
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 02 May 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1115870
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2022 View all 39 articles
81-year-old female presented with subacute right lower extremity edema due to iliac vein compression by a markedly enlarged external iliac lymph node later identified as newly relapsed metastatic endometrial carcinoma. The patient underwent a full evaluation of the iliac vein lesion and cancer and had an intravenous stent placed with complete resolution of symptoms post-procedure.
An 81-year-old Caucasian female with a history of stage IB high-grade undifferentiated endometrial carcinoma diagnosed 2 years prior and paroxysmal atrial fibrillation (AF) on anticoagulation presented with a two-week history of right leg swelling (Table 1). The edema extended to her knee and was not relieved by conservative measures, including compression stockings. Of note, the patient's endometrial carcinoma was initially treated with hysterectomy, bilateral salpingo-oophorectomy, vaginal brachytherapy, and 6 cycles of chemotherapy with Carboplatin and Paclitaxel. The patient had since been in remission. Her family history was significant for breast cancer and dementia in her mother, atrial fibrillation (AF) and leukemia in her father, and unspecified cancer in her brother. The patient had multiple risk factors for AF—family history of AF, sedentary lifestyle, obesity, hypertension, left atrial enlargement, and previous exposure to carboplatin. She had no significant smoking, alcohol, or illicit drug history. She retired from accounting 20 years ago.
• To broaden the differential diagnosis of unilateral peripheral edema in a patient with a history of cancer.
• To understand the comprehensive evaluation and treatment of NIVL.
The differential diagnosis of unilateral leg edema includes deep vein thrombosis, cellulitis, Baker's cyst, lower extremity trauma, lymphedema, venous insufficiency, and May-Thurner syndrome.
The first step in the patient's diagnostic work-up was a venous Duplex ultrasound test of the right lower extremity, which revealed marked right external iliac vein narrowing with abnormal Doppler waveforms, suggestive of a hemodynamically significant obstructive lesion secondary to external compression. A transthoracic echocardiogram revealed normal left ventricular size and systolic function, mild left atrial dilation, grade 1 diastolic dysfunction, moderate mitral regurgitation, and an elevated estimated right atrial pressure based on the dilated inferior vena cava. To measure the intracardiac pressures, rule out pulmonary hypertension, and better characterize the iliac vein stenosis, the patient underwent right heart catheterization (RHC) with iliac venography. The iliac venogram revealed severe stenosis of the right external iliac vein (Figure 1A), while RHC showed normal intracardiac pressures (normal RAP, mean PAP, and PCWP).
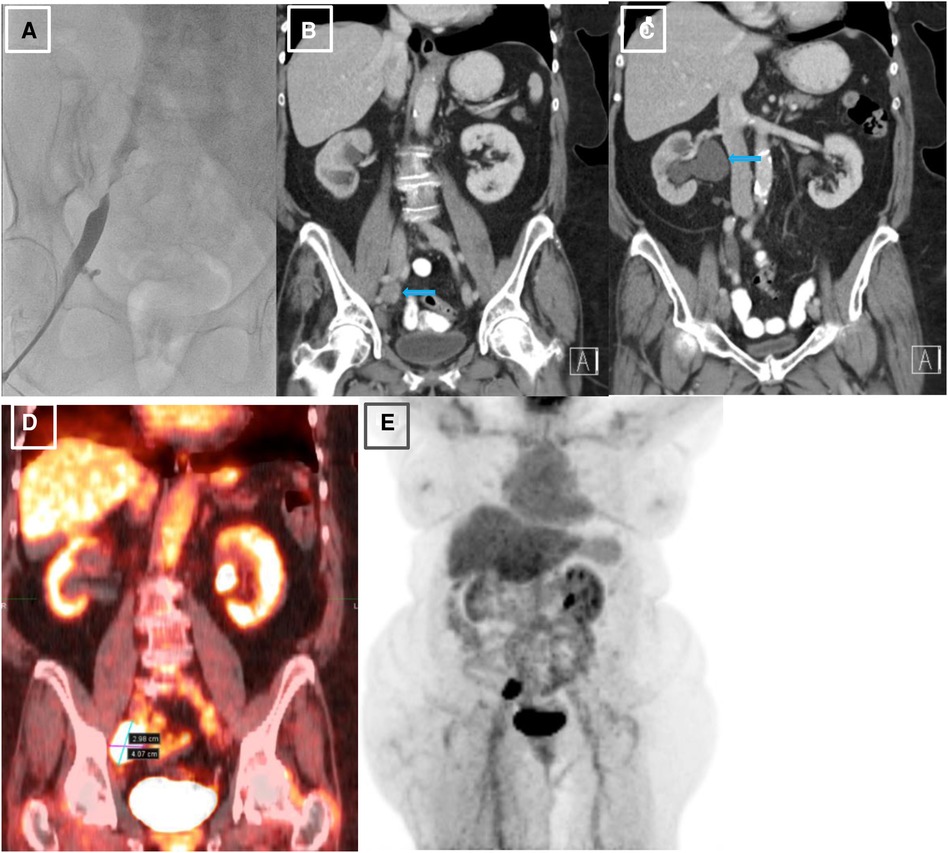
Figure 1. Initial venography, CT and PET imaging of the right external iliac vein. (A). Iliac venography demonstrating severe stenosis of right external iliac vein. (B). CT abdomen pelvis revealing right external iliac lymphadenopathy (LAD), measuring 2.5 × 3.2 × 2.7 cm, marked by blue arrow. (C). CT abdomen pelvis demonstrating severe right sided hydronephrosis due toR ureteral external compression by mass burden, marked by blue arrow. (D, E). PET scan redemonstrating R external iliac LAD.
Given her history of endometrial carcinoma, the possibility of extrinsic compression of the iliac vein by tumor was considered. Thus, a computed tomography (CT) scan of the abdomen/pelvis with and without intravenous (IV) contrast was obtained. The results indicated a markedly enlarged and necrotic right external iliac lymph node (2.5 × 3.2 × 2.7 cm) which was causing external compression of, and likely invading, the right iliac vein (Figure 1B). Significant compression of the right ureter associated with severe right-sided hydronephrosis (Figure 1C) and right pelvic sidewall lymphadenopathy were seen as well. Next, a full-body PET scan was ordered for staging purposes, demonstrating metabolic activity in the right external iliac lymph node and an area of increased activity in the left adrenal gland (Figures 1D, E). After a multidisciplinary discussion, the patient was offered an endovascular intervention of the iliac vein with stenting to improve her refractory right lower extremity edema.
Since there was a high probability of cancer invading the vessel wall and the right ureter, a decision was made for palliative endovascular stent placement to provide symptomatic relief and to avoid the risks of an open surgical approach, including intra-operative and post-operative bleeding. The patient was taken to the cardiac catheterization laboratory, where an intravascular ultrasound (IVUS) was performed to visualize the lesion and to measure the lesion length and the reference diameter for choosing the correct size of the stent. After the lesion was crossed with a 0.035 angled glide guide wire, the patient underwent right external iliac vein balloon angioplasty followed by the successful placement of a VICI 14 × 90 mm stent, VENITI, INC., Fremont, CA. Post-dilation was done with a 10 × 60 mm balloon at 8 atmospheres and a 14 × 60 mm balloon at 4 atmospheres. The follow-up IVUS showed a re-canalized iliac vein with normal venous flow and 100 mm2 of luminal gain at the previously occluded point. The re-canalization was additionally re-demonstrated on an abdominal/pelvic CT scan (Figures 2A, B).
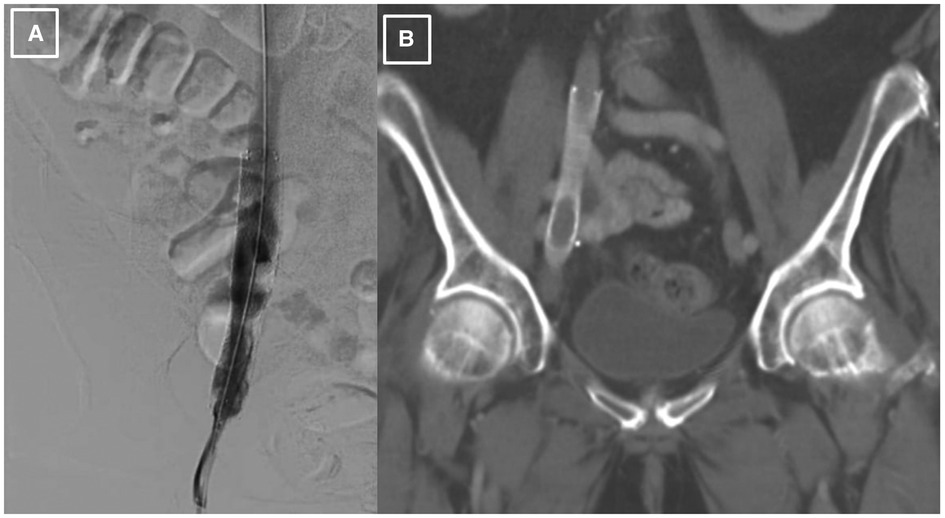
Figure 2. Post-procedural imaging of the stented right external iliac vein. (A). Iliac venography status post IVUS-guided visualization and right external iliac vein balloon-angioplasty with successful placement of VICI 14 × 90 mm stent and resultant patency. (B). CT abdomen pelvis demonstrating presence of stent within the R external iliac vein prior to abdominal debulking procedure.
The patient's right lower extremity edema completely resolved after the intervention. The patient was started on aspirin 81 mg daily for three months to prevent stent thrombosis and allow its endothelization. A few weeks later, the patient underwent laparoscopy with retroperitoneal lymph node biopsy and tumor debulking. The surgeon released the ureteral compression but was unable to remove the obstructing lymph node, as it was invading the iliac vein wall with a high risk of bleeding. The patient was followed by interventional cardiology every six months with Doppler imaging, with vein patency noted during two years of follow-up.
The development of lower extremity edema, as other types of edema, occurs by three main mechanisms, all of which are caused by fluid shifts from the intravascular to the interstitial space: an increase in intravascular hydrostatic pressure, a decrease in hydrostatic oncotic pressure or increased capillary permeability. The diagnostic work-up for leg swelling depends on the time of onset and its laterality. As seen in this patient, the development of an acute or subacute unilateral lower extremity edema can occur over the span of hours or weeks, and the differential diagnosis includes deep vein thrombosis (DVT), musculoskeletal injury, ruptured popliteal cyst, or venous compression (1). Patients with cancer have a high risk of developing venous thromboembolism (VTE) for several reasons: cancer is associated with high a pro-inflammatory and hypercoagulable state, tumor mass can cause venous external compression, and certain anticancer drugs can increase the risk of VTE. Anticancer drugs that have been reported to increase VTE risk include Thalidomide, Lenalidomide, and Bevacizumab (2). These medications are linked to an increased risk of VTE including DVT and arterial events (2). Thalidomide, particularly when used with dexamethasone, has been associated with up to 28% DVT rates, with additional risk factors including combined use with Doxorubicin, newly diagnosed disease, and Chromosome 11 abnormalities (2). Lenalidomide may have VTE rates as high as 75% (2). Bevacizumab, an anti-angiogenic, has been linked to an increased risk of arterial and venous events (2). Platinum-based compounds, such as cisplatin and carboplatin, can also cause arterial and venous thromboembolism (3). In patients with unprovoked DVT, age-appropriate cancer screening should follow the treatment of DVT. In the case of bilateral lower extremity edema in a patient with cancer, the differential diagnosis should also include thrombosis within, or extrinsic compression of, the inferior vena cava.
Iliac vein compression syndrome, a subtype of NIVL, also known as May-Thurner syndrome (MTS) is a condition mostly seen in young women who present with left leg swelling due to the compression of the left common iliac vein by the right common iliac artery at the level of the lumbar spine. The prevalence of MTS is estimated to be between 15%–30% based on autopsy data. The vast majority of these patients remain asymptomatic with only 2%–5% of patients developing a DVT. Long-standing MTS often results in chronic venous disease (4). In contrast to MTS, NIVL can occur in any segment of the iliac vein caused by other etiologies such as extrinsic compression by tumor, mass, spine spurs, or calcified atherosclerotic iliac arteries (5).
The first-line imaging modality used in diagnosing NIVL or evaluating unilateral leg swelling in patients with cancer is Doppler ultrasound of the iliac and other lower extremity veins. The test is noninvasive, easily accessible, and readily available. However, its diagnostic value may be limited in lesions with lower severity or in patients with obesity due to technical limitations. For typical MTS, CT pelvis with IV contrast is not sensitive enough to diagnose the compressed iliac vein due to limited visibility, but it is a good modality for ruling out extrinsic compression as in NIVL. CT venography with contrast is useful to further differentiate thrombotic from non-thrombotic intravenous lesions (6). Invasive venography is a useful diagnostic tool for identifying occlusion or severe stenosis of iliac veins, as demonstrated in our case. However, its effectiveness may be limited in detecting luminal narrowing, resulting in potentially missed diagnoses of NIVL due to its low sensitivity in this regard. Specifically, the diagnostic quality may be compromised in cases of non-occlusive or partially narrowed iliac vein lesions. Therefore, in instances where clinical indications strongly suggest NIVL, such as in our case, the use of intravascular ultrasound (IVUS) is warranted for a more accurate diagnosis as IVUS can provide a 360o view within the vessel itself, which provides greater anatomic detail of the affected region (7). In fact, it has been demonstrated to approximate the morphology of the lesion, affording greater predictive value for stent sizing compared with the CT venogram alone (8, 9). IVUS was found to have a diagnostic sensitivity of > 90% for lesion detection when compared with CT venography, which has a 68% sensitivity (6).
The spectrum of treatments available for NIVL largely depends on the severity and persistence of the patient's symptoms. Conservative management includes compression stockings with leg elevation and calf muscle exercises. In refractory cases, endovascular repair with stent placement is pursued (10). Many studies have demonstrated an overall improvement in the quality of life and reduction in symptom severity with the use of venous stenting, such that it is nearly considered to be the gold standard for patients with high symptom burden (5, 9, 11).
A specific subgroup of NIVL called cancer-associated vein obstruction (CAVO) has recently garnered attention (12). A few studies have shown that percutaneous intervention is effective in reducing symptoms of swelling and discomfort with a low risk of complications (13). One group studied the efficacy of treating CAVO with an endovascular venous stent combined with linear Iodine-125 radioactive seeds strand. The new technique yielded longer patency of the stent and lower symptom burden without affecting survival (14).
In patients with cancer, DVT is the most likely cause of acute or subacute unilateral leg edema and should be ruled out first before considering other less common etiologies, such as NIVL. The latter condition may not be widely recognized by general providers. The recommended diagnostic workup consists of a duplex ultrasound of the deep iliac venous system, which often demonstrates abnormal venous Doppler waveforms, and a CT venogram of the pelvis to potentially visualize the iliac vein compression. It is important to remember that a negative CT venogram does not rule out NIVL due to its low diagnostic sensitivity. When there is a high index of suspicion for NIVL, invasive iliac venography with IVUS evaluation is instrumental in leading to the correct diagnosis and offering the option of therapeutic endovascular venous stenting. After multidisciplinary discussion and shared decision-making with the patient, endovascular intervention vs. surgical approach vs. medical management is chosen depending on the clinical situation.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
JZ, RK—writting sections of the article equally JL—revision and editing mainly of the interventional part of the article and the discussion AK—supervision of the composition of the whole manuscript, editing the article, consulting with other authors during this process. All authors contributed to the article and approved the submitted version.
The article publishing charge has been funded by the Letts O'Brien Fund for Breast Cancer Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
(NIVL), Non-thrombotic iliac vein lesion; (RHC), right heart catheterization; (CT), computed tomography; (PET), positron emission tomography; (IVUS), intravascular ultrasound; (DVT), deep vein thrombosis; (MTS), May-Thurner Syndrome; (LAD), lymphadenopathy.
1. Gasparis AP, Kim PS, Dean SM, Khilnani NM, Labropoulos N. Diagnostic approach to lower limb edema. Phlebology. (2020) 35(9):650–5. doi: 10.1177/0268355520938283
2. Qureshi W, Ali Z, Amjad W, Alirhayim Z, Farooq H, Qadir S, et al. Venous thromboembolism in cancer: an update of treatment and prevention in the era of newer anticoagulants. Front Cardiovasc Med. (2016) 3:24. doi: 10.3389/fcvm.2016.00024
3. Grover SP, Hisada YM, Kasthuri RS, Reeves BN, Mackman N. Cancer therapy-associated thrombosis. Arterioscler Thromb Vasc Biol. (2021) 41(4):1291–305. doi: 10.1161/ATVBAHA.120.314378
4. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. (2006) 44(1):136–44. doi: 10.1016/j.jvs.2006.02.065
5. Radaideh Q, Patel NM, Shammas NW. Iliac vein compression: epidemiology, diagnosis and treatment. Vasc Health Risk Manag. (2019) 15:115–22. doi: 10.2147/VHRM.S203349
6. Liu Z, Gao N, Shen L, Yang J, Zhu Y, Li Z, et al. Endovascular treatment for symptomatic iliac vein compression syndrome: a prospective consecutive series of 48 patients. Ann Vasc Surg. (2014) 28(3):695–704. doi: 10.1016/j.avsg.2013.05.019
7. McLafferty RB. The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol. (2012) 29(1):10–5. doi: 10.1055/s-0032-1302446
8. Montminy ML, Thomasson JD, Tanaka GJ, Lamanilao LM, Crim W, Raju S. A comparison between intravascular ultrasound and venography in identifying key parameters essential for iliac vein stenting. J Vasc Surg Venous Lymphat Disord. (2019) 7(6):801–7. doi: 10.1016/j.jvsv.2019.03.015
9. Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. (2002) 35(4):694–700. doi: 10.1067/mva.2002.121127
10. Razavi MK, Black S, Gagne P, Chiacchierini R, Nicolini P, Marston M, et al. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. (2019) 12(12):e008268. doi: 10.1161/CIRCINTERVENTIONS.119.008268
11. Chen Z, Zhang XC, Sun Y, Xu M. Diagnosis and treatment of nonthrombotic right iliac vein compression syndrome. Ann Vasc Surg. (2019) 61:363–70. doi: 10.1016/j.avsg.2019.05.033
12. Xiao L, Tong JJ, Shen J. Endoluminal treatment for venous vascular complications of malignant tumors. Exp Ther Med. (2012) 4(2):323–8. doi: 10.3892/etm.2012.589
13. O'Sullivan GJ, Waldron D, Mannion E, Keane M, Donnellan PP. Thrombolysis and iliofemoral vein stent placement in cancer patients with lower extremity swelling attributed to lymphedema. J Vasc Interv Radiol. (2015) 26(1):39–45. doi: 10.1016/j.jvir.2014.10.010
Keywords: peripheral edema, cancer, imaging, vascular disease, intravascular ultrasonography (IVUS)
Citation: Zeman J, Kompella R, Lee J and Kim AS (2023) Case report: Non-thrombotic iliac vein lesion: an unusual cause of unilateral leg swelling in a patient with endometrial carcinoma. Front. Cardiovasc. Med. 10:1115870. doi: 10.3389/fcvm.2023.1115870
Received: 4 December 2022; Accepted: 12 April 2023;
Published: 2 May 2023.
Edited by:
Reto Asmis, Wake Forest University, United StatesReviewed by:
Yung-Wei Chi, University of California, Davis, United States© 2023 Zeman, Kompella, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agnes S. Kim YWtpbUB1Y2hjLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.