
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 21 March 2023
Sec. Heart Valve Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1113908
This article is part of the Research Topic Comprehensive Insights into Mitral Valve Prolapse: From biology to future perspectives of treatment, passing through diagnostic tools, surgical techniques and transcatheter options View all 12 articles
 Antonia van Kampen1,2
Antonia van Kampen1,2 Guillaume Goudot3
Guillaume Goudot3 Sophie Butte1
Sophie Butte1 Dane C. Paneitz1
Dane C. Paneitz1 Michael A. Borger2
Michael A. Borger2 Vinay Badhwar4
Vinay Badhwar4 Thoralf M. Sundt1
Thoralf M. Sundt1 Nathaniel B. Langer1
Nathaniel B. Langer1 Serguei Melnitchouk1*
Serguei Melnitchouk1*
Background: Patients with mitral valve prolapse (MVP) requiring surgical repair (MVr) are increasingly operated using minimally invasive strategies. Skill acquisition may be facilitated by a dedicated MVr program. We present here our institutional experience in establishing minimally invasive MVr (starting in 2014), laying the foundation to introduce robotic MVr.
Methods: We reviewed all patients that had undergone MVr for MVP via sternotomy or mini-thoracotomy between January 2013 and December 2020 at our institution. In addition, all cases of robotic MVr between January 2021 and August 2022 were analyzed. Case complexity, repair techniques, and outcomes are presented for the conventional sternotomy, right mini-thoracotomy and robotic approaches. A subgroup analysis comparing only isolated MVr cases via sternotomy vs. right mini-thoracotomy was conducted using propensity score matching.
Results: Between 2013 and 2020, 799 patients were operated for native MVP at our institution, of which 761 (95.2%) received planned MVr (263 [34.6%] via mini-thoracotomy) and 38 (4.8%) received planned MV replacement. With increasing proportions of minimally invasive procedures (2014: 14.8%, 2020: 46.5%), we observed a continuous growth in overall institutional volume of MVP (n = 69 in 2013; n = 127 in 2020) and markedly improved institutional rates of successful MVr, with 95.4% in 2013 vs. 99.2% in 2020. Over this period, a higher complexity of cases were treated minimally-invasively and increased use of neochord implantation ± limited leaflet resection was observed. Patients operated minimally invasively had longer aortic cross-clamp times (94 vs. 88 min, p = 0.001) but shorter ventilation times (4.4 vs. 4.8 h, p = 0.002) and hospital stays (5 vs. 6 days, p < 0.001) than those operated via sternotomy, with no significant differences in other outcome variables. A total of 16 patients underwent robotically assisted MVr with successful repair in all cases.
Conclusion: A focused approach towards minimally invasive MVr has transformed the overall MVr strategy (incision; repair techniques) at our institution, leading to a growth in MVr volume and improved repair rates without significant complications. On this foundation, robotic MVr was first introduced at our institution in 2021 with excellent outcomes. This emphasizes the importance of building a competent team to perform these challenging operations, especially during the initial learning curve.
Cardiac surgery faces the challenge of integrating ongoing innovations, allowing constant progress, while requiring a significant effort to update and train surgeons in technically challenging and unforgiving procedures. Mitral valve (MV) surgery represents an archetype of this challenge, since for this procedure, conventional full sternotomy has been increasingly replaced by minimally invasive and robotic approaches that aim to limit postoperative complications and allow faster patient recovery while maintaining a high level of technical success (1, 2). The literature leaves little room for doubt: minimally invasive MV surgery has become a standard approach with excellent short- and long-term results (3–6). However, the integration of these procedures, still often limited to tertiary care centers, has been slowed down by learning curves, the need for a time-consuming training program, and the risk of a transient increase in postoperative complications (5). Recently, robotically assisted mitral valve surgery has been gaining increasing popularity, due to potentially even smaller incisions and certain advances in surgical exposure.
Massachusetts General Hospital (MGH) established a focused MV surgery innovation program in 2014, dedicated to mitral valve repair (MVr) via a minimally invasive approach and the introduction of the robotic approach as further innovation. Previously published studies demonstrated that learning curves for minimally invasive MVr can be overcome safely (4, 7) and adverse events can potentially be reduced by dedicated and standardized teaching procedures (8). We aim to analyze the results of our MVr program over eight years in terms of the number and type of mitral procedures, as well as peri- and postoperative outcomes, and present our experience with introducing the robotic MVr approach.
All patients that had undergone MV surgery at Massachusetts General Hospital between January 2013 and December 2020 were identified via interrogation of the Society of Thoracic Surgeons database. In addition, we included all patients that had received robotically assisted MVr at our institution in 2021 and 2022 to compare the initial outcomes of that innovative approach to previous outcomes. All perioperative notes, surgical reports, and imaging findings were reviewed. The planned operative strategy (repair vs. replacement, minimally invasive vs. conventional approach) was obtained from the perioperative notes, and intraoperative complications or changes in strategy were registered as reported by the individual surgeon. Standard preoperative transthoracic and intraoperative transesophageal echocardiography reports were utilized to evaluate MV pathology and procedural success. Successful repair was defined as MVr without requiring intraoperative conversion to MV replacement. The study was approved, and individual informed consent waived by the Institutional Review Board of Massachusetts General Hospital. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Patients referred for MVr undergo routine preoperative transthoracic echocardiography and either coronary angiography or ECG-gated CT angiography for coronary assessment. In addition, CT angiography of the entire aorta and iliac vessels is added if a patient is considered for a minimally invasive approach. Patients are then scheduled to have a full sternotomy approach if additional valve surgery or coronary artery bypass grafting (CABG) is indicated. The most common exclusion criteria for minimally invasive MVr include dilation of the ascending aorta > 45 mm, significant mitral annular calcification, presence of breast implants, significant pectus excavatum, left diaphragm paralysis, and previous right thoracotomy or sternotomy. Patients with low left ventricular ejection fraction (LVEF) or a predicted risk of mortality > 4% (moderate surgical risk category) by the Society of Thoracic Surgeons (STS PROM) risk score are also preferably operated on via a full sternotomy, to keep aortic cross-clamp times as short as possible. Furthermore, patients with significant calcifications or soft plaques in the descending aorta are not eligible for minimally invasive MVr at our institution, because of the need for retrograde aortic perfusion. Among our staff, 2 individuals perform minimally invasive MVr, while the other surgeons continue to use the sternotomy approach in all MVP patients referred to their practice.
To directly compare outcomes of conventional and minimally invasive MVr, patients undergoing isolated MVr were identified from the larger patient cohort excluding those that had major concomitant procedures prohibiting minimally invasive access at our institution i.e., CABG, surgery on aortic, tricuspid, or pulmonary valve, surgery on the ascending aorta or aortic arch, those with acute endocarditis, and those with severe mitral annular calcification requiring debridement and annular patch repair (Figure 1). Non-exclusionary concomitant procedures included closure of an atrial septal defect or a persistent foramen ovale (PFO), the Cox-Maze procedure, and occlusion of the left atrial appendage.
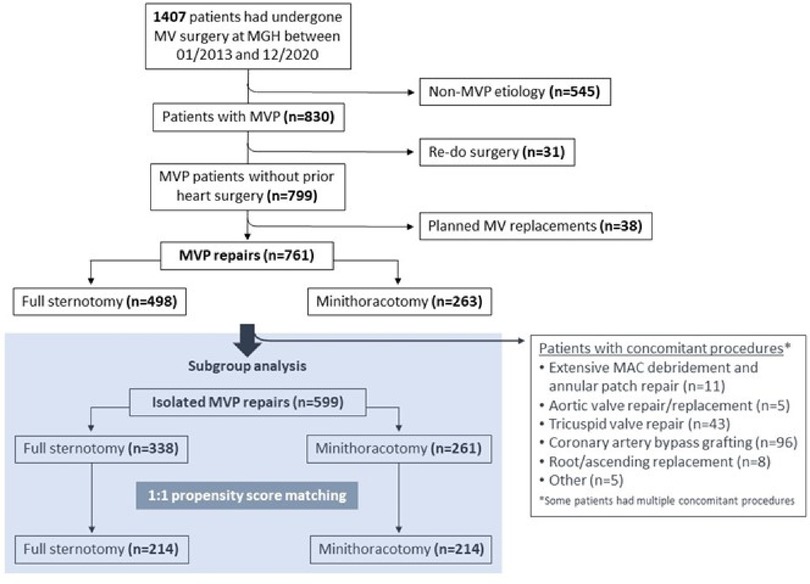
Figure 1. CONSORT diagram of patient selection for minimally invasive vs. sternotomy MVr. MAC = mitral annular calcificiation; MVP = mitral valve prolapse.
The extensive expertise gained throughout the years of successfully performing minimally invasive MVr was the essential foundation to launch a robotic MVr program in 2021, with the goal of obtaining similar excellent outcomes without compromising patient safety and facilitating certain steps of the procedure. Due to less restricted mobility and orientation of the surgical instruments during robotic surgery, exposure of and access to the papillary muscles for neochord implantation is superior to standard minimally-invasive approaches with long-shafted one-directional instruments. Despite initially expected longer procedural times, we believe that by introducing the robotic approach, we can further improve patient care and will eventually achieve equal or even shorter procedural times as with the established techniques.
At our institution, the most experienced minimally-invasive MV surgeon and team consisting of a co-surgeon, a scrub technician, a perfusionist, and an anesthesiologist, underwent comprehensive training via a society-supported formal training program including simulator and cadaver training. In-person proctoring was done for the first 5 cases, and remote proctoring for the sixth, seventh, and eighth cases. This stepwise approach (Figure 2) led to a successful launch of our robotic MVr program (9). For the initial cases included in this analysis, we added selection criteria to the ones mentioned above for minimally invasive surgery: Because we suspected initially prolonged procedural times, we included patients with very low perioperative risk (STS PROM <1%) and focused on pathologies deemed uncomplicated to repair based on preoperative echocardiography. Robotic MVr is currently available on a weekly basis at our institution and offered to all patients eligible for minimally invasive MVr, as described above. All patients that had undergone robotic MVr at our institution until August of 2022 were included in this analysis.
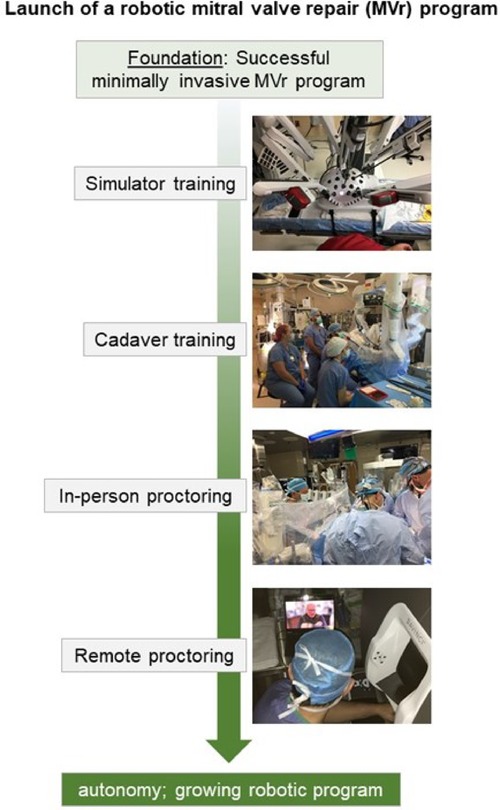
Figure 2. Stepwise approach to launching a robotic mitral valve repair program at Massachusetts general hospital.
After assessment for normal distribution, continuous variables were expressed as median with interquartile range [25th—75th percentile], and categorical variables as numbers with percentages. Unmatched group comparisons were conducted using the Wilcoxon rank sum test or Fisher's exact test, as appropriate. For the subgroup analysis, propensity scores were calculated using logistic regression. A 1:1 propensity score analysis was conducted using the “nearest neighbor” algorithm, with a caliper setting of 0.1 standard deviations and without replacement. Before matching, multiple imputations was used to compensate for missing data. Standardized mean differences were used to evaluate the balancing of covariates after matching. Variables used to calculate the propensity score were age, sex, Barlow's valve, arterial hypertension, diabetes, peripheral arterial disease, previous stroke, chronic obstructive lung disease, preoperative LVEF, body mass index, and STS PROM.
After matching, continuous variables were reported as median with interquartile range and compared using Wilcoxon signed rank test; categorical variables were reported as numbers with percentages and compared using McNemar's test. Long-term freedom from re-operation and death was examined using Kaplan-Meier method with log-rank test. Data analysis was conducted using R software (R-Studio, version 3.4.1, Boston, MA, United States).
A total of 1407 patients underwent MV surgery via full sternotomy or right anterolateral mini-thoracotomy at our institution between January 2013 and December 2020. Of them, 830 patients were operated for mitral valve prolapse (MVP), of which 38 received a planned MV replacement (common reasons were too complex valve anatomy, severe mitral annular calcifications, or patient preference) and 31 were re-operations. Surgical repair of native MVP was thus performed in 761 patients, and those were included for further analysis (Figure 1). Table 1 shows the preoperative characteristics of all 761 patients. In summary, the median age was 64 [55–72] years, and 245 patients (32.2%) were female. Mitral regurgitation was severe in 720 (94.9%), moderate in 37 patients (4.9%), and less than moderate in 4 patients (0.2%) (MVP was not the primary indication for surgery in those latter two groups). Significant mitral annular calcification was present in 38 patients (5%). Moderate or more aortic stenosis was found in 12 patients (1.6%), moderate or more aortic regurgitation in 19 patients (2.8%), and moderate or more tricuspid regurgitation in 112 patients (15%). A concomitant diagnosis of coronary artery disease existed in 102 patients (13.4%), and of atrial fibrillation in 177 patients (23.3%). Twelve patients (1.6%) had a dilated ascending aorta. Table 2 provides an overview of mitral valve repair details in the unmatched sternotomy and mini-thoracotomy groups.
As expected, patients of the sternotomy group underwent significantly more concomitant procedures, as depicted in Supplementary Table S1. Briefly, in the sternotomy group, 98 patients (19.7%) underwent concomitant CABG, 5 patients (1%) aortic valve repair or replacement, and 8 patients (1.6%) ascending aortic replacement with or without root replacement. Regarding concomitant procedures that are feasible via sternotomy as well as via a mini-thoracotomy approach, LAA occlusion was performed more frequently in the sternotomy group (228 patients (45.8%) vs. 10 patients (3.8%) in the mini-thoracotomy group, p < 0.001), as well as the bi-atrial Cox-Maze procedure (85 patients (17.1%) vs. 2 patients (0.8%), p < 0.001). The left Cox-Maze procedure was performed in similar proportions in both groups (37 patients (7.4%) in the sternotomy group, 15 patients (5.7%) in the mini-thoracotomy group, p = 0.5). A PFO was closed in 66 patients of the sternotomy group (13.3%) and 41 patients of the mini-thoracotomy group (15.6%; p = 0.4).
We observed a continuous growth in the overall institutional volume of surgery for MVP with increasing proportions of minimally invasive operations since 2014, when the mini-thoracotomy approach was first introduced at our institution (Figure 3). Overall native MVP case volume was n = 69 in 2013 and n = 127 in 2020. With the start of our dedicated minimally invasive MVr program, institutional repair success rates improved markedly over the years included in this analysis, with 95.4% in 2013 (before the introduction of minimally invasive MVr) vs. 99.2% in 2020 (Figure 4). Residual MR was mild or more in 9.2% of patients in 2013 vs. 2% in 2020, with a continuous reduction over the years (Figure 5).
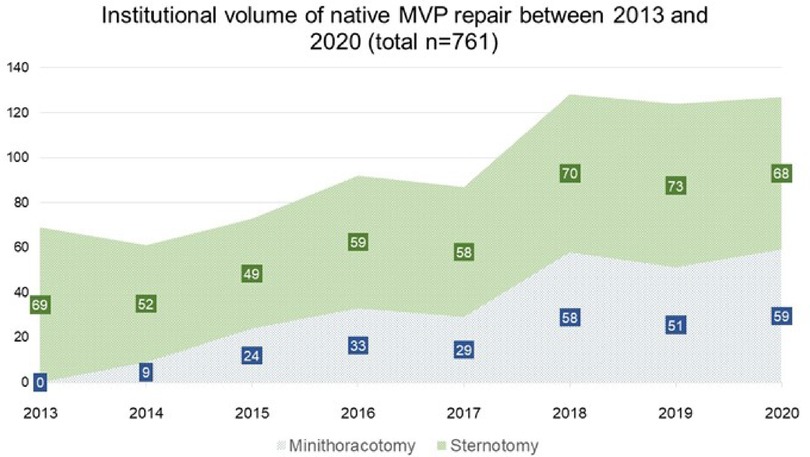
Figure 3. Development of institutional volumes after introduction of minimally invasive MVr program. MVP: Mitral Valve Prolapse.
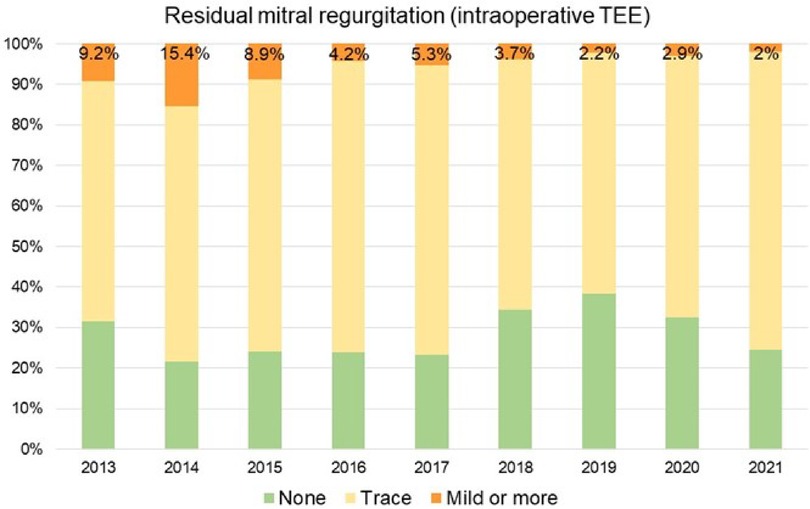
Figure 5. Proportion of patients with mild or more residual mitral regurgitation. TEE: Transesophageal Echocardiography.
Since the start of our minimally invasive MVr program, there has been a continuous growth in the proportion of surgically more complex patients [i.e., anterior mitral leaflet (AML) or bileaflet prolapse], with proportions similar to before 2014 (Figure 6). In addition, a steady increase in the utilization of chordal reconstruction with GoreTex neochords and a combination of limited leaflet resection and neochord insertion could be observed. At the same time, a purely resectional technique was utilized less commonly in recent years (Figure 7).
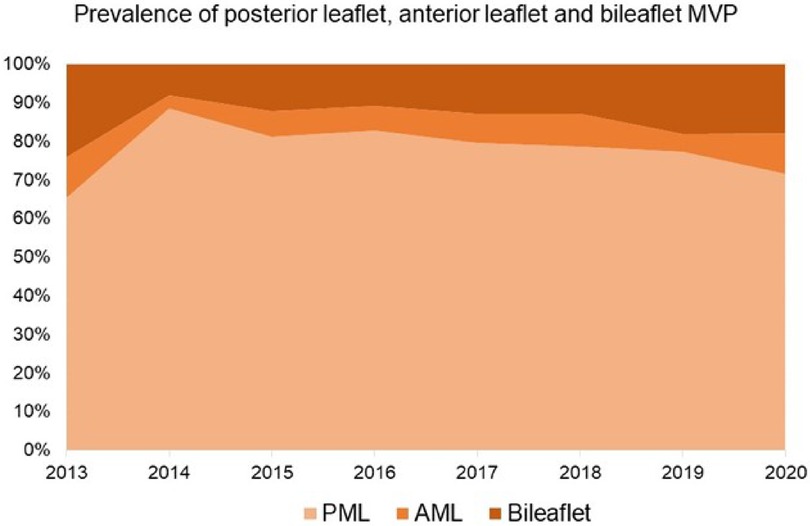
Figure 6. Proportions of patients with posterior leaflet, anterior leaflet, and bileaflet prolapse. AML: Anterior Mitral Leaflet; PML: Posterior Mitral Leaflet. MVP: Mitral Valve Prolapse.
The repair success rate with the minimally invasive approach was 100%, and only 3/263 patients (1.1%) required intraoperative conversion to full sternotomy. Reasons for conversion were bleeding from the aortic root in 2 and an intraoperative localized aortic dissection at the insertion point of the cardioplegia cannula in 1 patient. All 3 patients were discharged home with no significant increase in their hospital stay or further complications.
For the subgroup analysis of isolated MVr via sternotomy vs. mini-thoracotomy, we identified 599 patients that received MVr without major concomitant procedures (Figure 1). Of those, 346 were operated via sternotomy and 261 via right anterolateral mini-thoracotomy. Propensity matching resulted in 214 matched pairs with comparable baseline characteristics (Table 3 and Figure 8). Outcomes of the unmatched groups are summarized in Supplementary Table S2. Between the matched groups, there were significant differences in repair techniques: The neochords + annuloplasty technique was used more frequently in the mini-thoracotomy group (81.8% vs. 38.3% in the sternotomy group, OR 2.1, 95%-CI 1.6–2.8, p < 0.001) while the resection + annuloplasty technique was used less often (4.7% vs. 43.5% in the sternotomy group, OR 0.1, 95%-CI 0.05–0.2, p < 0.001). Occlusion of the left atrial appendage was also done less frequently in the mini-thoracotomy group (3.3% vs. 40.7% in the sternotomy group, OR 0.09, 95%-CI 0.04–0.2, p < 0.001) as well as the bi-atrial Cox-Maze procedure (0.9% vs. 11.7% in the sternotomy group, OR 0.08, 95%-CI 0.009–0.3, p = 0.01). There were no significant differences in the rates of PFO closure or the left Cox-Maze procedure. One patient in the sternotomy group required emergency CABG to the circumflex artery, and 1 patient each of the sternotomy and minithoracotomy groups required emergency replacement of the ascending aorta because of an intraoperative localized aortic dissection. Patients operated via minithoracotomy had significantly increased cardiopulmonary bypass times of 161 min (IQR 147–185) vs. 116 (98–145, p < 0.001); and increased aortic cross-clamp times of 94 min (84–113.8) vs. 88 (70–109, p = 0.001). Information on operative details between the matched groups is displayed in Table 4. MVr was successful in 210 patients (98.1%) of the sternotomy and 214 patients (100%) of the mini-thoracotomy group (p = 0.9), with no significant differences in residual MR and mean mitral valve pressure gradient (see Table 5).
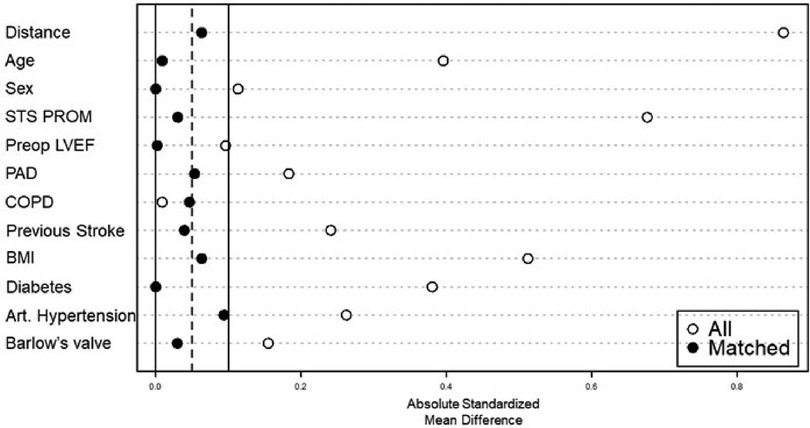
Figure 8. Balancing of covariates by propensity score matching. STS PROM: society of thoracic surgeons–predicted risk of mortality; LVEF: left ventricular ejection fraction; PAD: peripheral artery disease: COPD: chronic obstructive pulmonary disease; BMI: body mass Index.
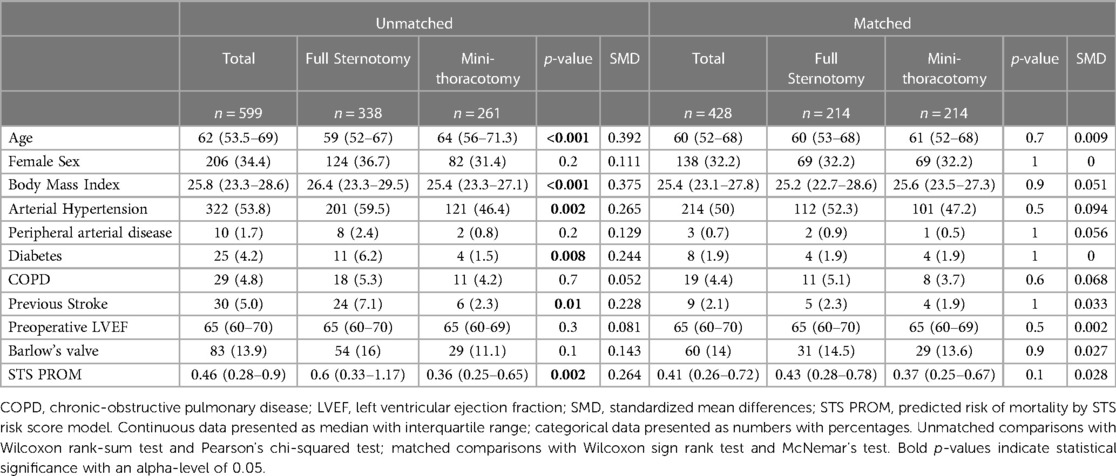
Table 3. Baseline characteristics of the isolated MVr groups before and after propensity score matching.
Patients operated via mini-thoracotomy had a slightly higher postoperative LVEF than those operated via sternotomy (62% [57–66] vs. 62% [55–66], p = 0.03), required significantly shorter time on mechanical ventilation (4.4 h [2.4–6.3] vs. 4.8 h [3.3–7.5], p = 0.002) and were hospitalized significantly shorter (5 days [4–5] vs. 6 days [5–7], p < 0.001). There were no significant differences between the matched groups regarding postoperative complications. Operative mortality was 0% in both groups, while 30-day mortality was 0.9% in the sternotomy and 0% in the minithoracotomy group (p = 0.5). Kaplan-Meier method with log-rank test revealed no difference regarding long-term freedom from re-operation and death (p = 0.19, Supplementary Figure S1). Postoperative outcomes of the matched groups are summarized in Table 6.
We included all patients (n = 16) that underwent robotically assisted MVr at our institution. Repair success rate was 100%, with one patient requiring a second CPB run for residual mild-moderate MR after the initial repair attempt. Repair techniques included resection + annuloplasty, neochord implantation + annuloplasty as well as the combined technique. Three patients had no residual MR and 13 had trace residual MR after robotic MVr. Concomitant PFO closure was done in 4 patients (25%). Mean CPB and aortic cross-clamp times were 239 ± 59 min and 153 ± 48 min respectively, which is substantially longer than in the matched minimally invasive and full sternotomy groups of isolated MVr. Mean hospitalization time was 5.4 ± 1.8 days and there were no relevant postoperative complications during the hospitalization. Preoperative characteristics and results of the robotic cohort are displayed in Table 7.
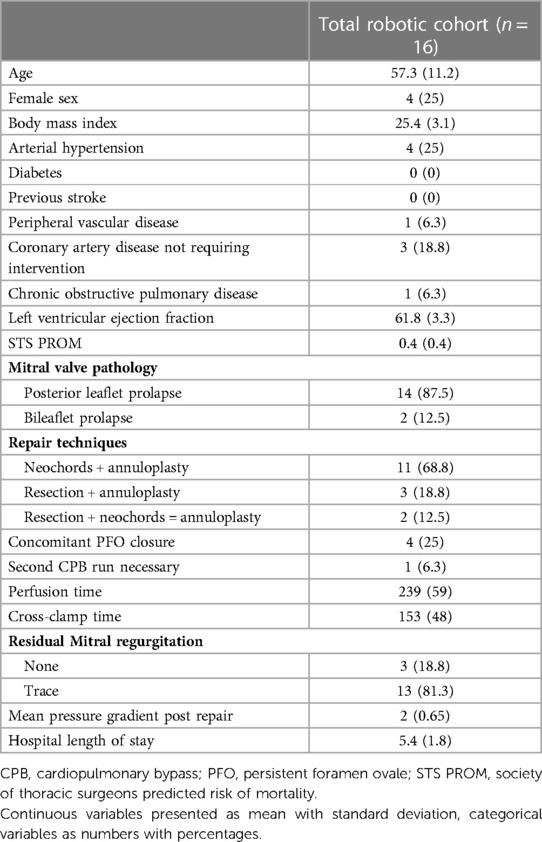
Table 7. Preoperative characteristics, surgical details, and outcomes of the first 16 robotic MVr patients.
Since the first reported case of minimally invasive mitral surgery in 1996 (10), minimally invasive MVr has undergone a significant and continuous expansion (11), supported by large series demonstrating the safety and benefit of this type of procedure (3–6). However, full sternotomy remains the most used approach for MVr. In this retrospective analysis, we observed that setting up a program dedicated to the incorporation of mini-thoracotomy for MVr has made it possible to change the management standard in an efficient and controlled manner without significant increases in complication rates. As shown by many groups, the benefits of minimally invasive MVr can be shorter postoperative ventilation times and shorter overall hospital stay without increased postoperative complications, and at similar procedural costs compared to the sternotomy approach (12–15). Our results are in line with these previous studies, showing significantly decreased postoperative ventilation time and hospital length of stay. A possible reason for shorter ventilation time, which we were not able to explore based on the presented data, may be less postoperative pain due to the smaller incision and less intraoperative spreading with quicker recovery to sufficient breathing patterns triggering earlier extubation. Another important benefit of the minimally invasive approach lies in an expedited recovery of these patients from an open-heart surgery. Without sternotomy-related precautions with regards to mobility, these otherwise healthy adults enjoy an earlier resumption of their active lifestyle as well as an earlier return to their work environment.
An important observation was the increase in overall MVr volume at our institution after starting the mini-thoracotomy program. This resulted in regular interaction of a team of experienced MV surgeons and echocardiographers for preoperative case planning and intraoperative consultation which we believe significantly contributes to patient safety and improved repair success rates (Figures 4, 5). This is in line with several prior reports that detected a strong relationship between individual surgeon and institutional case volumes with repair success rates. We believe that this benefit has a wider beneficial effect as well. Chikwe et al. found that even surgeons with lower MVr volumes (<25/year) have higher repair success rates when operating at the same institutions as higher-volume surgeons (>50/year) when compared to working at other institutions. Newell et al. also recently showed that at an institution with high MVr volumes, outcomes of low- and high-volume surgeons were comparable (16–19). Higher volumes also improve educational experiences with regular trainee exposure to complex MVr, allowing for stepwise teaching opportunities.
It should be emphasized that the quality of MVr was not negatively affected by choosing a minimally invasive approach, with a repair success rate of 100% in this cohort and no differences in residual MR compared to patients operated via sternotomy. In addition, neochord insertion, which might have superior long-term success rates (1, 20, 21), was more frequently used in mini-thoracotomy patients of our cohort. Longer lines of coaptation found after the neochord technique may translate into improved long-term outcomes (22, 23). As displayed in Figure 7, increasing expertise in complex MVr via mini-thoracotomy has changed our institutional approaches to MVP and a combination of resection and neochord insertion is not uncommon. In our opinion, it is crucial to be able to provide a wide range of repair techniques tailored to individual valve anatomy, without necessarily restricting the approach to either “resecting” or “respecting”.
With growing institutional and individual experience regarding minimally invasive MVr, a growth in proportion of more complex MVP cases was observed at MGH, such as anterior leaflet and bileaflet prolapse, undergoing MVr (Figure 6). While isolated posterior leaflet prolapse is the predominant lesion in MVP and associated with excellent repair rates, anterior and bileaflet prolapse present a more challenging surgical task. Yet, repairing these pathologies is feasible with similar long-term outcomes as MVr for isolated posterior leaflet prolapse and should be the primary goal for treatment of MVP (20, 24).
Especially in recent years, cardiac surgeons are confronted with the utilization of robotic support within a growing range of surgical specialties, sparking interest of patients and referring cardiologists. Perceived as an advance of established minimally invasive procedure, introducing robotically assisted cardiac surgery faces similar challenges as surgical training in general (9, 25). Robotic MV surgery was first reported on in 1998 (10, 26), with subsequent encouraging multicenter studies on the success of robotic MVr (27). However, robotic deployment, in addition to the difficulties inherent to a new procedure, is even more limited by its cost and low availability of equipment. Nevertheless, the learning curves appear to be acceptable, with reported rapid reductions in cross-clamp times and stabilization after 20–30 cases (28, 29). As mentioned above, facilitation of papillary muscle and mitral valve exposure and free multi-dimensional movement of surgical instruments are advantages of robotic MV surgery compared to the established methods.
At MGH, a stepwise approach is now followed to shift from standard minimally invasive to robotic MVr. Badhwar et al. proposed that institutional cardiac surgery case volumes be >250 cases/year, that an experienced team of anesthesiologists and perfusionists be present, and that the individual surgeon have at least 15 minimally invasive MVr cases in his or her record, to allow for safe initiation of a robotic MVr program. These criteria were by far exceeded in our case: Institutional yearly volume = ca. 1900 cases with an established team of cardiac anesthesiologists and perfusionists; individual volume of minimally invasive MVr >250 cases. Use of robotic platform for MVr was introduced only when minimally invasive MVr outcomes were excellent and comparable to those of sternotomy MVr, demonstrated by our subgroup analysis. Through guidance of experienced robotic MVr surgeons at all stages of training (theoretical, simulator, and cadaver training) as well as in-person and remote proctoring (Figure 2), we were able to launch a successful robotic MVr program that thus far has yielded satisfying results with a 100% repair success rate. As previously published, a decrease in aortic cross-clamp time can be expected over the next phase of robotic MVr cases. Especially through development of advanced simulation settings, adaptation of robotic surgery can be facilitated for surgeons already competent in complex minimally invasive MVr (30–32).
In an era of the rapid growth of transcatheter techniques, new percutaneous approaches to MVP repair are being proposed (33). However, the possibility of repairing all aspects of MVP (leaflet, annular and subvalvular abnormalities) and especially the availability of durable and safe annuloplasty currently favors surgical MVr. By reducing invasiveness and tissue trauma using minimally invasive and robotic approaches, earlier discharge and reduced use of rehabilitation facilities are possible, with reduced scarring to improve quality of life and patient satisfaction (34, 35).
It is our hope that the very good results of the presented cohort at an institution that fairly recently had started a minimally invasive MVr program might encourage centers to enable experienced surgeons and trainees to gain competence in minimally invasive MVr via formal training programs and interinstitutional proctoring support.
This work presents a retrospective analysis limited to a tertiary care center. Although propensity score matching was used to compare outcomes of minimally invasive and conventional MVr, remaining selection bias as well as surgeon bias need to be considered for the evaluation of the presented results. However, careful application of exclusion criteria and matching served to compare contemporary patient cohorts at a large-volume center. Regarding training aspects and institutional program, the surgical team at an institution like ours is large enough to have a reference surgeon in this type of procedure, and the training of residents is well-structured and standardized. The presented results and educational aspects might therefore not be entirely applicable to non-academic and lower volume centers.
Implementing a minimally invasive mitral surgery approach in the framework of a dedicated support program allows for rapid modification of institutional approaches to complex MVr, with improved institutional outcomes and diversification of repair techniques. Based on an established and successful minimally invasive MVr program, the introduction of robotic support can then be accomplished without compromising patient safety, if a team-based stepwise training protocol is followed. In-person and remote proctoring are key elements for learning new surgical techniques and can be utilized to accomplish a more wide-spread use of less-invasive MVr.
The data underlying the analysis cannot be shared due to data protection of the included individuals. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Massachusetts General Hospital. Written informed consent was waived by the Institutional Review Board of Massachusetts General Hospital. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
AVK, GG, MAB, TMS and SM contributed to conception and design of the study. AVK, SB, DP, TMS, NLB and SM were involved in data acquisition and organization. AVK performed the statistical analysis. AVK and GG wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
MB's hospital receives speaker's honoraria and/or consulting fees on his behalf from Edwards Lifesciences, Medtronic, Abbott and Artivion. SM receives speaker's honoraria from Medtronic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1113908/full#supplementary-material.
1. Bonaros N, Hoefer D, Oezpeker C, Gollmann-Tepeköylü C, Holfeld J, Dumfarth J, et al. Predictors of safety and success in minimally invasive surgery for degenerative mitral disease. Eur J Cardiothorac Surg. (2022) 61:637–44. doi: 10.1093/ejcts/ezab438
2. Van Praet KM, Stamm C, Sündermann SH, Meyer A, Unbehaun A, Montagner M, et al. Minimally invasive surgical mitral valve repair: state of the art review. Interv Cardiol. (2018) 13:14–9. doi: 10.15420/icr.2017:30:1
3. Axtell AL, Moonsamy P, Dal-Bianco JP, Passeri JJ, Sundt TM, Melnitchouk S. Minimally invasive nonresectional mitral valve repair can be performed with excellent outcomes. Ann Thorac Surg. (2020) 109:437–44. doi: 10.1016/j.athoracsur.2019.07.029
4. Mazine A, Vistarini N, Ghoneim A, Lebon J-SS, Demers P, Jeanmart H, et al. Very high repair rate using minimally invasive surgery for the treatment of degenerative mitral insufficiency. Can J Cardiol. (2015) 31:744–51. doi: 10.1016/j.cjca.2014.12.029
5. Holzhey DM, Seeburger J, Misfeld M, Borger MA, Mohr FW. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation. (2013) 128:483–91. doi: 10.1161/CIRCULATIONAHA.112.001402
6. Davierwala PM, Seeburger J, Pfannmueller B, Garbade J, Misfeld M, Borger MA, et al. Minimally invasive mitral valve surgery: "the Leipzig experience". Ann Cardiothorac Surg. (2013) 2:744–50. doi: 10.3978/j.issn.2225-319X.2013.10.14
7. Soppa G, Yates M, Viviano A, Smelt J, Valencia O, Van Besouw JP, et al. Trainees can learn minimally invasive aortic valve replacement without compromising safety. Interact Cardiovasc Thorac Surg. (2015) 20:458–62. doi: 10.1093/icvts/ivu428
8. Pisano GP, Bohmer RMJ, Edmondson AC. Organizational differences in learning: evidence from the adoption of minimally invasive cardiac surgery. Management. (2001) 47(6):735–879. doi: 10.1287/mnsc.47.6.752.9811.
9. Badhwar V, Wei LM, Geirsson A, Dearani JA, Grossi EA, Guy TS, et al. Contemporary robotic cardiac surgical training. J Thorac Cardiovasc Surg. (2021) 165(2):779–83. doi: 10.1016/j.jtcvs.2021.11.005
10. Carpentier A, Loulmet D, Carpentier A, Le Bret E, Haugades B, Dassier P, et al. Chirurgie A coeur ouvert par video-chirurgie et Mini-Thoracotomie premier cas (valvuloplastie mitrale) opere avec succes. Comptes Rendus L’Academie des Sci-Ser III. (1996) 319:219–223. PMID: 8761668.
11. Casselman FP, Van Slycke S, Dom H, Lambrechts DL, Vermeulen Y, Vanermen H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg. (2003) 125:273–82. doi: 10.1067/mtc.2003.19
12. Maier RH, Kasim AS, Zacharias J, Vale L, Graham R, Walker A, et al. Minimally invasive versus conventional sternotomy for mitral valve repair: protocol for a multicentre randomised controlled trial (UK Mini mitral). BMJ Open. (2021) 11:e047676. doi: 10.1136/bmjopen-2020-047676
13. Hage A, Hage F, Al-Amodi H, Gupta S, Papatheodorou SI, Hawkins R, et al. Minimally invasive versus sternotomy for mitral surgery in the elderly: a systematic review and meta-analysis. Innov Technol Tech Cardiothorac Vasc Surg. (2021) 16:310–6. doi: 10.1177/15569845211000332
14. Downs EA, Johnston LE, LaPar DJ, Ghanta RK, Kron IL, Speir AM, et al. Minimally invasive mitral valve surgery provides excellent outcomes without increased cost: a multi-institutional analysis. Ann Thorac Surg. (2016) 102:14–21. doi: 10.1016/j.athoracsur.2016.01.084
15. Cao C, Gupta S, Chandrakumar D, Nienaber TA, Indraratna P, Ang SC, et al. A meta-analysis of minimally invasive versus conventional mitral valve repair for patients with degenerative mitral disease. Ann Cardiothorac Surg. (2013) 2:693–703. doi: 10.3978/j.issn.2225-319X.2013.11.08
16. Chikwe J, Toyoda N, Anyanwu AC, Itagaki S, Egorova NN, Boateng P, et al. Relation of mitral valve surgery volume to repair rate, durability, and survival. J Am Coll Cardiol. (2017) 69:2397–2406. doi: 10.1016/j.jacc.2017.02.026
17. Badhwar V, Vemulapalli S, Mack MA, Gillinov AM, Chikwe J, Dearani JA, et al. Volume-Outcome association of mitral valve surgery in the United States. JAMA Cardiol. (2020) 5:1092–101. doi: 10.1001/jamacardio.2020.2221
18. Newell P, Percy E, Hirji S, Harloff M, Mcgurk S, Malarczyk A, et al. Outcomes of mitral valve repair among high- and low-volume surgeons within a high-volume institution. Ann Thorac Surg. (2022) 115:1–8. doi: 10.1016/j.athoracsur.2022.05.057
19. LaPar DJ, Ailawadi G, Isbell JM, Crosby IK, Kern JA, Rich JB, et al. Virginia Cardiac surgery quality initiative. Mitral valve repair rates correlate with surgeon and institutional experience. J Thorac Cardiovasc Surg. (2014) 148:995–1003; discussion 1003–4. doi: 10.1016/j.jtcvs.2014.06.039
20. Pfannmüller B, Seeburger J, Misfeld M, Borger MA, Garbade J, Mohr FW. Minimally invasive mitral valve repair for anterior leaflet prolapse. J Thorac Cardiovasc Surg. (2013) 146:109–13. doi: 10.1016/j.jtcvs.2012.06.044
21. David TE, David CM, Lafreniere-Roula M, Manlhiot C. Long-term outcomes of chordal replacement with expanded polytetrafluoroethylene sutures to repair mitral leaflet prolapse. Journal of Thoracic and Cardiovascular Surgery. J Thorac Cardiovasc Surg. (2020) 160:385–394.e1. doi: 10.1016/j.jtcvs.2019.08.006
22. Falk V, Seeburger J, Czesla M, Borger MA, Willige J, Kuntze T, et al. How does the use of polytetrafluoroethylene neochordae for posterior mitral valve prolapse (loop technique) compare with leaflet resection? A prospective randomized trial. J Thorac Cardiovasc Surg. (2008) 136:1200–6. doi: 10.1016/j.jtcvs.2008.07.028
23. Pfannmueller B, Misfeld M, Verevkin A, Garbade J, Holzhey DM, Davierwala P, et al. Loop neochord versus leaflet resection techniques for minimally invasive mitral valve repair: long-term results. Eur J Cardio-Thoracic Surg. (2021) 59:180–6. doi: 10.1093/ejcts/ezaa255
24. Seeburger J, Borger MA, Doll N, Walther T, Passage J, Falk V, et al. Comparison of outcomes of minimally invasive mitral valve surgery for posterior, anterior and bileaflet prolapse. Eur J Cardio-Thoracic Surg. (2009) 36:532–8. doi: 10.1016/j.ejcts.2009.03.058
25. Athanasiou T, Ashrafian H, Rowland SP, Casula R. Robotic cardiac surgery: advanced minimally invasive technology hindered by barriers to adoption. Future Cardiol. (2011) 7:511–22. doi: 10.2217/fca.11.40
26. Mohr FW, Falk V, Diegeler A, Walther T, Van Son JAM, Autschbach R, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg. (1998) 115:567–76. doi: 10.1016/S0022-5223(98)70320-4
27. Nifong LW, Chitwood WR, Pappas PS, Smith CR, Argenziano M, Starnes VA, et al. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg. (2005) 129:1395–404. doi: 10.1016/j.jtcvs.2004.07.050
28. Güllü A, Senay S, Kocyigit M, Zencirci E, Akyol A, Degirmencioglu A, et al. An analysis of the learning curve for robotic-assisted mitral valve repair. J Card Surg. (2021) 36:624–8. doi: 10.1111/jocs.15281
29. Yaffee DW, Loulmet DF, Kelly LA, Ward AF, Ursomanno PA, Rabinovich AE, et al. Can the learning curve of totally endoscopic robotic mitral valve repair be short-circuited? Innovations (Phila). (2014) 9:43–8. doi: 10.1097/IMI.0000000000000039
30. Engelhardt S, Sauerzapf S, Brčić A, Karck M, Wolf I, De Simone R. Replicated mitral valve models from real patients offer training opportunities for minimally invasive mitral valve repair. Interact Cardiovasc Thorac Surg. (2019) 29:43–50. doi: 10.1093/icvts/ivz008
31. Jebran AF, Saha S, Waezi N, Al-Ahmad A, Niehaus H, Danner BC, et al. Design and training effects of a physical reality simulator for minimally invasive mitral valve surgery. Interact Cardiovasc Thorac Surg. (2019) 29:409–15. doi: 10.1093/icvts/ivz112
32. Nia PS, Heuts S, Daemen JHT, Olsthoorn JR, Randolph Chitwood W, Maessen JG. The EACTS simulation-based training course for endoscopic mitral valve repair: an air-pilot training concept in action. Interact Cardiovasc Thorac Surg. (2020) 30:691–8. doi: 10.1093/icvts/ivz323
33. Maisano F, Alfieri O, Banai S, Buchbinder M, Colombo A, Falk V, et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. Replacement? Eur Heart J. (2015) 36:1651–9. doi: 10.1093/eurheartj/ehv123
34. Dogan S, Aybek T, Risteski PS, Detho F, Rapp A, Wimmer-Greinecker G, et al. Minimally invasive port access versus conventional mitral valve surgery: prospective randomized study. Ann Thorac Surg. (2005) 79:492–8. doi: 10.1016/j.athoracsur.2004.08.066
Keywords: mitral valve (MV) repair, mitral valve prolapse, minimally invasive cardiac surgery, minimally invasive mitral valve repair, robotic cardiac surgery, robotic mitral valve repair
Citation: van Kampen A, Goudot G, Butte S, Paneitz DC, Borger MA, Badhwar V, Sundt TM, Langer NB and Melnitchouk S (2023) Building a successful minimally invasive mitral valve repair program before introducing the robotic approach: The Massachusetts General Hospital experience. Front. Cardiovasc. Med. 10:1113908. doi: 10.3389/fcvm.2023.1113908
Received: 1 December 2022; Accepted: 2 March 2023;
Published: 21 March 2023.
Edited by:
Claudia Maria Loardi, Centre Hospitalier Universitaire de Tours, FranceReviewed by:
Matteo Pettinari, East Limburg Hospital, Belgium Bernhard Winkler, Vienna Health Association, Austria© 2023 van Kampen, Goudot, Butte, Paneitz, Borger, Badhwar, Sundt, Langer and Melnitchouk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serguei Melnitchouk c21lbG5pdGNob3VrQG1naC5oYXJ2YXJkLmVkdQ==
Specialty Section: This article was submitted to Heart Valve Disease, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.