- 1Department of Emergency Medicine, New Taipei Municipal Tucheng Hospital, New Taipei City, Taiwan
- 2Department of Emergency Medicine, Lin-Kou Medical Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 3College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 4Department of Medical Imaging and Intervention, New Taipei Municipal Tucheng Hospital, New Taipei City, Taiwan
Aortoesophageal fistula (AEF) is an extremely rare cardiovascular etiology of hematemesis and upper gastrointestinal bleeding. As such, its recognition and diagnosis are challenging and may be delayed when such patients present to the emergency department (ED). Without timely surgical intervention, AEF is almost always fatal. Awareness of AEF as a possible diagnosis and consequently early identification of these patients presenting to the ED are therefore crucial in optimizing clinical outcomes. We report a 45-year-old male presenting to the ED with the classical triad of an AEF (Chiari's triad)—midthoracic pain or dysphagia, a sentinel episode of minor hematemesis, then massive hematemesis with risk of exsanguination. The case report highlights the importance of considering the differential diagnosis of AEF when evaluating patients presenting to the ED with hematemesis, especially if they have predisposing risk factors such as prior aortic or esophageal surgeries, aortic aneurysms, or thoracic malignancies. Patients suspected of having AEF should be prioritized for early computed tomography angiography to expedite diagnosis and treatment.
Introduction
Gastrointestinal bleeding is a common presentation seen in the emergency department (ED). Upper gastrointestinal bleeding (UGIB), in which the source of bleeding is proximal to the ligament of Treitz, accounts for approximately 70%–80% of all gastrointestinal hemorrhages (1). UGIB typically manifests as hematemesis, occasionally accompanied by hematochezia and melena. Since peptic ulcer disease (i.e., non-variceal) and esophageal varices represent the vast majority of UGIB etiologies, the possibility of vascular abnormalities is often overlooked (2).
One such rare vascular etiology causing hematemesis is aortoesophageal fistula (AEF). AEFs can be classified as primary or secondary. Primary AEFs directly originate from the native aorta due to various circumstances such as aortic aneurysm (54.2%), foreign body ingestion (19.2%), and advanced esophageal carcinoma (17.0%), in addition to radiotherapy and infections (e.g., syphilis, tuberculosis); secondary AEFs are sequelae of prior vascular interventions such as thoracic aortic or esophageal surgeries (4.7%) and graft placement (3, 4). We report a patient with underlying esophageal cancer who presented to the ED with hematemesis and was subsequently diagnosed with a primary AEF.
Case report
A 45-year-old Chinese male with underlying recently diagnosed squamous cell carcinoma of the esophagus (stage T4bN2M0) presented to the ED with frank hematemesis. He was hypotensive (blood pressure 95/51 mmHg) and tachycardic (pulse rate 129 beats/min) on ED arrival, while point-of-care full blood count revealed gross anemia (Hb 3.6 g/dl). The patient was resuscitated accordingly with intravenous fluid boluses pending activation of a massive transfusion protocol. He was also treated for the provisional diagnosis of UGIB secondary to bleeding esophageal tumor with tranexamic acid and proton pump inhibitors. The other hematological and biochemical blood investigations returned normal. Further review of his past medical records revealed that the patient had just completed his first cycle of concurrent chemoradiotherapy a month prior, with the initial tumor staging imaging studies showing no tumor invasion of the adjacent vascular structures.
The patient was transfused with 6 units of packed cells in the ward. There was a symptom-free latent interval of 6 h, until the patient developed another bout of hematemesis and suffered a cardiovascular collapse while awaiting esophagoduodenoscopy. Cardiopulmonary resuscitation was performed in accordance with Advanced Cardiac Life Support protocols. He eventually achieved a return of spontaneous circulation after 36 min but required intubation and inotropic support. The patient was transfused with another 6 units of packed cells and 12 units of fresh frozen plasma. Computed tomography angiography (CTA) thereafter demonstrated an AEF with aortic pseudoaneurysm, as well as massive contrast extravasation at the distal esophagus suggestive of an active hemorrhage (Figures 1, 2). Yet another 6 units of packed cells, 6 units of fresh frozen plasma, and 12 units of platelets were transfused. Nevertheless, he finally succumbed to recurrent hematemesis leading to fatal exsanguination before definitive surgical intervention could be performed (9 h post herald bleed).
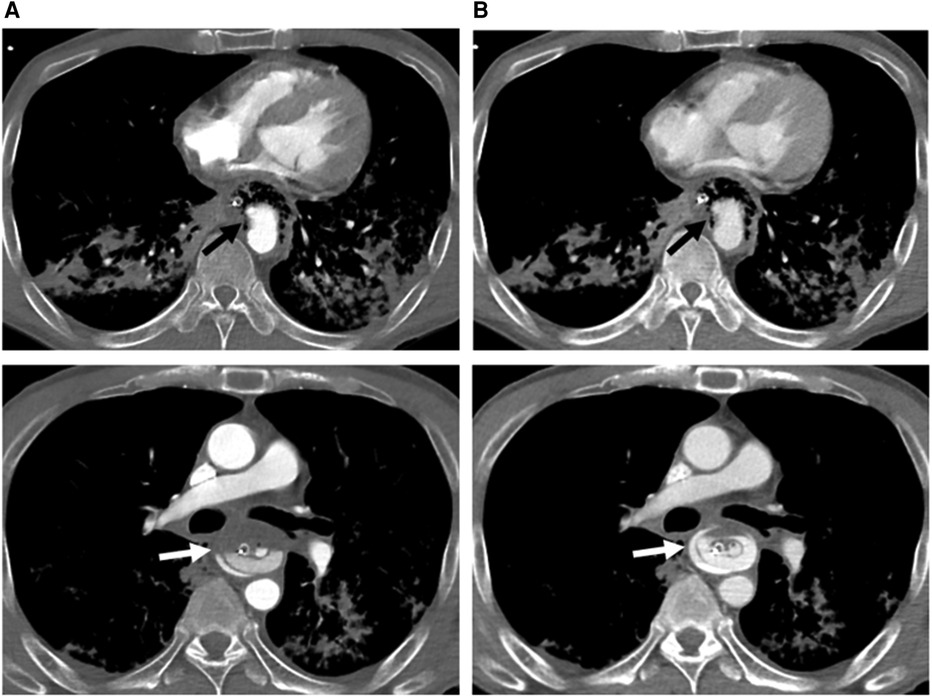
Figure 1. Axial computed tomography angiography of arterial (A) and venous (B) phases at two different levels below the carina, demonstrating a descending aortic pseudoaneurysm (black arrow) protruding into the esophagus (with a nasogastric tube in situ) through an aortoesophageal fistula (evidenced by direct communication of esophagus and aorta). There is a progressive increase in amount of contrast material (white arrow) in the esophagus, indicating rupture of the pseudoaneurysm and active bleeding.
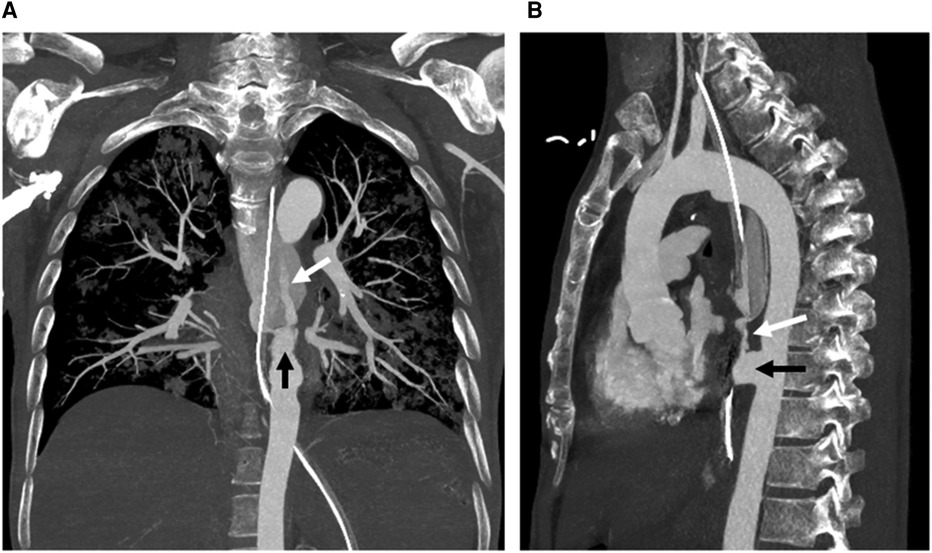
Figure 2. Coronal (A) and sagittal (B) maximum intensity projection images from computed tomography angiography show a descending aortic pseudoaneurysm (black arrow) at the level of the aortoesophageal fistula, complicated with rupture and contrast material extravasation (white arrow) into the esophagus (with a nasogastric tube in situ).
Discussion
AEF is an extremely rare cardiovascular etiology of hematemesis and UGIB. As such, its recognition and diagnosis are challenging and may be delayed when such patients present to the ED. Without timely surgical treatment, AEF is almost always fatal; even with surgical intervention, AEF patients face a high mortality rate of 77% (5). Awareness of AEF as a possible diagnosis and consequently early identification of these patients presenting to the ED are therefore crucial in optimizing their clinical outcomes.
On retrospective review of our patient's clinical course, his progression illustrates the classical triad of an AEF (Chiari's triad)—midthoracic pain or dysphagia, a sentinel episode of minor hematemesis, and a symptom-free interval followed by fatal exsanguination due to recurrent hematemesis. The symptom-free interval during which there is spontaneous cessation of hematemesis has been described in up to 80% of AEF patients (6). In our case, this latent interval lasted approximately 6 h, possibly attributable to the transient occlusion of the AEF via a combination of periaortic hematomas, intravascular hypotension, and arterial wall spasm (7).
CTA is the investigation modality of choice to confirm the diagnosis of AEF, as it can objectively demonstrate contrast extravasation. OGDS may be useful in excluding other common causes of UGIB, as well as revealing a bulging pulsatile lesion or submucosal hematoma via direct visualization that is suggestive of bleeding into the esophageal wall (8, 9). Nevertheless, esophagoduodenoscopy can be hazardous due to the risk of dislodging the occluding periaortic hematoma responsible for hemostasis and precipitating fatal hemorrhage (10–12).
Clinicians should keep in mind that the diagnosis of AEF is possible in patients presenting with hematemesis to the ED, especially if they have underlying risk factors of previous aortic surgery, aortic aneurysms, and thoracic cancer. Patients suspected to have AEF can be prioritized for CTA to clinch the definitive diagnosis, and subsequent arrangements for surgical interventions can be expedited. While an earlier CTA may or may not have improved the survival chances of our patient, establishing the diagnosis quickly would have been beneficial in allowing our ED team to counsel the patient's family regarding his prognosis accordingly—in recognition of this, our ED now prioritizes CTA over esophagoduodenoscopy in patients who present with frank hematemesis and concurrently have known risk factors for AEF (aortic aneurysm, foreign body ingestion, and advanced esophageal carcinoma).
The definitive treatment of AEFs is usually a combination of aortic (thoracic endovascular aortic repair, graft replacement, graft repair) and esophageal (esophagectomy, esophageal stent, esophageal repair) surgeries (13). In the acute setting of massive hematemesis, the Sengstaken-Blakemore tube (SBT) has been reported to be effective in securing hemostasis via the gastroesophageal balloon's tamponade effect, to buy time for definitive surgery (7, 14). Nevertheless, deploying the SBT is not without its complications, such as aspiration pneumonitis, airway obstruction, mucosal ulceration, esophageal perforation, and broncho-esophageal fistulas (15–17).
Surgical treatment options of AEF include open surgery and thoracic endovascular aortic repair (TEVAR); the latter is a minimally invasive technique which deploys an endoluminal aortic stent to rapidly control the bleeding with a favorable 30-day mortality rate of 27.5% (18, 19). TEVAR however does not address the esophageal lesion in AEFs, which may form a nidus for infections and subsequently lead to stent graft infection, mediastinitis, sepsis, re-hemorrhage, and stroke (20, 21). In contrast, open surgery allows for the debridement of infected mediastinum and esophageal repair in addition to aortic wall reconstruction; the trade-off is a high operative mortality rate of up to 55% (22). Combining TEVAR as bridging therapy with follow-up definitive open repair has been found to yield the lowest mortality rate at 25% (19), though esophageal cancer patients like ours may benefit more from palliative esophageal stents with survival of up to 8 months (23).
Conclusion
AEF is a rare and life-threatening cardiovascular cause of UGIB. It should be included in the list of differential diagnoses when evaluating patients presenting to the ED with hematemesis, especially if they have predisposing risk factors such as prior aortic or esophageal surgeries, aortic aneurysms, or thoracic malignancies. Patients suspected of having AEF should be prioritized for early CTA to expedite the diagnosis. Minimally invasive procedures such as SBT or TEVAR are pivotal to achieve initial hemostasis, which should be followed by definitive open surgery once the patient is stable.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of the Chang Gung Memorial Hospital (IRB no: 2212130011). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization: ACW, Y-MC, ZNL G, K-FC, C-JS. Data curation: Y-MC, K-FC, C-JS. Funding acquisition: C-JS. Methodology: ACW, Y-MC, ZNLG, K-FC, C-JS. Investigation: Y-MC, K-FC, C-JS. Resources: C-JS. Supervision: C-JS. Validation: ZNLG, C-JS. Visualization: ZNLG, C-JS. Writing- original draft: ACW, ZNLG, K-FC, C-JS. Writing—review & editing: C-JS, ZNLG. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Ministry of Science and Technology of Taiwan [MOST 109-2314-B-182A-102-] and Chang Gung Memorial Hospital in Taiwan [CMRPVVL0071 and CORPVVL0061]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. DeLaney M, Greene CJ. Emergency department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. (2015) 17:1–18.26291048
2. Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. Br Med J. (2019) 364:I536. doi: 10.1136/bmj.l536
3. Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. (1991) 91:279–87. doi: 10.1016/0002-9343(91)90129-L
4. Barrios Carvajal M, Díaz-Tobarra M, Martí-Obiol R, López Mozos F, Ortega Serrano J. Combined treatment of an aortoesophageal fistula after aortoplasty for aortic stenosis. Ann Thorac Surg. (2015) 100:1091–3. doi: 10.1016/j.athoracsur.2014.11.038
5. Yang Y, Hu D, Peng D. Primary aortoesophageal fistula: a fatal outcome. Am J Emerg Med. (2018) 36:343.e1–e3. doi: 10.1016/j.ajem.2017.11.008
6. Carter R, Mulder GA, Snyder EN Jr, Brewer LA 3rd. Aortoesophageal fistula. Am J Surg. (1978) 136:26–30. doi: 10.1016/0002-9610(78)90195-2
7. Seet E, Beevee S, Cheng A, Lim E. The sengstaken-blakemore tube: uses and abuses. Singapore Med J. (2008) 49:e195–7.18756331
8. Saers SJF, Scheltinga MRM. Primary aortoenteric fistula. Br J Surg. (2005) 92:142–52. doi: 10.1002/bjs.4928
9. Maher MM, Murphy J, Dervan P, O’Connell D. Aorto-oesophageal fistula presenting as a submucosal oesophageal haematoma. Br J Radiol. (1998) 71:972–4. doi: 10.1259/bjr.71.849.10195014
10. Ctercteko G, Mok CK. Aorta-esophageal fistula induced by a foreign body: the first recorded survival. J Thorac Cardiovasc Surg. (1980) 80:233–5. doi: 10.1016/S0022-5223(19)37796-7
11. Baron RL, Koehler RE, Gutierrez FR, Forrest JV, Weyman PJ. Clinical and radiographic manifestations of aortoesophageal fistulas. Radiology. (1981) 141:599–605. doi: 10.1148/radiology.141.3.7302210
12. Benson MJ, Rouse D, van Someren N, Wingate DL, Swain CP. Fatal hemorrhage from an aorto-esophageal fistula precipitated by flexible endoscopy. Gastrointest Endosc. (1991) 37:193–6. doi: 10.1016/S0016-5107(91)70686-X
13. Takeno S, Ishii H, Nanashima A, Nakamura K. Aortoesophageal fistula: review of trends in the last decade. Surg Today. (2020) 50:1551–9. doi: 10.1007/s00595-019-01937-z
14. Assink J, Vierhout BP, Snellen JP, Benner PM, Paul MA, Cuesta MA, et al. Emergency endovascular repair of an aortoesophageal fistula caused by a foreign body. J Endovasc Ther. (2005) 12:129–33. doi: 10.1583/04-1401R.1
15. Avgerinos A, Armonis A. Balloon tamponade technique and efficacy in variceal haemorrhage. Scand J Gastroenterol Suppl. (1994) 207:11–6. doi: 10.3109/00365529409104188
16. Chong CF. Esophageal rupture due to sengstaken-blakemore tube misplacement. World J Gastroenterol. (2005) 11:6563–5. doi: 10.3748/wjg.v11.i41.6563
17. Chien JY, Yu CJ. Images in clinical medicine. Malposition of a sengstaken-blakemore tube. N Engl J Med. (2005) 352:e7. doi: 10.1056/NEJMicm040003
18. Li S, Gao F, Hu HO, Shi J, Zhang J. Risk factors for mortality in patients with aortoesophageal fistula related to aortic lesions. Gastroenterol Res Pract. (2020) 2020:4850287. doi: 10.1155/2020/4850287
19. Canaud L, Ozdemir BA, Bee WW, Bahia S, Holt P, Thompson M. Thoracic endovascular aortic repair in management of aortoesophageal fistulas. J Vasc Surg. (2014) 59:248–54. doi: 10.1016/j.jvs.2013.07.117
20. Zhan Y, Xu Z. Massive hemorrhage from an aortoesophageal fistula caused by esophageal stent implantation: a case report and literature review. Medicine (Baltimore). (2019) 98:e18303. doi: 10.1097/MD.0000000000018303
21. Lee S, Srinivasa RN, Rigberg DA, Yanagawa J, Benharash P, Moriarty JM. Aortoesophageal fistula involving the central aortic arch salvaged with emergent percutaneous TEVAR, great vessel coverage and in vivo graft fenestration. Diagn Interv Radiol. (2021) 27:122–5. doi: 10.5152/dir.2020.20033
22. Kieffer E, Chiche L, Gomes D. Aortoesophageal fistula: value of in situ aortic allograft replacement. Ann Surg. (2003) 238:283–90. doi: 10.1097/01.sla.0000080828.37493.e0
Keywords: aortoesophageal fistula, haematemesis, upper gastrointestinal bleeding, Chiari's triad, emergency department, computed tomography angiography
Citation: Wong AC, Chou Y-M, Goh ZNL, Chang K-F and Seak C-J (2023) Case report: Aortoesophageal fistula—an extremely rare but life-threatening cardiovascular cause of hematemesis. Front. Cardiovasc. Med. 10:1123305. doi: 10.3389/fcvm.2023.1123305
Received: 13 December 2022; Accepted: 3 April 2023;
Published: 20 April 2023.
Edited by:
Pietro Enea Lazzerini, University of Siena, ItalyReviewed by:
Ulrich Ronellenfitsch, University Hospital Halle (Saale), GermanyCassius Iyad Ochoa Chaar, Yale University, United States
© 2023 Wong, Chou, Goh, Chang and Seak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-June Seak anVsaWFuc2Vha0Bob3RtYWlsLmNvbQ==
 Alexis Ching Wong
Alexis Ching Wong Yu-Mou Chou
Yu-Mou Chou Zhong Ning Leonard Goh
Zhong Ning Leonard Goh Kuang-Fu Chang3,4
Kuang-Fu Chang3,4 Chen-June Seak
Chen-June Seak