- 1Department of Nephrology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2National Clinical Research Center for Geriatric Disorders, Xuanwu Hospital, Capital Medical University, Beijing, China
Background: Cardiac valve calcification (CVC) is highly prevalent and a risk factor for adverse outcomes in patients with chronic kidney disease (CKD). This meta-analysis aimed to investigate the risk factors for CVC and association between CVC and mortality in CKD patients.
Method: Three electronic databases including PubMed, Embase, and Web of Science were searched for relevant studies up to November 2022. Hazard ratios (HR), odds ratios (OR), and 95% confidence intervals (CI) were pooled using random-effect meta-analyses.
Results: 22 studies were included in the meta-analysis. Pooled analyses showed that CKD patients with CVC were relatively older, had a higher body mass index, left atrial dimension, C-reaction protein level, and a declined ejection fraction. Calcium and phosphate metabolism dysfunction, diabetes, coronary heart disease, and duration of dialysis were all predictors for CVC in CKD patients. The presence of CVC (both aortic valve and mitral valve) increased the risk of all-cause and cardiovascular mortality in CKD patients. However, the prognostic value of CVC for mortality was not significant anymore in patients with peritoneal dialysis.
Conclusion: CKD patients with CVC had a greater risk of all-cause and cardiovascular mortality. Multiple associated factors for development of CVC in CKD patients should be taken into consideration by healthcare professionals to improve prognosis.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier [CRD42022364970].
1. Introduction
Chronic kidney disease (CKD), defined as the occurrence of renal structural alteration and dysfunction more than three months, affects approximately 15% of the adult population worldwide, which puts a huge burden on public health (1). Cardiovascular diseases (CVD) remain a most serious complication to CKD, causing an increasing morbidity and mortality for patients with CKD (2–4). Accumulating evidence suggested that vascular calcification is one of the major causes of CVD in CKD patients (5), and represents a strong predictor for mortality (6–8).
Patients with CKD exhibit a higher cardiovascular risk compared to non-CKD cohort (9). Non-traditional risk factors should be emphasized to identify the mechanism of accelerated atherosclerosis, apart from traditional risk factors, such as age, male, hypertension, hypercholesterolemia and obesity. The Kidney Disease Improving Global Outcome (KDIGO) Guideline suggested that cardiac valve calcification (CVC) should be emphasized in the risk stratification of CVD in CKD patients (10).
Uremic toxins might be a key regulator of calcification for end-stage renal disease patients (11). Numerous studies reported a highly prevalence of CVC and stenosis development in patients undergoing renal replace therapy (12). Aortic valve calcification and aortic stenosis may occur in the early stages of kidney failure in a GFR-dependent manner (13, 14). CVC was recognized as a contributor to all-cause and cardiovascular mortality in hemodialysis patients (15, 16), however, the prognostic value was not reliable (17, 18). Multiple risk factors for CVC have been explored, such as traditional factors (age, hypertension, and diabetes) and non-traditional factors (hyperphosphatemia, calcium phosphate product, fibroblast growth factor 23, inflammation and malnutrition) (19–21). It is crucial to identify independent risk factors for CVC in CKD patients. However, the pathophysiology of CVC is not completely understood.
To our knowledge, analysis of the impact of CVC on mortality of CKD patients has not yet been performed. The aim of the meta-analysis was to investigate the clinical prognosis of CVC, in addition to the associated factors, in patients with CKD.
2. Method
2.1. Search strategies
We conducted a systematic review, prospectively registered on PROSPERO (ID: CRD42022364970). This systematic review was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (22). We searched the PubMed, Embase, and Web of Science database up to November 2022. The search strategies were shown in Supplementary Material. The references of selected articles were searched manually for additional eligible studies.
2.2. Study selection
Two researchers screened all abstracts to verify potentially relevant articles for this review. Any disagreement was settled by another independent reviewer. Studies met the following criteria were eligible: (1) involving participants with CKD regardless of dialysis; reporting the association of CVC and mortality, including associated risk factors of CVC; (2) reporting of the hazard ratio (HR) or odds ratio (OR) with 95% confidence interval (CI); (3) cohort studies (prospective or retrospective). CVC was defined as bright echoes on one or more cusps of more than 1 mm in either mitral or aortic valves or both by echocardiographs. All echocardiographic data were acquired according to the guidelines of the American Society of Echocardiography (23). Studies without adjustment for specific potential confounders and non-English studies were excluded.
2.3. Data extraction and statistical analyses
Data were collected by two authors from the eligible studies, including first author's last name, publication year, country, study design, demographic characteristics of patients and outcomes. All statistical analyses were conducted in Review Manager 5.3 software. Binomial factors are presented as OR with 95% CI, while continuous variables are presented as standardized mean difference (SMD) with 95% CI. HR adjusted for confounding variables and 95% CI were extracted from included studies in terms of all-cause mortality, CV mortality and CV events. Statistical heterogeneity among studies was evaluated using the I2 index (24). A random-effect model was applied to due to a significant heterogeneity, otherwise a fixed-effect model was applied. The Newcastle–Ottawa Scale (NOS) for cohort studies was applied to assess the quality of cohort study (25). Subgroup analysis was performed according to the type of cardiac valve and dialysis modality. Publication bias was explored by Egger test. P < 0.05 was considered to be statistically significant.
3. Result
3.1. Baseline characteristics of the included studies
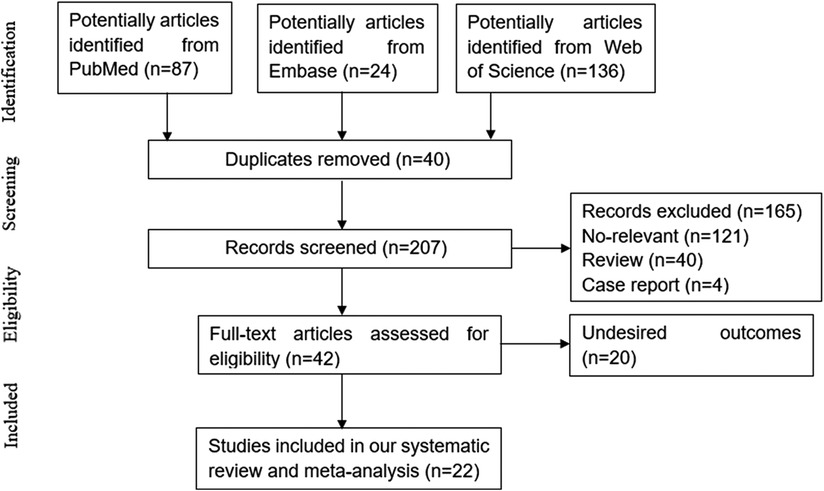
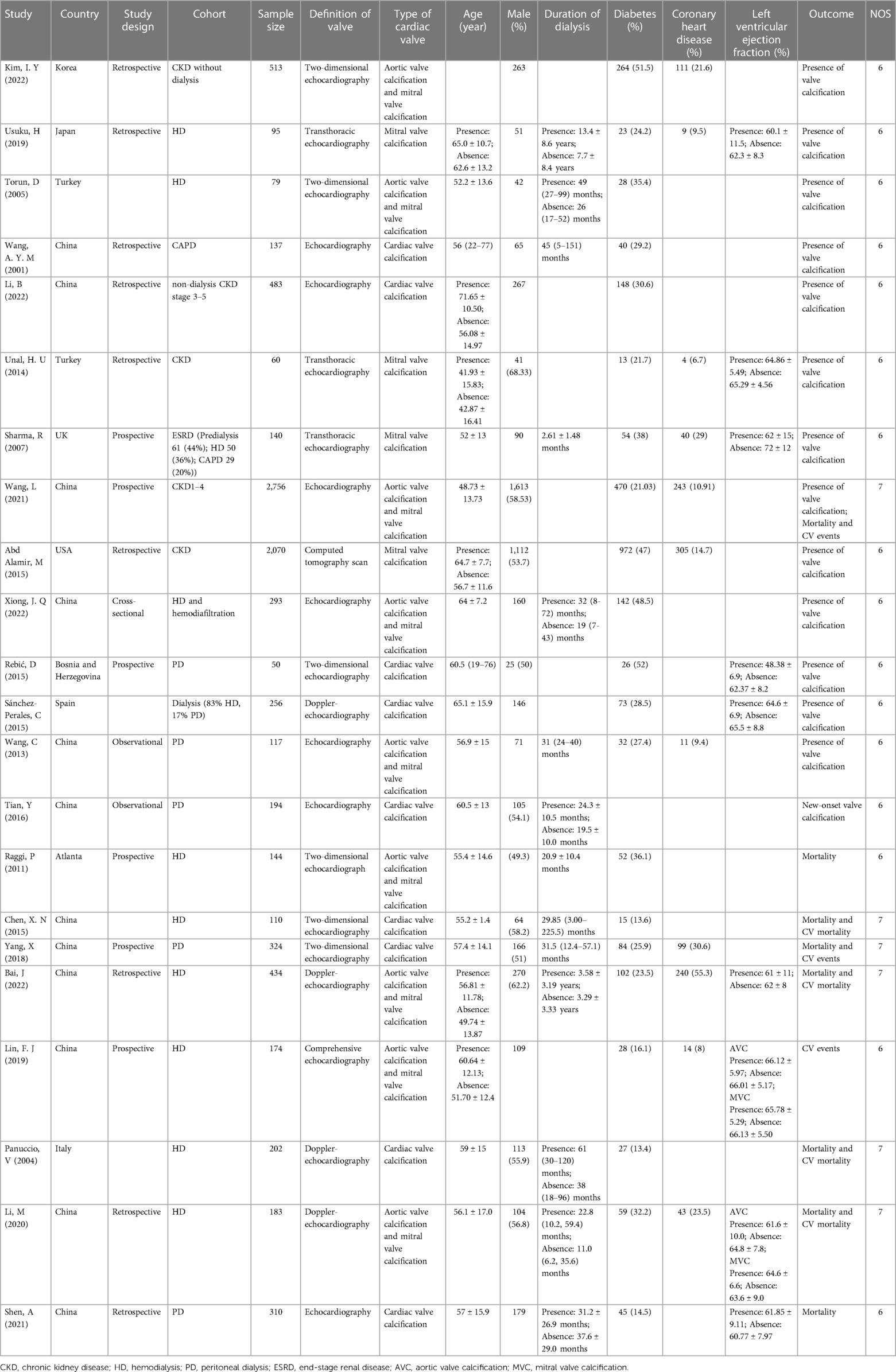
Following the inclusion criteria, 22 (12, 15–18, 26–42) articles were eventually included in our study. The study selection process is depicted in Figure 1. Among those including articles, 13 studies only reported associated factors for development of CVC, while the other 9 (15–18, 38–42) studies also explored the association of CVC and mortality meanwhile. Patients treated with hemodialysis were enrolled in 9 studies, while patients treated with peritoneal dialysis were enrolled in 6 studies. 3 studies only included patients with non-dialysis CKD, while 2 involved both hemodialysis and peritoneal dialysis patients. The basic characteristics of the included studies at baseline are listed in Table 1.
3.2. Comparing basic demographic and echocardiography characteristic between CKD patients with and without CVC
Advanced age (SMD: 9.35, 95% CI: 6.45, 12.25), higher BMI (SMD: 0.85, 95% CI: 0.17, 1.53), and higher systolic blood pressure (SMD: 9.31, 95% CI: 4.58, 14.05) were significantly associated with a development of CVC in CKD patients. Studies also evaluated several echocardiographic markers, including left atrial dimension, left ventricular ejection fraction (LVEF), early diastolic transmitral flow velocity to atrial contraction transmitral flow velocity ratio (E/a ratio), and early diastolic transmitral flow velocity to diastolic early mitral annular velocity ratio (E/e′ ratio). CKD cohorts with CVC were more likely to be accompanied with a higher E/e′ ratio (SMD: 2.97, 95% CI: 0.31, 5.62) by meta-analysis of three studies, and higher left atrial dimension (SMD: 2.15, 95% CI: 1.01, 3.29) by meta-analysis of five studies. Four studies reported a lower E/a ratio (SMD: −0.17, 95% CI: −0.25, −0.09) while nine studies reported a lower LVEF (SMD: −2.19, 95% CI: −4.06, −0.32) in patients with CVC (shown in Supplementary Figure S1).
3.3. Basic characteristics and comorbidities predictors for CVC in patients with CKD
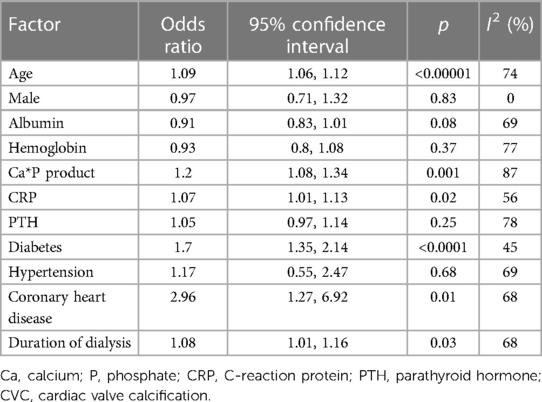
We compared demographic characteristics between CKD patients with and without CVC. Older age (OR: 1.09, 95% CI: 1.06–1.12), and longer duration of dialysis (OR: 1.08, 95% CI: 1.01–1.16) were significantly associated with the presence of CVC. CKD patients with diabetes as shown in Figure 2, coronary heart disease (OR:2.96, 95% CI: 1.27-6.92) (OR: 1.7, 95% CI: 1.35–2.14), coronary heart disease (OR: 1.09, 95% CI: 1.06–1.12) had a higher risk for development of CVC. The effect of male sex and hypertension on CVC in CKD patients was not significant (asshown in Figure 2 and Table 2).
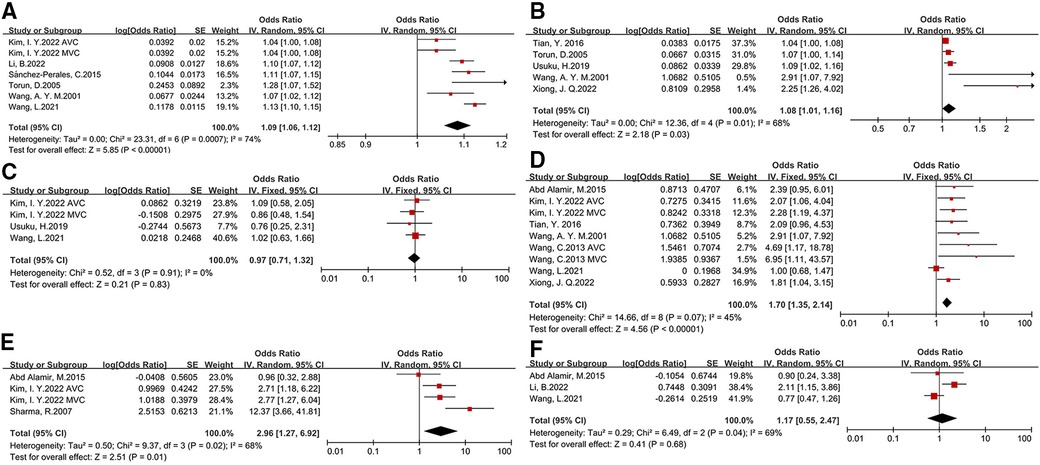
Figure 2. Basic characteristics and comorbidity risk factors for development of CVC in CKD patients [(A) age, (B) duration of dialysis, (C) male sex, (D) diabetes, (E)coronary heart disease, (F) hypertension].
3.4. Laboratory tests predictors for CVC in patients with CKD
We investigated several laboratory examinations of CKD patients in this meta-analysis (as shown in Figure 3 and Table 2). The results suggested that an increased calcium*phosphate product (OR: 1.2, 95% CI: 1.08–1.34) is associated with a higher risk of CVC. The association of parathyroid hormone and CVC was slight. There is a tight link among malnutrition, inflammation and atherosclerosis (MIA) in CKD patients. We only found C-reaction protein (CRP) (OR: 1.07, 95% CI: 1.01–1.13), but not albumin and hemoglobin, is associated with a higher risk of CVC. Besides, the level of total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and triglyceride between patients with CVC and without CVC was relatively comparable (shown in Supplementary Figure S2).
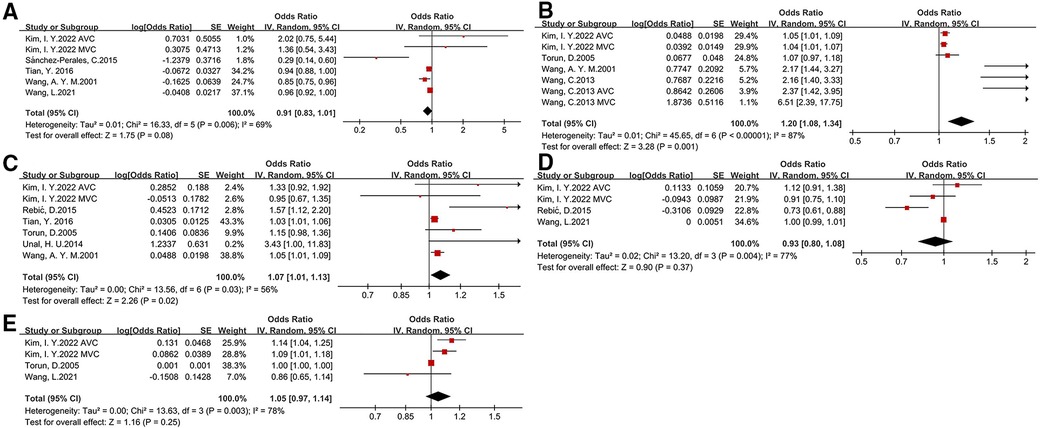
Figure 3. Laboratory risk factors for development of CVC in CKD patients [(A) albumin, (B) calcium*phosphate, (C) C-reaction protein, (D) hemoglobin, (E)parathyroid hormone].
3.5. Association between CVC and all-cause mortality and cardiovascular mortality
The relationship between CVC and prognosis in CKD patients were assessed covering all-cause deaths (8 studies), cardiovascular deaths (4 studies), and cardiovascular events (5 studies). Upon meta-analysis of including studies, we found that the presence of CVC was associated with greater risk of all-cause mortality (HR: 1.46, 95% CI: 1.26–1.69) and cardiovascular mortality (HR: 2.31, 95% CI: 1.86–2.88) (as shown in Figure 4). All studies included in the analysis utilized a fixed effects model because of low heterogeneity (I2 = 0%, I2 = 12%, respectively). Nonsignificant publication bias was examined with Egger test (p = 0.535). However, the effect of CVC and cardiovascular events was slight in CKD patients (HR: 2.1, 95% CI: 0.96–4.59). Due to the small number of studies addressing other clinical outcomes, subgroup analysis was only performed for the association of CVC and all-cause mortality. By subgroup analysis according to the different type of valve calcification, we found significant association between valve calcification and all-cause mortality in both aortic valve (HR: 1.49, 95% CI: 1.15–1.94) and mitral valve (HR: 1.59, 95% CI: 1.24–2.03). However, when stratified by the patients type, the predictive value of CVC for mortality was significant only in hemodialysis patients (HR: 1.51, 95% CI: 1.29–1.78), but not peritoneal dialysis patients (HR: 1.38, 95% CI: 0.93–2.03) (as shown in Supplementary Material).
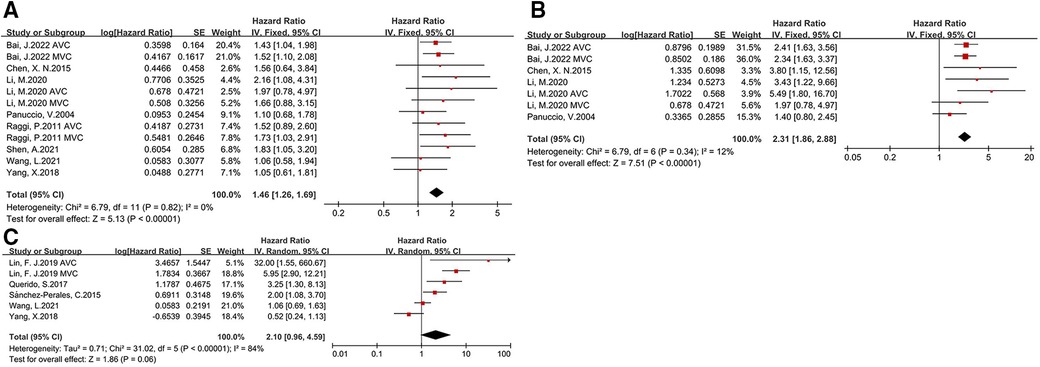
Figure 4. Forest plot of clinical outcomes for CKD patients with CVC [(A) all-cause mortality, (B) cardiovascular mortality, (C) cardiovascular events].
4. Discussion
Our findings demonstrated that CVC increased the risk of all-cause mortality and cardiovascular mortality in patients with CKD, regardless of aortic valve or mitral valve. Various factors were firstly reported to be probably related to the development of CVC, including age, comorbidities (coronary heart disease and diabetes), CRP and calcium-phosphate metabolism.
Vascular calcification is defined as a form of calcium-phosphate complexes deposition in the vasculature, mainly including intimal calcification, Mönckeberg medial arterial calcification, and valvular calcification (43). The echocardiographic evaluation of CVC is recommended as a routine examination by the KDIGO guidelines (44). CVC is increasingly common in dialysis patients (45). However, the predictive value of calcified valves for mortality remains controversial (46).
By meta-analysis, we found CKD patients with CVC were at a higher hazard for all-cause mortality and cardiovascular mortality, which is similar to another meta-analysis (47). However, in our study, we included more updated articles and found the effect of CVC on cardiovascular events was limited, probably due to a different definition of cardiovascular events in individual study. CVC is more frequently observed as the decline of residual renal function, including both aortic valve calcification and mitral valve calcification (48). In a multicenter study of stage 5 CKD patients, mitral valve calcification remained associated with all-cause mortality after adjusting for confounding, while aortic valve calcification was not (15). In our subgroup analysis, we found both aortic valve and mitral valve calcification could predict all-cause mortality in CKD patients, effectively. The mitral valve calcification may lead to mitral regurgitation and stenosis, whereas aortic valve calcification is more likely to cause aortic stenosis. Nevertheless, the predictive value of CVC was obvious only in hemodialysis patients, but not peritoneal dialysis patients, which is not consistent with the results of Wang, Z (47). In another publication involving peritoneal dialysis patients, the significance of CVC associated with all-cause mortality was lost when adjusting for covariates (39). Valve calcification was more frequently found in hemodialysis compared to peritoneal dialysis patients (49). The residual renal function and the electrolyte imbalance in peritoneal dialysis patients might be different from that in hemodialysis patients. Peritoneal dialysis patients may have a greater time-averaged exposure to phosphate than hemodialysis (50). The calcium balance between hemodialysis and peritoneal dialysis needs to be further explored. On echocardiography, CVC patients had a higher left ventricle mass index, pulmonary artery pressure, and a lower ejection fraction (51). CVC has also been associated with markers of atherosclerosis and severity of coronary artery disease (52), including carotid intima-media thickness, and plaque in the carotid arteries (53, 54). CVC on routine echocardiography can be used for risk assessment in CKD patients to reduce cardiovascular events. Verifying the risk factors for CVC is important to improve prognosis.
The pathogenetic features including mechanical stress, metabolic disorder and inflammatory disturbances were described. However, the pathophysiology and clinical impact of CVC was still unknown. In this meta-analysis, we found CKD patients with CVC were older, with a lower ejection fraction and relatively worse diastolic function. Several diseases could lead to a progressive left atrial enlargement, involving heart failure and hypertension. CVC might alter volume overload to induce left atrial dysfunction. A large left atrial diameter was an independent risk factor for mortality in hemodialysis patients or renal allograft recipients (55, 56). Mitral valve replacement in patients with mitral valve stenosis could improve left atrial contractile function by inhibiting apoptotic process (57). E/e ratio was associated with mortality and cardiovascular hospitalization in patients with heart failure with preserved ejection fraction (58). A review also confirmed that an increased E/e ratio was associated with an increased risk for chronic kidney disease progression (59). In patients with aortic valve stenosis, LVEF were significantly lower than that of patients with normal aortic valve (60). Left ventricular diastolic dysfunction was another predictor for all-cause mortality and cardiovascular outcomes in pre-dialysis CKD patients, while a stringent linear correlation was also clarified between E/e ratio and mortality (61). Our study corroborates the notion that several echocardiography parameters could be a marker of CVC in CKD patients.
Diabetes and hypertension are common causes of CKD. Diabetic nephropathy probably had an independent effect on E/e ratio, left ventricular diastolic dysfunction and cardiac valve regurgitation (62). In addition, diabetes patients are with a higher coronary artery calcification (CAC) score, and incidence rate of major adverse cardiovascular events (63). A higher CAC score was significantly associated with fibroblast growth factor 23, parathyroid hormone, and inflammation index (64). High glucose conditions can exacerbate oxidative stress and calcification through the induction of CD36 scavenger receptors (65) and endothelial dysfunction (66). The alteration involved several pathways activated by hyperglycemia advanced glycation-end products.
Chronic kidney disease-mineral and bone disease, a common complication of CKD, is shown as biochemical abnormalities of calcium, phosphate, vitamin D, parathyroid hormone (PTH), bone disorders, and calcification. In our study, we found higher level of Ca*P product was a significant risk factor for CVC in CKD patients, although the predictive value of PTH was weak. Aortic valve calcification might be mediated through a process of osteoblast-like differentiation (67). Evidence showed that hyperphosphatemia, hypercalcemia could promote osteogenic/chondrogenic differentiation, apoptosis of VSMC (6). An elevated Ca concentration probably stimulate VSMC mineralization by elevating Ca*P product (68). Considering the number of studies exploring the association between serum Ca, serum P and CVC was small, we could not perform a meta-analysis in this study. Meaningfully, we prompted that imbalanced Ca and P metabolism probably contributes to CVC.
CKD patients are under a chronic low-grade inflammatory and malnutrition state, resulting in a higher risk of morbidity and mortality (69). The term MIA syndrome has been proposed. In our study, CRP was a predictor for CVC in patients with CKD. Chronic inflammation with activation of CRP and proinflammatory cytokines is related to an aggravated oxidative stress and endothelial dysfunction. Various inflammatory biomarkers were significantly associated with arterial calcification in CKD patients (70), including β2-microglobulin, interleukin 2, interleukin 8 and interleukin 18. The increase in the concentration of CRP is proportional to the decrease of renal function (71) and progression of abdominal aortic calcification in hemodialysis patients (72). The activation of dendritic cell function by CRP might be involved during atherogenesis (73). However, the predictive value of albumin, hemoglobin, cholesterol, and triglyceride for CVC was poor in our meta-analysis. More index considering nutrition and inflammation should be addressed in the future study.
As far as we know, this is the first meta-analysis to explore risk factors for development of CVC in CKD patients. Our meta-analysis included relatively recent studies, and were larger and more extensive in number and risk factors. In addition, we confirmed that both aortic valve and mitral valve calcification could predict poor prognosis in CKD patients. Our findings suggested that a comprehensive evaluation of various risk factors and routine echocardiography should not be overlooked. Our study had some limitations. The adjust covariates were different in individual study, which might affect the stability of our study. Besides, the sample size of several including studies were relatively small, probably leading to a bias. Due to the limitation of the data from original articles, we could not explore the definite associated factors for calcified aortic valve and mitral valve, separately. Finally, the number of studies reporting the association of CVC and other outcomes was small, in particular coronary heart disease, stroke and cerebrovascular disease. More larger sample size clinical studies are preferred to further confirm the reliability of our results.
5. Conclusion
Our meta-analysis indicated that both aortic valve and mitral valve calcification could predict all-cause mortality and cardiovascular mortality in patients with CKD. Various risk factors should be considered to prevent development and progression of CVC, including advanced age, diabetes, coronary heart disease, CRP and calcium-phosphate metabolism disorder. Further research is required to determine the mechanisms for CVC increasing the risk of mortality in CKD patients through large multicenter studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AZ: contributed to the study concept and design; JZ, QP, AZ: contributed to data collection; JZ, QP, SW, LW: contributed to the statistical analysis; JZ: contributed to the original draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Ministry of Education & National Natural Science Foundation of Beijing (grant no. KZ 202110025038), National Natural Science Foundation of China (grant no. 81873619), the Xuanwu Hospital Huizhi talent leader training program to AZ, and National Natural Science Foundation of China (grant no. 82000710).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1120634/full#supplementary-material.
References
1. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, et al. US renal data system 2018 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. (2019) 73(3 Suppl 1):A7–8. doi: 10.1053/j.ajkd.2019.01.001
2. Okuno S, Ishimura E, Kitatani K, Fujino Y, Kohno K, Maeno Y, et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2007) 49(3):417–25. doi: 10.1053/j.ajkd.2006.12.017
3. Parikh NI, Hwang SJ, Larson MG, Levy D, Fox CS. Chronic kidney disease as a predictor of cardiovascular disease (from the framingham heart study). Am J Cardiol. (2008) 102(1):47–53. doi: 10.1016/j.amjcard.2008.02.095
4. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. (2021) 143(11):1157–72. doi: 10.1161/CIRCULATIONAHA.120.050686
5. Brandenburg VM, Schuh A, Kramann R. Valvular calcification in chronic kidney disease. Adv Chronic Kidney Dis. (2019) 26(6):464–71. doi: 10.1053/j.ackd.2019.10.004
6. Shanahan CM, Crouthamel MH, Kapustin A, Giachelli CM. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. (2011) 109(6):697–711. doi: 10.1161/CIRCRESAHA.110.234914
7. Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. (2009) 5(1):185–97. doi: 10.2147/VHRM.S4822
8. Shantouf RS, Budoff MJ, Ahmadi N, Ghaffari A, Flores F, Gopal A, et al. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. (2010) 31(5):419–25. doi: 10.1159/000294405
9. Noels H, Boor P, Goettsch C, Hohl M, Jahnen-Dechent W, Jankowski V, et al. The new SFB/TRR219 research centre. Eur Heart J. (2018) 39(12):975–7. doi: 10.1093/eurheartj/ehy083
10. Marwick TH, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and valvular heart disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2019) 96(4):836–49. doi: 10.1016/j.kint.2019.06.025
11. Zickler D, Willy K, Girndt M, Fiedler R, Martus P, Storr M, et al. High cut-off dialysis in chronic haemodialysis patients reduces serum procalcific activity. Nephrol Dial Transplant. (2016) 31(10):1706–12. doi: 10.1093/ndt/gfw293
12. Xiong JQ, Chen XM, Liang CT, Guo W, Wu BL, Du XG. Prognosis and risk factors for cardiac valve calcification in Chinese end-stage kidney disease patients on combination therapy with hemodialysis and hemodiafiltration. Ren Fail. (2022) 44(1):224–32. doi: 10.1080/0886022X.2022.2032742
13. Guerraty MA, Chai B, Hsu JY, Ojo AO, Gao Y, Yang W, et al. Relation of aortic valve calcium to chronic kidney disease (from the chronic renal insufficiency cohort study). Am J Cardiol. (2015) 115(9):1281–6. doi: 10.1016/j.amjcard.2015.02.011
14. Vavilis G, Bäck M, Occhino G, Trevisan M, Bellocco R, Evans M, et al. Kidney dysfunction and the risk of developing aortic stenosis. J Am Coll Cardiol. (2019) 73(3):305–14. doi: 10.1016/j.jacc.2018.10.068
15. Raggi P, Bellasi A, Gamboa C, Ferramosca E, Ratti C, Block GA, et al. All-cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol. (2011) 6(8):1990–5. doi: 10.2215/CJN.01140211
16. Bai J, Zhang X, Zhang A, Zhang Y, Ren K, Ren Z, et al. Cardiac valve calcification is associated with mortality in hemodialysis patients: a retrospective cohort study. BMC Nephrol. (2022) 23(1):43. doi: 10.1186/s12882-022-02670-5
17. Wang L, Cheng H, Zou X, Yuan J, Wu W, Han S, et al. Prevalence and correlates of cardiovascular calcification and its prognostic effects among patients with chronic kidney disease: results from the C-STRIDE study. Front Public Health. (2021) 9:762370. doi: 10.3389/fpubh.2021.762370
18. Panuccio V, Tripepi R, Tripepi G, Mallamaci F, Benedetto FA, Cataliotti A, et al. Heart valve calcifications, survival, and cardiovascular risk in hemodialysis patients. Am J Kidney Dis. (2004) 43(3):479–84. doi: 10.1053/j.ajkd.2003.11.009
19. Plytzanopoulou P, Papasotiriou M, Politis P, Parissis C, Paraskevopoulou P, Kehagias I, et al. Malnutrition as a risk factor for cardiac valve calcification in patients under maintenance dialysis: a cross-sectional study. Int Urol Nephrol. (2020) 52(11):2205–12. doi: 10.1007/s11255-020-02590-z
20. Petrović D, Obrenović R, Stojimirović B. Risk factors for aortic valve calcification in patients on regular hemodialysis. Int J Artif Organs. (2009) 32(3):173–9. doi: 10.1177/039139880903200308
21. Chen Y, Chen YX, Huang C, Duan ZB, Xu CY. The clinical value of klotho and FGF23 in cardiac valve calcification among patients with chronic kidney disease. Int J Gen Med. (2021) 14:857–66. doi: 10.2147/IJGM.S299197
22. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4(1):1. doi: 10.1186/2046-4053-4-1
23. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
25. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
26. Kim IY, Ye BM, Kim MJ, Kim SR, Lee DW, Kim HJ, et al. 1,25-dihydroxyvitamin D deficiency is independently associated with cardiac valve calcification in patients with chronic kidney disease. Sci Rep. (2022) 12(1):915. doi: 10.1038/s41598-022-04981-x
27. Usuku H, Yamamoto E, Arima Y, Takashio S, Araki S, Sueta D, et al. Accumulation of coronary risk factors is associated with progression of mitral annular calcification in patients undergoing dialysis therapy: a long-term follow-up study. Int J Cardiol. (2019) 293:248–53. doi: 10.1016/j.ijcard.2019.05.001
28. Torun D, Sezer S, Baltali M, Adam FU, Erdem A, Ozdemir FN, et al. Association of cardiac valve calcification and inflammation in patients on hemodialysis. Ren Fail. (2005) 27(2):221–6. doi: 10.1081/JDI-51837
29. Wang AYM, Woo J, Wang M, Sea MMM, Ip R, Li PKT, et al. Association of inflammation and malnutrition with cardiac valve calcification in continuous ambulatory peritoneal dialysis patients. J Am Soc Nephrol. (2001) 12(9):1927–36. doi: 10.1681/ASN.V1291927
30. Li B, Hu X, Pan H, Xiao Y, Dong J, Bao Y, et al. Cardiac valve calcification prevalence and association with neutrophil-to-lymphocyte ratio in newly diagnosed patients with non-dialysis chronic kidney disease stage 3–5. Bratisl Lek Listy. (2022) 123(7):523–7. doi: 10.4149/BLL_2022_084
31. Unal HU, Çelik M, Gökoğlan Y, Çetinkaya H, Gök M, Karaman M, et al. Mitral annular calcification and the serum osteocalcin level in patients with chronic kidney disease. Ren Fail. (2014) 36(10):1481–5. doi: 10.3109/0886022X.2014.962421
32. Sharma R, Pellerin D, Gaze DC, Mehta RL, Gregson H, Streather CP, et al. Mitral annular calcification predicts mortality and coronary artery disease in end stage renal disease. Atherosclerosis. (2007) 191(2):348–54. doi: 10.1016/j.atherosclerosis.2006.03.033
33. Abd Alamir M, Radulescu V, Goyfman M, Mohler ER III, Gao YL, Budoff MJ. Prevalence and correlates of mitral annular calcification in adults with chronic kidney disease: results from CRIC study. Atherosclerosis. (2015) 242(1):117–22. doi: 10.1016/j.atherosclerosis.2015.07.013
34. Rebić D, Rašić S, Hamzić-Mehmedbašić A, Džemidžić J, Kurtalić E. Valvular calcification and left ventricular modifying in peritoneal dialysis patients. Ren Fail. (2015) 37(8):1316–22. doi: 10.3109/0886022X.2015.1073495
35. Sánchez-Perales C, Vázquez Ruiz de Castroviejo E, García-Cortés MJ, Biechy Mdel M, Gil-Cunquero JM, Borrego-Hinojosa J, et al. Valvular calcifications at the start of dialysis predict the onset of cardiovascular events in the course of follow-up. Nefrologia. (2015) 35(2):157–63. doi: 10.1016/j.nefro.2015.05.017
36. Wang C, Jiang L, Feng S, Shi Y, Shen H, Shi X, et al. Risk factor analysis of calcification in aortic and mitral valves in maintenance peritoneal dialysis patients. Kidney Blood Press Res. (2013) 37(4–5):488–95. doi: 10.1159/000355729
37. Tian Y, Feng S, Zhan Z, Lu Y, Wang Y, Jiang S, et al. Risk factors for new-onset cardiac valve calcification in patients on maintenance peritoneal dialysis. Cardiorenal Med. (2016) 6(2):150–8. doi: 10.1159/000443620
38. Chen XN, Chen ZJ, Ma XB, Ding B, Ling HW, Shi ZW, et al. Aortic artery and cardiac valve calcification are associated with mortality in Chinese hemodialysis patients: a 3.5 years follow-up. Chin Med J (Engl). (2015) 128(20):2764–71. doi: 10.4103/0366-6999.167315
39. Yang X, Zhang H, Shi Y, Yu Z, Yan H, Ni Z, et al. Association of serum angiopoietin-2 with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients: a prospective cohort study. J Transl Med. (2018) 16(1):312. doi: 10.1186/s12967-018-1687-0
40. Lin FJ, Zhang X, Huang LS, Zhou X, Ji G, Luo R, et al. De novo cardiac valve calcification after hemodialysis in end-stage renal disease patients predicts future cardiovascular events: a longitudinal cohort study. Cardiorenal Med. (2019) 9(4):229–39. doi: 10.1159/000494701
41. Li M, Ye ZC, Li CM, Zhao WB, Tang H, Liu X, et al. The influence of cardiac valvular calcification on all-cause and cardiovascular mortality in maintenance hemodialysis patients. Int Urol Nephrol. (2020) 52(5):943–51. doi: 10.1007/s11255-020-02448-4
42. Shen A, Jiang L, Tian Y, Lu Y, Wang Z, Shen H, et al. Valvular calcific deposits and mortality in peritoneal dialysis patients: a propensity score-matched cohort analysis. Cardiorenal Med. (2021) 11(4):200–7. doi: 10.1159/000516285
43. Phadwal K, Feng D, Zhu D, MacRae VE. Autophagy as a novel therapeutic target in vascular calcification. Pharmacol Ther. (2020) 206:107430. doi: 10.1016/j.pharmthera.2019.107430
44. KDIGO guideline. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. (2009) (113):S1–130. doi: 10.1038/ki.2009.188
45. Straumann E, Meyer B, Misteli M, Blumberg A, Jenzer HR. Aortic and mitral valve disease in patients with end stage renal failure on long-term haemodialysis. Br Heart J. (1992) 67(3):236–9. doi: 10.1136/hrt.67.3.236
46. Mohamed BA, Yang W, Litt H, Rosas SE. Valvular calcification, inflammation, and mortality in dialysis patients. J Heart Valve Dis. (2013) 22(4):584–90.24224425
47. Wang Z, Jiang A, Wei F, Chen H. Cardiac valve calcification and risk of cardiovascular or all-cause mortality in dialysis patients: a meta-analysis. BMC Cardiovasc Disord. (2018) 18(1):12. doi: 10.1186/s12872-018-0747-y
48. Ureña-Torres P, D'Marco L, Raggi P, García-Moll X, Brandenburg V, Mazzaferro S, et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dial Transplant. (2020) 35(12):2046–53. doi: 10.1093/ndt/gfz133
49. Rroji M, Seferi S, Cafka M, Petrela E, Likaj E, Barbullushi M, et al. Is residual renal function and better phosphate control in peritoneal dialysis an answer for the lower prevalence of valve calcification compared to hemodialysis patients? Int Urol Nephrol. (2014) 46(1):175–82. doi: 10.1007/s11255-013-0438-7
50. Evenepoel P, Meijers BK, Bammens B, Viaene L, Claes K, Sprangers B, et al. Phosphorus metabolism in peritoneal dialysis- and haemodialysis-treated patients. Nephrol Dial Transplant. (2016) 31(9):1508–14. doi: 10.1093/ndt/gfv414
51. Sayarlioglu H, Acar G, Sahin M, Altunoren O, Coskun Yavuz Y, Nacar AB, et al. Prevalence and risk factors of valvular calcification in hemodialysis patients. Iran J Kidney Dis. (2013) 7(2):129–34.23485537
52. Kim IY, Kim MJ, Lee DW, Lee SB, Shin MJ, Rhee H, et al. Cardiac valve calcification is associated with presence and severity of coronary artery disease in patients with pre-dialysis chronic kidney disease. Clin Exp Nephrol. (2015) 19(6):1090–7. doi: 10.1007/s10157-015-1104-4
53. Wang AY, Ho SS, Wang M, Liu EK, Ho S, Li PK, et al. Cardiac valvular calcification as a marker of atherosclerosis and arterial calcification in end-stage renal disease. Arch Intern Med. (2005) 165(3):327–32. doi: 10.1001/archinte.165.3.327
54. Arita Y, Nakayama M, Matsukuma Y, Yoshitomi R, Seki M, Fukui A, et al. Association of aortic valve calcification with carotid artery lesions and peripheral artery disease in patients with chronic kidney disease: a cross-sectional study. BMC Nephrol. (2020) 21(1):203. doi: 10.1186/s12882-020-01864-z
55. Stosovic MD, Petrovic MZ, Vujisic-Tesic BD, Stanojevic M, Simic-Ogrizovic SP, Jovanovic DB, et al. Predictive value of echocardiography and its relation to Kt/V and anthropometric parameters in hemodialysis patients. Ren Fail. (2015) 37(4):589–96. doi: 10.3109/0886022X.2015.1007821
56. Kainz A, Goliasch G, Wiesbauer F, Binder T, Maurer G, Nesser HJ, et al. Left atrial diameter and survival among renal allograft recipients. Clin J Am Soc Nephrol. (2013) 8(12):2100–5. doi: 10.2215/CJN.04300413
57. Trikas A, Papathanasiou S, Tousoulis D, Tentolouris K, Vasiliadou K, Antoniades C, et al. Left atrial function, cytokines and soluble apoptotic markers in mitral stenosis: effects of valvular replacement. Int J Cardiol. (2005) 99(1):111–5. doi: 10.1016/j.ijcard.2004.01.018
58. Nauta JF, Hummel YM, van der Meer P, Lam CSP, Voors AA, van Melle JP. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: a systematic review in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. (2018) 20(9):1303–11. doi: 10.1002/ejhf.1220
59. Kang E, Lee SW, Ryu H, Kang M, Kim S, Park SK, et al. Left ventricular diastolic dysfunction and progression of chronic kidney disease: analysis of KNOW-CKD data. J Am Heart Assoc. (2022) 11(13):e025554. doi: 10.1161/JAHA.122.025554
60. Rodríguez-Carbó J, Torres-Arellano JM, Ávila-Vanzzini N, Springall R, Bojalil R, Infante O, et al. Association of the heart rate variability response to active standing with the severity of calcific aortic valve disease: novel insights of a neurocardiovascular pathology. J Clin Med. (2022) 11(16):4771. doi: 10.3390/jcm11164771
61. Suh SH, Oh TR, Choi HS, Kim CS, Bae EH, Oh KH, et al. Association of left ventricular diastolic dysfunction with cardiovascular outcomes in patients with pre-dialysis chronic kidney disease: findings from KNOW-CKD study. Front Cardiovasc Med. (2022) 9:844312. doi: 10.3389/fcvm.2022.844312
62. Tang C, Ouyang H, Huang J, Zhu J, Gu X. Differences between diabetic and non-diabetic nephropathy patients in cardiac structure and function at the beginning of hemodialysis and their impact on the prediction of mortality. J Int Med Res. (2021) 49(3):300060521997588. doi: 10.1177/0300060521997588
63. Zhu L, Liu J, Gao C, Zhao W, Que J, Wang X, et al. Comparison of coronary plaque, coronary artery calcification and major adverse cardiac events in Chinese outpatients with and without type 2 diabetes. Springerplus. (2016) 5(1):1678. doi: 10.1186/s40064-016-3373-0
64. Bundy JD, Chen J, Yang W, Budoff M, Go AS, Grunwald JE, et al. Risk factors for progression of coronary artery calcification in patients with chronic kidney disease: the CRIC study. Atherosclerosis. (2018) 271:53–60. doi: 10.1016/j.atherosclerosis.2018.02.009
65. Navas-Madroñal M, Castelblanco E, Camacho M, Consegal M, Ramirez-Morros A, Sarrias MR, et al. Role of the scavenger receptor CD36 in accelerated diabetic atherosclerosis. Int J Mol Sci. (2020) 21(19):7360. doi: 10.3390/ijms21197360
66. Dhananjayan R, Koundinya KS, Malati T, Kutala VK. Endothelial dysfunction in type 2 diabetes Mellitus. Indian J Clin Biochem. (2016) 31(4):372–9. doi: 10.1007/s12291-015-0516-y
67. Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. (2003) 107(17):2181–4. doi: 10.1161/01.CIR.0000070591.21548.69
68. Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. (2004) 66(6):2293–9. doi: 10.1111/j.1523-1755.2004.66015.x
69. Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD. Nutrients. (2017) 9(3):208. doi: 10.3390/nu9030208
70. Kiu Weber CI, Duchateau-Nguyen G, Solier C, Schell-Steven A, Hermosilla R, Nogoceke E, et al. Cardiovascular risk markers associated with arterial calcification in patients with chronic kidney disease stages 3 and 4. Clin Kidney J. (2014) 7(2):167–73. doi: 10.1093/ckj/sfu017
71. Gupta J, Mitra N, Kanetsky PA, Devaney J, Wing MR, Reilly M, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol. (2012) 7(12):1938–46. doi: 10.2215/CJN.03500412
72. Choi SR, Lee YK, Cho AJ, Park HC, Han CH, Choi MJ, et al. Malnutrition, inflammation, progression of vascular calcification and survival: inter-relationships in hemodialysis patients. PLoS One. (2019) 14(5):e0216415. doi: 10.1371/journal.pone.0216415
Keywords: cardiac valve calcification, cardiovascular mortality, chronic kidney disease, mortality, risk factor
Citation: Zhang J, Pang Q, Wang S, Wu L and Zhang A (2023) Associated factors of cardiac valve calcification and its prognostic effects among patients with chronic kidney disease: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1120634. doi: 10.3389/fcvm.2023.1120634
Received: 10 December 2022; Accepted: 10 April 2023;
Published: 26 April 2023.
Edited by:
Pompilio Faggiano, Fondazione Poliambulanza Istituto Ospedaliero, ItalyReviewed by:
Antonio Bellasi, Ente Ospedaliero Cantonale (EOC), SwitzerlandMarios Papasotiriou, University of Patras, Greece
© 2023 Zhang, Pang, Wang, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aihua Zhang cm9zZXpoYW5nOTk4QGhvdG1haWwuY29t
 Jialing Zhang
Jialing Zhang Qi Pang1
Qi Pang1 Aihua Zhang
Aihua Zhang