by published studies. For instance, ononin, a natural isoflavone glycoside, could alleviate endoplasmic reticulum stress in AIC by modulating apoptosis-related signaling pathways (78). Astragaloside is reported as an active agent for AIC inhibition by inhibiting autophagy and oxidative stress (79–81). From target prediction and pathway analysis, the pharmacological mechanism of SQFZ against AIC may associate with microRNAs in cancer, Rap1 signaling pathway, MAPK signaling pathway and lipid and atherosclerosis. MicroRNAs play an essential role in gene regulation associated with cardiotoxicity-related cell death, apoptosis, and differentiation (82). Several miRNAs were identified as biomarkers of AIC, such as miR-1, miR-126, and miR-210 (83). Recently studies also indicated that the targeted therapy of microRNA could alleviate AIC (84, 85) Moreover, research shows that inhibition of the MAPK signaling pathway could effectively alleviate AIC by regulating apoptosis (86). And the lipid-lowering drugs were confirmed as promising cardioprotective agents in AIC patients (87). In the next step, experimental validation could be carried out for a more confirmed mechanism of SQFZ against AIC.
AI is the extraction of Radix Astragali, and is generally used in viral myocarditis, enteritis, and hepatitis (88). CI is the extraction of cinobufotalin (Chinese name: Huachansu), which is the major anti-tumor component isolated from toad venom and has been used clinically for various cancers (89). These were both seen to be commonly used CPMs in clinical practice, as complementary treatment to recommended Western therapies. AI could enhance myocardial contractility, improve circulation, protect myocardial cells and regulate immunity (90). CI has a shortening effect on action potential duration and an inhibitory effect on Na+, K (+)-ATPase activity along with its cardiotonic effect (91). The network pharmacological analysis collected 53 components and 651 potential targets of AI + CI against AIC. The top 10 components identified from the C-T network of AI + CI against AIC have shown anti-cardiotoxicity activity in published studies, indicating their potentiality in anti-ACT (92). From target prediction and pathway analysis, there are several co-pathways of AI + CI, SM, and SQFZ in treating AIC. For the anti-AIC mechanism of AI + CI and SM, pathways of EGFR tyrosine kinase inhibitor resistance and the AGE-RAGE signaling pathway in diabetic complications both play important roles. As for the anti-AIC mechanism of AI + CI and SQFZ, microRNAs in cancer and the Rap1 signaling pathway were significantly enriched. It may relate to some same ingredients of herbs in these CPMs. Notably, the calcium signaling pathway is the unique enrichment pathway of AI + CI against AIC, compared with SM and SQFZ. Calcium ion overload is well known to be vital in the pathogenesis of heart dysfunctions, especially arrhythmia. The pathological cellular Ca2+ overload could lead to an accelerated beating rhythm in ventricular myocytes and caused arrhythmia (93). Moreover, the doxorubicin metabolite could target calsequestrin type 2, increasing cytoplasmic Ca2+ concentration, and triggering an arrhythmogenic state (94). These results might explain why AI + CI treatment has advantages in improving ECG abnormalities of cardiotoxic substances.
Inevitably, there are some limitations in our study. Firstly, the effectiveness of DEX + CPMs treatments against AIC still needs further validation with more long-term and high-quality population samples. Secondly, it is inevitable to choose multiple-drug chemotherapy schemes considering the complex condition of tumor patients in clinically, RCTs that use only anthracycline chemotherapy regimens need to be included as much as possible in the future, in order to eliminate interference from other factors. Thirdly, included studies had inadequate descriptions of randomization, allocation concealment, and blinding method, which may lead to baseline wander. Finally, this study was based on data analysis only, and further in vivo and in vitro experiments are necessary to support our results.
We conducted a network meta-analysis integrated with network pharmacology analysis to compare the efficacy of different DEX + CPMs treatments against AIC, and explore their potential pharmacological mechanisms. Multifaceted evidence revealed that DEX + CPMs treatments have obvious advantages over DEX alone in anti-AIC. DEX + SM, DEX + SQFZ, and DEX + AI + CI might be the preferred intervention for improving LVEF value, CK-MB level, and ECG abnormalities. And network pharmacology discovered that CPMs has the characteristics of multiple pathways, multiple components, and multiple targets in alleviating AIC. Collectively, the present study will provide clinicians and researchers with detailed comparisons of therapeutic strategies and references.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
BZ conceived the study. YR, YW, and XD independently extracted data. YY and ZL checked the accuracy of the data and assessed the quality of the included studies. YR and YW wrote the manuscript. XZ and ZL revised the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (No. 82274117).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1126110/full#supplementary-material.
1. Narezkina A, Nasim K. Anthracycline cardiotoxicity. Circ Heart Fail. (2019) 12:e005910. doi: 10.1161/CIRCHEARTFAILURE.119.005910
2. Wang Y, Rao Y, Lin Z, Sa R, Yin Y, Zhang X, et al. Current status and trends of research on anthracycline-induced cardiotoxicity from 2002 to 2021: a twenty-year bibliometric and visualization analysis. Oxid Med Cell Longev. (2022) 2022:6260243. doi: 10.1155/2022/6260243
3. Henriksen PA. Anthracycline cardiotoxicity: an update on mechanisms, monitoring and prevention. Heart. (2018) 104:971–7. doi: 10.1136/heartjnl-2017-312103
4. Cai F, Luis MAF, Lin X, Wang M, Cai L, Cen C, et al. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: preventive strategies and treatment. Mol Clin Oncol. (2019) 11:15–23. doi: 10.3892/mco.2019.1854
5. Zhang H, Wang Z, Liu Z, Du K, Lu X. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front Cardiovasc Med. (2021) 8:685434. doi: 10.3389/fcvm.2021.685434
6. Zamorano JL, Lancellotti P, Muñoz DR, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines. Kardiol Pol. (2016) 74:1193–233. doi: 10.5603/KP.2016.0156
7. Shin SS. Development of integrated traditional Chinese and western medicine and change of medical policy in China. Uisahak. (1999) 8:207–32.12216556
8. Lampe H, Halle B, Freund M. Western and Chinese medicine in oncology and hematology. Forsch Komplementmed. (2011) 18:185–91. doi: 10.1159/000330935
9. Guo XY, Liu J, Liu J, Li HJ, Qi Y, Qin LP, et al. Use of traditional Chinese medicine in Chinese patients with coronary heart disease. Biomed Environ Sci. (2013) 26:303–10. doi: 10.3967/0895-3988.2013.04.009
10. Gao J, Wang X, Li L, Zhang H, He R, Han B, et al. Block matching pyramid algorithm-based analysis on efficacy of shexiang baoxin pills guided by echocardiogram (ECG) on patients with angina pectoris in coronary heart disease. J Healthc Eng. (2021) 6:3819900. doi: 10.1155/2021/3819900
11. Liu Z, Li G, Ma Y, Lin L. The effects of aspirin with combined compound danshen dropping pills on hemorheology and blood lipids in middle-aged and elderly patients with CHD: a systematic review and meta-analysis. Front Public Health. (2021) 9:664841. doi: 10.3389/fpubh.2021.664841
12. Wang WJ, Zhang T. Integration of traditional Chinese medicine and western medicine in the era of precision medicine. J Integr Med. (2017) 15:1–7. doi: 10.1016/S2095-4964(17)60314-5
13. Chen T, Shen HM, Deng ZY, Yang ZZ, Zhao RL, Wang L, et al. A herbal formula, SYKT, reverses doxorubicin-induced myelosuppression and cardiotoxicity by inhibiting ROS-mediated apoptosis. Mol Med Rep. (2017) 15:2057–66. doi: 10.3892/mmr.2017.6272
14. Sideri S, Papageorgiou SN, Eliades T. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J Clin Epidemiol. (2018) 100:103–10. doi: 10.1016/j.jclinepi.2018.01.003
15. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
16. He S, Wang T, Shi C, Wang Z, Fu X. Network pharmacology-based approach to understand the effect and mechanism of Danshen against anemia. J Ethnopharmacol. (2022) 282:114615. doi: 10.1016/j.jep.2021.114615
17. Ru J, Li P, Wang J, Zhou W, Li B, Huang C, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. (2014) 6:13. doi: 10.1186/1758-2946-6-13
18. Daina A, Michielin O, Zoete V. Swisstargetprediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. (2019) 47(W1):W357–64. doi: 10.1093/nar/gkz382
19. Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, et al. Pubchem in 2021: new data content and improved web interfaces. Nucleic Acids Res. (2021) 49(D1):D1388–95. doi: 10.1093/nar/gkaa971
20. Consortium U. Uniprot: the universal protein knowledgebase in 2021. Nucleic Acids Res. (2021) 49:D480–9. doi: 10.1093/nar/gkaa1100
21. Jia A, Xu L, Wang Y. Venn diagrams in bioinformatics. Brief Bioinform. (2021) 22:bbab108. doi: 10.1093/bib/bbab108
22. Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. (2021) 49:D605–12. doi: 10.1093/nar/gkaa1074
23. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. (2003) 13:2498–504. doi: 10.1101/gr.1239303
24. Zhu Y, Yu J, Zhang K, Feng Y, Guo K, Sun L, et al. Network pharmacology analysis to explore the pharmacological mechanism of effective Chinese medicines in treating metastatic colorectal cancer using meta-analysis approach. Am J Chin Med. (2021) 49(8):1839–70. doi: 10.1142/S0192415X21500877
25. Ren JL, Wu S, Jia HY. Clinical treatment of dextramine combined with wenxinkeli to reduce the cardiotoxicity of anthracycline induced breast cancer patients. Chin J Integr Med on Cardio Cerebrovasc Dis. (2020) 16:2747–9. doi: 10.12102/j.issn.1672-1349.2020.16.051
26. Wang SY, Wang XC, Chen SY, Wang WL, Lv JY, Gao WB. A multicenter prospective clinical study of shenqi fuzheng injection in preventing and treating anthracycline cardiotoxicity in elderly patients. J Inner Mongolia Minzu Univer (Nat Sci). (2020) 4:336–9. doi: 10.14045/j.cnki.15-1220.2020.04.011
27. Jiang ZH, Zhang GJ, Xu LL, Cao ZY. Effect of dexamethasone combined with astragalus injection on cardiac toxicity after Adriamycin chemotherapy. Int J Lab Med. (2018) 11:1383–5. doi: 10.3969/j.issn.1673-4130.2018.11.029
28. Lv ZH, Jiang SQ. Influence of combined treatment of dexrazoxane and shenmai injection on reducing cardiotoxicity in the acute leukemia chemotherapy. J Trop Med. (2017) 5:633–6. doi: 10.3969/j.issn.1672-3619.2017.05.021
29. Wang X, Fang JF, Mu XM. Clinical study of dexrazoxane combined with shen mai injection for protecting the heart before chemotherapy of hematological neoplasms. Chin Comm Doctors. (2017) 13:69–70. doi: 10.3969/j.issn.1007-614x.2017.13.43
30. Chen SZ, Qi XM, Qian DY, Yuan BJ. Clinical observation on shenqi fuzheng injection in preventing cardiotoxicity of anthracycline. J Clin Med Pract. (2017) 9:163–164+167. doi: 10.7619/jcmp.201709049
31. Lin WB. Observation on protective effect of dexrazoxane combined with shenmai injection on cardiac toxicity induced by anthracyclines drugs. Clin Med Eng. (2016) 12:1661–2. doi: 10.3969/j.issn.1674-4659.2016.12.1661
32. Zhang ZJ. The value of dexrazoxane and shenmai injection in reducing the central organ toxicity of chemotherapy in patients with acute leukemia. Contemp Med Symp. (2016) 20:99–100. CNKI:SUN:QYWA.0.2016-20-073
33. Zhang GW. Influences of dexrazoxane monotherapy combined with huachansu injection on Adriamycin induced cardiotoxicity in malignant tumor patients. Pract J Cancer. (2016) 7:1202–6. doi: 10.3969/j.issn.1001-5930.2016.07.050
34. Yu W, Guo ZQ, Xie RX, Fan F. Clinical observation of the reduction of anthracycline-induced cardiotoxicity in patients with breast cancer by dexrazoxane combined with shenmai injection. China Pharm. (2015) 32:4562–4. doi: 10.6039/j.issn.1001-0408.2015.32.35
35. Wang X. Study of dexrazoxane combined with shenmai injection in treatment of cardiotoxicity for patients with acute leukemia during chemotherapy. Med J Chin People’s Health. (2015) 12:86–8. doi: 10.3969/j.issn.1672-0369.2015.12.048
36. Luo RF, Liu M, Zhang SC. Protective effects of Astragalus injection/huachansu injection combined with dexrazoxane against Adriamycin related cardiac toxicity. Chin J Hosp Pharm. (2015) 7:629–32. doi: 10.13286/j.cnki.chinhosppharmacyj.2015.07.18
37. Feng Q. Clinical study on the reduction of cardiotoxicity in breast cancer patients treated with FAC chemotherapy regimen by dexrazoxane combined with shenqi fuzheng injection. Jilin J Tradit Chin Med. (2014) 3:256–9. doi: 10.13463/j.cnki.jlzyy.2014.03.014
38. Wang X. Clinical study on dextrimide combined with shenmai to reduce the cardiotoxicity of anthracycline. Med J Chin People’s Health. (2014) 5:47–8. doi: 10.3969/j.issn.1672-0369.2014.05.022
39. Zhuang HF, Zhang Y, Cai JH, Shen JP. Clinical study on anthracycline cardiotoxicity reduction by dexrazoxane combined with shen mai injection. Chin J Clin Oncol. (2012) 6:348–51. doi: 10.3969/j.issn.1000-8179.2012.06.013
40. Stachowiak P, Milchert-Leszczyńska M, Falco M, Polakowska M, Wojtarowicz A, Kaliszczak R, et al. The role of biochemical markers with special regard to troponin, CK-MB, NT-proBNP as early biomarkers of cardiotoxicity among women after chemotherapy due to breast cancer. Przegl Lek. (2016) 73:359–63. 9668198
41. Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med. (2020) 7:26. doi: 10.3389/fcvm.2020.00026
42. Kinoshita T, Yuzawa H, Natori K, Wada R, Yao S, Yano K, et al. Early electrocardiographic indices for predicting chronic doxorubicin-induced cardiotoxicity. J Cardiol. (2021) 77:388–94. doi: 10.1016/j.jjcc.2020.10.007
43. Buttà C, Zappia L, Laterra G, Roberto M. Diagnostic and prognostic role of electrocardiogram in acute myocarditis: a comprehensive review. Ann Noninvasive Electrocardiol. (2020) 25:e12726. doi: 10.1111/anec.12726
44. Totzeck M, Schuler M, Stuschke M, Heusch G, Rassaf T. Cardio-oncology—strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. (2019) 280:163–75. doi: 10.1016/j.ijcard.2019.01.038
45. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. (2015) 131:1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777
46. Shi J, Li J, Li J, Li R, Wu X, Gao F, et al. Synergistic breast cancer suppression efficacy of doxorubicin by combination with glycyrrhetinic acid as an angiogenesis inhibitor. Phytomedicine. (2021) 81:153408. doi: 10.1016/j.phymed.2020.153408
47. Qu J, Ke F, Yang X, Wang Y, Xu H, Li Q, et al. Induction of P-glycoprotein expression by dandelion in tumor and heart tissues: impact on the anti-tumor activity and cardiotoxicity of doxorubicin. Phytomedicine. (2022) 104:154275. doi: 10.1016/j.phymed.2022.154275
48. Hayden JA, Ellis J, Ogilvie R, Stewart SA, Bagg MK, Stanojevic S, et al. Some types of exercise are more effective than others in people with chronic low back pain: a network meta-analysis. J Physiother. (2021) 67:252–62. doi: 10.1016/j.jphys.2021.09.004
49. Yuan M, Zang L, Xu A, Gong M, Liu Q, Huo B, et al. Dynamic changes of serum heart type-fatty acid binding protein in cancer patients treated with immune checkpoint inhibitors. Front Pharmacol. (2021) 12:748677. doi: 10.3389/fphar.2021.748677
50. Kim J, Hashim IA. The clinical utility of CK-MB measurement in patients suspected of acute coronary syndrome. Clin Chim Acta. (2016) 456:89–92. doi: 10.1016/j.cca.2016.02.030
51. Ananthan K, Lyon AR. The role of biomarkers in cardio-oncology. J Cardiovasc Transl Res. (2020) 13:431–50. doi: 10.1007/s12265-020-10042-3
52. Posch F, Niedrist T, Glantschnig T, Firla S, Moik F, Kolesnik E, et al. Left ventricular ejection fraction and cardiac biomarkers for dynamic prediction of cardiotoxicity in early breast cancer. Front Cardiovasc Med. (2022) 9:933428. doi: 10.3389/fcvm.2022.933428
53. John AD, Fleisher LA. Electrocardiography: the ECG. Anesthesiol Clin. (2006) 24:697–715. doi: 10.1016/j.atc.2006.08.005
54. Bagliani G, Della Rocca DG, De Ponti R, Capucci A, Padeletti M, Natale A. Ectopic beats: insights from timing and morphology. Card Electrophysiol Clin. (2018) 10:257–75. doi: 10.1016/j.ccep.2018.02.013
55. Atallah-Yunes SA, Kadado AJ, Kaufman GP, Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. (2019) 145:1527–57. doi: 10.1007/s00432-019-02927-x
56. Niu M, Zhang S, Zhang B, Yang G, Li Y. Interpretation of “guidelines for web-based pharmacologic evaluation methods. Chin Herb Med. (2021) 52:4119–29. doi: 10.7501/j.issn.0253-2670.2021.14.001
57. Rao VA. Iron chelators with topoisomerase-inhibitory activity and their anticancer applications. Antioxid Redox Signal. (2013) 18:930–55. doi: 10.1089/ars.2012.4877
58. Liu WY, Zhang JW, Yao XQ, Jiang C, He JC, Ni P, et al. Shenmai injection enhances the cytotoxicity of chemotherapeutic drugs against colorectal cancers via improving their subcellular distribution. Acta Pharmacol Sin. (2017) 38:264–76. doi: 10.1038/aps.2016.99
59. Li L, Li J, Wang Q, Zhao X, Yang D, Niu L, et al. Shenmai injection protects against doxorubicin-induced cardiotoxicity via maintaining mitochondrial homeostasis. Front Pharmacol. (2020) 11:815. doi: 10.3389/fphar.2020.00815
60. Zhang S, You ZQ, Yang L, Li LL, Wu YP, Gu LQ, et al. Protective effect of shenmai injection on doxorubicin-induced cardiotoxicity via regulation of inflammatory mediators. BMC Complement Altern Med. (2019) 19:317. doi: 10.1186/s12906-019-2686-2
61. Shao M, Wang M, Ma L, Wang Q, Gao P, Tian X, et al. β-elemene blocks lipid-induced inflammatory pathways via PPARβ activation in heart failure. Eur J Pharmacol. (2021) 910:174450. doi: 10.1016/j.ejphar.2021.174450
62. Kukreja RC, Salloum FN, Das A. Cyclic guanosine monophosphate signaling and phosphodiesterase-5 inhibitors in cardioprotection. J Am Coll Cardiol. (2012) 59:1921–7. doi: 10.1016/j.jacc.2011.09.086
63. Morgan LV, Petry F, Scatolin M, de Oliveira PV, Alves BO, Zilli GAL, et al. Investigation of the anti-inflammatory effects of stigmasterol in mice: insight into its mechanism of action. Behav Pharmacol. (2021) 32:640–51. doi: 10.1097/FBP.0000000000000658
64. Choudhary SP, Tran LS. Phytosterols: perspectives in human nutrition and clinical therapy. Curr Med Chem. (2011) 18:4557–67. doi: 10.2174/092986711797287593
65. Wan Y, Wang J, Xu JF, Tang F, Chen L, Tan YZ, et al. Panax ginseng and its ginsenosides: potential candidates for the prevention and treatment of chemotherapy-induced side effects. J Ginseng Res. (2021) 45:617–30. doi: 10.1016/j.jgr.2021.03.001
66. Li C, Gou X, Gao H. Doxorubicin nanomedicine based on ginsenoside Rg1 with alleviated cardiotoxicity and enhanced antitumor activity. Nanomedicine (Lond). (2021) 16:2587–604. doi: 10.2217/nnm-2021-0329
67. Chen CT, Wang ZH, Hsu CC, Lin HH, Chen JH. In vivo protective effects of diosgenin against doxorubicin-induced cardiotoxicity. Nutrients. (2015) 7:4938–54. doi: 10.3390/nu7064938
68. Dirks-Naylor AJ. The role of autophagy in doxorubicin-induced cardiotoxicity. Life Sci. (2013) 93:913–6. doi: 10.1016/j.lfs.2013.10.013
69. Fung C, Chen X, Grandis JR, Duvvuri U. EGFR tyrosine kinase inhibition induces autophagy in cancer cells. Cancer Biol Ther. (2012) 13:1417–24. doi: 10.4161/cbt.22002
70. Sereno M, Brunello A, Chiappori A, Barriuso J, Casado E, Belda C, et al. Cardiac toxicity: old and new issues in anti-cancer drugs. Clin Transl Oncol. (2008) 10:35–46. doi: 10.1007/s12094-008-0150-8
71. Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. (2010) 106:842–53. doi: 10.1161/CIRCRESAHA.109.212217
72. Kumar R, Bhargava P, Suchal K, Bhatia J, Arya DS. Targeting AGE-RAGE signaling pathway by saxagliptin prevents myocardial injury in isoproterenol challenged diabetic rats. Drug Dev Res. (2021) 82:589–97. doi: 10.1002/ddr.21779
73. Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes. (2014) 5:860–7. doi: 10.4239/wjd.v5.i6.860
74. Burr SD, Jr SJ. Extracellular matrix components isolated from diabetic mice alter cardiac fibroblast function through the AGE/RAGE signaling cascade. Life Sci. (2020) 250:117569. doi: 10.1016/j.lfs.2020.117569
75. Dong J, Su SY, Wang MY, Zhan Z. Shenqi fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res. (2010) 29:137. doi: 10.1186/1756-9966-29-137
76. Zhang H, Chen T, Shan L. Shenqi FuZheng injection as an adjunctive treatment to chemotherapy in breast cancer patients: a meta-analysis. Pharm Biol. (2019) 57:612–24. doi: 10.1080/13880209.2019.1660383
77. Liao J, Hao C, Huang W, Shao X, Song Y, Liu L, et al. Network pharmacology study reveals energy metabolism and apoptosis pathways-mediated cardioprotective effects of shenqi fuzheng. J Ethnopharmacol. (2018) 227:155–65. doi: 10.1016/j.jep.2018.08.029
78. Zhang H, Weng J, Sun S, Zhou J, Yang Q, Huang X, et al. Ononin alleviates endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity by activating SIRT3. Toxicol Appl Pharmacol. (2022) 452:116179. doi: 10.1016/j.taap.2022.116179
79. Qu PR, Jiang ZL, Song PP, Liu LC, Xiang M, Wang J. Saponins and their derivatives: potential candidates to alleviate anthracycline-induced cardiotoxicity and multidrug resistance. Pharmacol Res. (2022) 182:106352. doi: 10.1016/j.phrs.2022.106352
80. Luo LF, Qin LY, Wang JX, Guan P, Wang N, Ji ES. Astragaloside IV attenuates the myocardial injury caused by Adriamycin by inhibiting autophagy. Front Pharmacol. (2021) 12:669782. doi: 10.3389/fphar.2021.669782
81. Lin J, Fang L, Li H, Li Z, Lyu L, Wang H, et al. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur J Pharmacol. (2019) 859:172490. doi: 10.1016/j.ejphar.2019.172490
82. Pellegrini L, Sileno S, D'Agostino M, Foglio E, Florio MC, Guzzanti V, et al. MicroRNAs in cancer treatment-induced cardiotoxicity. Cancers (Basel). (2020) 12:704. doi: 10.3390/cancers12030704
83. Pereira JD, Tosatti JAG, Simões R, Luizon MR, Gomes KB, Alves MT. microRNAs associated to anthracycline-induced cardiotoxicity in women with breast cancer: a systematic review and pathway analysis. Biomed Pharmacother. (2020) 131:110709. doi: 10.1016/j.biopha.2020.110709
84. Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L, et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. (2018) 15:284–96. doi: 10.1016/j.redox.2017.12.013
85. Han D, Wang Y, Wang Y, Dai X, Zhou T, Chen J, et al. The tumor-suppressive human circular RNA CircITCH sponges miR-330-5p to ameliorate doxorubicin-induced cardiotoxicity through upregulating SIRT6, survivin, and SERCA2a. Circ Res. (2020) 127:e108–25. doi: 10.1161/CIRCRESAHA.119.316061
86. Zhang L, Feng M, Wang X, Zhang H, Ding J, Cheng Z, et al. Peptide szeto-schiller 31 ameliorates doxorubicin-induced cardiotoxicity by inhibiting the activation of the p38 MAPK signaling pathway. Int J Mol Med. (2021) 47:63. doi: 10.3892/ijmm.2021.4896
87. Haybar H, Goudarzi M, Mehrzadi S, Aminzadeh A, Khodayar MJ, Kalantar M, et al. Effect of gemfibrozil on cardiotoxicity induced by doxorubicin in male experimental rats. Biomed Pharmacother. (2019) 109:530–5. doi: 10.1016/j.biopha.2018.10.101
88. Liu K, Wan G, Jiang R, Zou L, Wan D, Zhu H, et al. Astragalus injection ameliorates lipopolysaccharide-induced cognitive decline via relieving acute neuroinflammation and BBB damage and upregulating the BDNF-CREB pathway in mice. Pharm Biol. (2022) 60:825–39. doi: 10.1080/13880209.2022.2062005
89. Qi J, Zulfiker AHM, Li C, Good D, Wei MQ. The development of toad toxins as potential therapeutic agents. Toxins (Basel). (2018) 10:336. doi: 10.3390/toxins10080336
90. Fu S, Zhang J, Menniti-Ippolito F, Gao X, Galeotti F, Massari M, et al. Huangqi injection (a traditional Chinese patent medicine) for chronic heart failure: a systematic review. PLoS One. (2011) 6:e19604. doi: 10.1371/journal.pone.0019604
91. Hirai Y, Morishita S, Ito C, Sakanashi M. Effects of bufadienolides and some kinds of cardiotonics on Guinea pig hearts. Nihon Yakurigaku Zasshi. (1992) 100:127–35. doi: 10.1254/fpj.100.127
92. Lu X, Lu L, Gao L, Wang Y, Wang W. Calycosin attenuates doxorubicin-induced cardiotoxicity via autophagy regulation in zebrafish models. Biomed Pharmacother. (2021) 137:111375. doi: 10.1016/j.biopha.2021.111375
93. Sun GB, Sun H, Meng XB, Hu J, Zhang Q, Liu B, et al. Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats. Toxicol Appl Pharmacol. (2014) 279:8–22. doi: 10.1016/j.taap.2014.05.005
Keywords: anthracycline-induced cardiotoxicity, Chinese patent medicines, network meta-analysis, network pharmacology, comparative efficacy, pharmacological mechanism
Citation: Rao Y, Wang Y, Lin Z, Zhang X, Ding X, Yang Y, Liu Z and Zhang B (2023) Comparative efficacy and pharmacological mechanism of Chinese patent medicines against anthracycline-induced cardiotoxicity: An integrated study of network meta-analysis and network pharmacology approach. Front. Cardiovasc. Med. 10:1126110. doi: 10.3389/fcvm.2023.1126110
Received: 17 December 2022; Accepted: 4 April 2023;
Published: 24 April 2023.
Edited by:
Yaozu Xiang, Tongji University, ChinaReviewed by:
Xiaoshan Zhao, Southern Medical University, China© 2023 Rao, Wang, Lin, Zhang, Ding, Yang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Zhang emhhbmdiQGJ1Y20uZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.