
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med., 19 June 2023
Sec. Cardiovascular Genetics and Systems Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1110392
This article is part of the Research TopicCase Reports in Cardiovascular Genetics and Systems Medicine: 2022View all 15 articles
 Jacopo Taurino1*
Jacopo Taurino1* Emanuele Micaglio2
Emanuele Micaglio2 Annalisa Russo Raucci3
Annalisa Russo Raucci3 Monica Zanussi3
Monica Zanussi3 Massimo Chessa4,5
Massimo Chessa4,5 Nathasha Samali Udugampolage1
Nathasha Samali Udugampolage1 Paola Carrera3,6
Paola Carrera3,6 Carlo Pappone2,5
Carlo Pappone2,5 Alessandro Pini1
Alessandro Pini1
Vascular Ehlers-Danlos syndrome (vEDS) is a genetic disease caused by a pathogenic mutation in the COL3A1 gene. Despite its severe course, the rarity and extreme clinical variability of the disease can pose significant obstacles to a timely diagnosis. Early and accurate diagnosis may lead to improved patient outcomes by providing access to targeted pharmacological treatments like celiprolol and enhancing the management of vEDS-related complications. Herein, we report a patient harboring a novel de novo COL3A1 missense variant, in which the diagnosis was only possible belatedly due to delayed referral for genetic evaluation. The patient developed pulmonary complications, aneurysms, and vascular malformations, and died at the age of 26 years due to massive pulmonary bleeding.
Vascular Ehlers-Danlos syndrome (vEDS) is a rare connective tissue disorder characterized by a severe course involving fragility and easy rupture of arteries and bowel, transparent skin, easy bruising, and distinct facial features such as protruding eyes, thin nose and lips, sunken cheeks, and lobeless ears (1).
Respiratory manifestations of vEDS are uncommon but have been described, including spontaneous pneumothorax, intrapulmonary bleeding causing recurrent hemoptysis, and bulla or bleb formation (2).
vEDS can be life-threatening at a very young age, and its diagnosis is challenging, particularly in the absence of a family history, due to the rarity and extreme clinical variability associated with the disorder (3). However, primary diagnosis of vEDS can be suspected through careful genetic counseling based on clinical presentation and then confirmed by identifying the pathogenic variant in the COL3A1 gene.
The patient was born preterm at the 35th week of gestation due to premature rupture of membranes. At just four months of age, he experienced an apparent life-threatening event (ALTE) while crying, characterized by sudden loss of consciousness, generalized hypotonia, and cyanosis (no documentation was available). Two months later, a similar event occurred, and a head CT scan revealed bilateral subdural hematoma that was resolved via craniotomy. As a result, the patient suffered reduced visual acuity in the left eye, believed to be secondary to compression of the ipsilateral optic nerve, but he did not develop any other permanent neurological deficits over time.
Starting at the age of 14 years, the patient began experiencing recurrent episodes of hemoptysis, accompanied by right-sided paresthesias, headache, and photophobia. A brain MRI revealed cerebellar tonsil ectasia through the foramen magnum. That same year, the patient suffered a spontaneous right-sided pneumothorax, which was treated with mechanical pleurodesis and exeresis of a bullous lesion. Another pneumothorax occurred at the age of 17 years, this time managed with chemical pleurodesis. Between these two events, he underwent saphenectomy to address multiple vein ectasia in his left leg.
At 22 years old, after an episode of severe hemoptysis, an exploratory thoracotomy was performed, revealing a massive spontaneous right-sided hemothorax. Following suspicion of a coagulation disorder, a hematologic screening which included CBC, PT, aPTT, PFA-100, coagulation factor assay, and vWF dosage—was carried out; all results were normal. At the time of testing, vEDS was not suspected and genetic counseling was not advised. Lacking a definite diagnosis, the patient continued his active lifestyle without adopting any cautious behavior, including smoking.
At the age of 25 years, the patient presented to the emergency department of our institute with acute pain in the right thoraco-abdominal area. A thoracic-abdominal CT scan revealed a massive retroperitoneal hematoma in the right perirenal region, extending from the subdiaphragmatic region to the pelvic cavity (maximum diameter of 30 cm), adjacent to a large anastomotic circle between the aorta and the right pulmonary artery. Multiple dilations of the peripheral branches of the pulmonary artery were also observed.
The right diaphragmatic artery appeared hypertrophic, circumvoluted, with two aneurysmal formations one of which was also partially thrombosed. These findings were confirmed by the angiograms (Figure 1A) and treated via selective coil embolization (Figure 1B). The angiograms also revealed a small dissection flap in the right external iliac artery, and a hypertrophic and circumvoluted 12th intercostal artery. Later, selective catheterization of this artery demonstrated arterial-venous shunts between it and the pulmonary vein for the right lower pulmonary lobe (Figure 2). The shunts were then treated via selective embolization as well (Figure 1B).
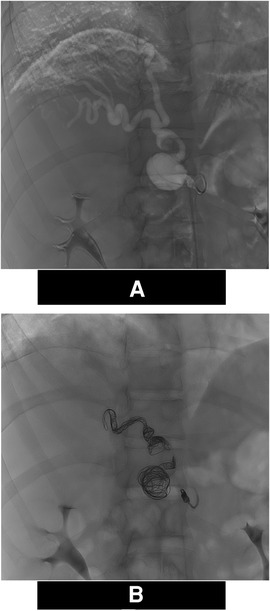
Figure 1. Angiograms of the right inferior diaphragmatic artery. (A) Digital Subtraction Angiography Hypertrophy of the right inferior diaphragmatic artery and evidence of 2 aneurisms (*). (B) Angiogram: Post Coil embolization using Interlock mechanically detachable coils (Boston Scientific, Natick, MA) and The Concerto™ Helix and 3D detachable coil systems (Medtronic Inc Minneapolis MN, US).
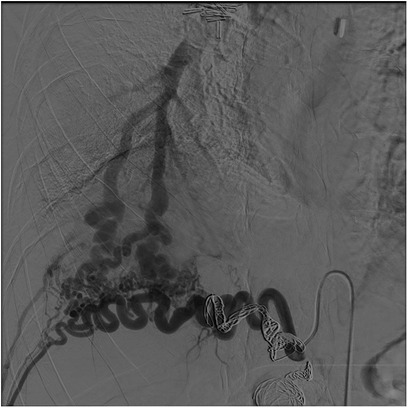
Figure 2. Digital subtraction angiography. Intercostal hypertrophic artery (*) and artero, venous fistula between this artery and the inferior right pulmonary vein (#).
One month later, the patient was readmitted to the emergency department due to another episode of hemoptysis. The chest CT scan revealed “ground glass” consolidation areas with a central consolidated component in the lung parenchyma, with the largest located at the left posterior costophrenic sinus, and several smaller ones in the left lower pulmonary lobe.
These findings were consistent with alveolar hemorrhage, likely originating from the hypertrophic left bronchial artery, where a pseudoaneurysm was recognizable. A subsequent angiography showed markedly tortuous and ectasic proximal sections of the bronchial arteries and hyperemia of the distal bronchial circles.
Superselective catheterization attempts to reach the bleeding site of the left bronchial artery were unsuccessful. Consequently, as the hemoptysis resolved, the patient was not subjected to an embolization procedure; instead, he was started on tranexamic acid therapy without a clinical indication for whole blood transfusion.
During this hospitalization, the patient was referred to clinical genetics for further evaluation. Upon physical examination, the patient exhibited a muscular constitution; his height was 177 cm, and his weight was 75 kg. The skin assessment was challenging due to the presence of multiple tattoos covering the trunk and limbs. No facial anomalies or signs of joint hypermobility were evident; however, a high-arched palate, absence of the lingual frenulum, and flat feet with hammer toes were observed. A connective tissue disorder was finally suspected.
To verify this hypothesis, the patient underwent genetic testing from peripheral blood extracted DNA using a panel of 20 genes (ACTA2, COL1A1, COL1A2, COL3A1, COL5A1, COL5A2, ELN, FBN1, FBN2, FLNA, MYH11, MYLK, NOTCH1, PLOD1, SMAD3, TGFB1, TGFB2, TGFBR1, TGFBR2, TNXB) via next-generation sequencing. The analysis identified the novel heterozygous missense variant c.1889G > A causing the amino-acid substitution p.(Gly630Glu) in COL3A1 (NM_000090.3) (Figure 3). Subsequent testing of parental samples demonstrated that the variant originated de novo (Supplementary Figure S1). A literature search (PubMed, Human Gene Mutation Database, LOVD, Ehlers-Danlos Syndrome variant Database, Internal data, ClinVar) confirmed that this variant has not been previously reported, indicating a novel mutation.
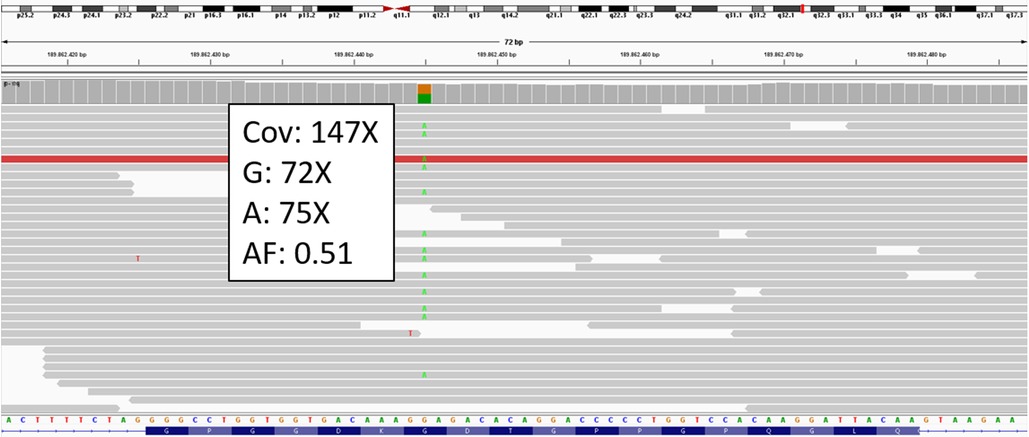
Figure 3. COL3A1 BAM from IGV. The c.1889G > A variant is detected in heterozygosity. Cov: coverage; AF: allelic fraction of alternative called base (A).
According to the ACMG standards for variant classification (4), the following criteria were applied to classify the variant as ‘likely pathogenic’: the variant was absent in both the unaffected parents (PS2), it is absent in the gnomAD database (PM2); it is located within the collagen triple helix repeat (PM1), and it is predicted in silico as deleterious by many different tools, including MetaRNN, M-CAP, MVP, Polyphen2 HDIV, Polyphen2 HVAR, SIFT, DEOGEN2, EIGEN, FATHMM, and MutPred (PP3).
The patient’s clinical presentation, along with imaging and genetic test results, led to a diagnosis of vEDS, and pharmacological therapy with celiprolol 200 mg daily was started.
The patient was subsequently evaluated by a multidisciplinary team to determine the best approach for treating the frequent pulmonary hemorrhages.
The only resolutive treatment proposed was heart-lung transplantation. However, the surgical team deemed this option impossible due to the patient's previous chemical pleurodesis and tissue fragility. Later that year, the patient experienced a new spontaneous pneumothorax, followed by a massive pulmonary hemorrhage that proved fatal at the age of 26 years.
Vascular Ehlers-Danlos syndrome (vEDS) is the most severe subtype among the Ehlers-Danlos syndromes (5); caused by pathogenic variants in the COL3A1 gene, which encodes the α1 chain of type III procollagen [proα1(III)].
vEDS carries a high risk of sudden death due to the rupture of large arteries or hollow organs, such as the bowel and uterus. The median age for the first major vascular event is 24.6 years, and the median survival is only 48 years due to the high rate of complications (6).
Clinical diagnostic criteria for vEDS include: a positive family history of the disorder, early onset of arterial rupture or dissection before age of 40 years, unexplained rupture of the sigmoid colon, unexplained spontaneous pneumothorax or carotid-cavernous sinus fistula formation without trauma, and minor vEDS features (e.g., tendon and muscle rupture, easy bruising, thin translucent skin, hypermobility of small joints, aged appearance of extremities particularly the hands, gingival recession, keratoconus, early onset varicose veins) (5).
Imaging, including computed tomography angiography and magnetic resonance angiography, is an essential tool for diagnosing and monitoring vEDS. Aneurysms, dissections, arterial ectasia, and occlusions are the most common radiologic findings, with abdominal vessels being the most frequently affected (34%), followed by thoracic (33%), and head/cervical vessels (17%) (6).
Genetic testing is the definitive method for confirming the diagnosis of vEDS, through the identification and characterization of a COL3A1 pathogenic variant, which may also offer a prognostic insight. According to Omar et al. (7), the majority of pathogenic variants in the COL3A1 gene (approximately 65%) are missense variants, resulting in the substitution of a glycine residue within the triple helical domain. Aberrant splicing, occurring in about 25% of patients, is the second most common mutation mechanism. Additionally, 5% of patients have nonsense or frameshift variants, leading to unstable RNA that is degraded through nonsense-mediated decay without producing protein (null mutations or haploinsufficiency).
According to Pepin et al. (6), splicing variants are associated with the most severe disease course, followed by missense variants involving glycine residues, and finally haploinsufficient variants are associated with a milder course, including delayed symptom onset and increased life expectancy.
Diagnosing vEDS can be challenging, especially because 50% of COL3A1 pathogenic variants occur de novo. As a result, vEDS is often only suspected after a significant or potentially fatal complication occurs (3).
For 70% of vEDS patients, the presenting sign is either a vascular rupture or an organ rupture (8). However, this means that a significant portion of patients have an atypical presentation of vEDS. Among these, 12% of patients have a spontaneous pneumothorax due to the rupture of a lung bleb as their first described manifestation (3). Additionally, pulmonary hemorrhages related to the rupture of small vascular nodules or arteriovenous fistulas have been reported as well (9, 2).
In 2015, Abrahamsen et al. (10) described a patient carrying a COL3A1 pathogenic variant whose disease onset occurred at the age of 19 years, characterized by recurrent episodes of spontaneous pneumothorax. The patient also had other typical signs of the disease, such as a tall and slender build, mild scoliosis, asymmetry of the chest, long fingers with hypermobility of the thumb joints, thin and translucent skin, prominent eyes, and keratoconus, which further supported genetic testing for COL3A1. The authors suggested that the atypical presentation of vEDS with pneumothorax and pulmonary bleeding may be more common in young adults.
Treatment for vEDS primarily focuses on avoiding high-risk activities and procedures such as arterial punctures, elective surgeries, and gastrointestinal and uterine endoscopies. These procedures should be limited to cases with urgent complications, including arterial or organ rupture. Blood pressure control is crucial, and the use of antiplatelet and anticoagulation medications should be avoided unless strictly indicated. It is essential for patients to understand their risk factors and implement necessary lifestyle changes to reduce the risk of complications, such as avoiding strenuous physical activity and contact sports (8).
Atypical presentations of vEDS may delay diagnosis, resulting in suboptimal patient management and serious clinical consequences. In this regard, Diebels et al. (11) described a 58-year-old patient with Stanford type B aortic dissection who underwent thoracic endovascular repair (TEVAR) but experienced a retrograde dissection with rupture of the ascending aorta just two days later, which ultimately proved fatal. In this case, diagnosis was not possible through clinical criteria, but was only revealed post-mortem thanks to the identification of a pathogenic mutation in the COL3A1 gene. The authors emphasized the importance of a conservative management approach for vascular events in patients even suspected of having vEDS, given the high fragility of their tissues and blood vessels, which can lead to very serious complications.
Currently, the only medical therapy that has been shown to improve outcomes for vEDS is the use of celiprolol, a β1-adrenoceptor antagonist and a β2-adrenoceptor agonist that reduces hemodynamic stress and the fragility of arterial walls (12).
The extent of evaluation at the time of initial diagnosis is currently a topic of ongoing debate. The most important step in managing vEDS is assembling an interdisciplinary care team, which should include a primary care physician, a geneticist, a vascular surgeon, and a general surgeon. This team will be responsible for coordinating both routine and emergency care (8).
In this work, we describe a patient with vEDS who died from the disease at a young age. Recurrent spontaneous pneumothorax and pulmonary hemorrhage in young individuals may indicate an atypical onset of vEDS and should warrant prompt genetic counseling, even in the absence of other common symptoms such as facial, skin, and joint anomalies.
Unfortunately, genetic counseling for our patient was recommended only after his condition worsened, and the delayed diagnosis precluded the proper clinical management that might have been potentially beneficial.
An earlier diagnosis might have allowed for timely treatment with celiprolol, which has been associated with better cardiovascular outcomes and longer life expectancy in vEDS patients. Furthermore, it might have led to alternative management strategies for the patient's recurrent pneumothoraces.
In this scenario, the typical tissue fragility of vEDS alone was not the reason to contraindicate heart and lung transplantation in our patient. However, this fragility, combined with the result of the previous chemical pleurodesis led the surgical team to deem any intervention unfeasible.
The usefulness of this case is to demonstrate the pivotal importance of referring to clinical genetics evaluation for all patients with a history of recurrent bleedings and spontaneous pneumothoraces. Early diagnosis of vEDS may enable better treatment choices, improved management, and ultimately, better outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AR, JT, and AP evaluated the patient and collected his clinical history; JT and AP conceived the clinical study; MC conducted neuroimaging analyses at follow-up; AR, MZ, NU, PC, critically revised the clinical data and contributed to assembling the manuscript; MZ and PC performed NGS analysis and interpretation; JT and EM wrote the manuscript; PC, AP and CP critically revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was partially supported by “Ricerca Corrente” funding from the Italian Ministry of Health to IRCCS Policlinico San Donato.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1110392/full#supplementary-material
1. Malfait F. Vascular aspects of the ehlers-danlos syndromes. Matrix Biol. (2018) 71-72:380–95. doi: 10.1016/j.matbio.2018.04.013
2. Kawabata Y, Watanabe A, Yamaguchi S, Aoshima M, Shiraki A, Hatamochi A, et al. Pleuropulmonary pathology of vascular ehlers-danlos syndrome: spontaneous laceration, haematoma and fibrous nodules. Histopathology. (2010) 56(7):944–50. doi: 10.1111/j.1365-2559.2010.03574.x
3. Byers PH, Belmont J, Black J, De Backer J, Frank M, Jeunemaitre X, et al. Diagnosis, natural history, and management in vascular ehlers-danlos syndrome. Am J Med Genet C Semin Med Genet. (2017) 175(1):40–7. doi: 10.1002/ajmg.c.31553
4. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17:405–23. doi: 10.1038/gim.2015.30
5. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the ehlers-danlos syndromes. Am J Med Genet C Semin Med Genet. (2017) 175(1):8–26. doi: 10.1002/ajmg.c.31552
6. Pepin MG, Schwarze U, Rice KM, Liu M, Leistritz D, Byers PH. Survival is affected by mutation type and molecular mechanism in vascular ehlers-danlos syndrome (EDS type IV). Genet Med. (2014) 16(12):881–8. doi: 10.1038/gim.2014.72
7. Omar R, Malfait F, Van Agtmael T. Four decades in the making: collagen III and mechanisms of vascular ehlers danlos syndrome. Matrix Biol Plus. (2021) 12:100090. doi: 10.1016/j.mbplus.2021.100090
8. Byers PH. Vascular ehlers-danlos syndrome. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. Genereviews®. Seattle (WA): University of Washington (1993–2023). Available at: https://www.ncbi.nlm.nih.gov/books/NBK1494/
9. Ishiguro T, Takayanagi N, Kawabata Y, Matsushima H, Yoshii Y, Harasawa K, et al. Ehlers-Danlos syndrome with recurrent spontaneous pneumothoraces and cavitary lesion on chest x-ray as the initial complications. Intern Med. (2009) 48(9):717–22. doi: 10.2169/internalmedicine.48.1818
10. Abrahamsen BJ, Kulseth MA, Paus B. A 19-year-old man with relapsing bilateral pneumothorax, hemoptysis, and intrapulmonary cavitary lesions diagnosed with vascular ehlers-danlos syndrome and a novel missense mutation in COL3A1. Chest. (2015) 147(5):e166–70. doi: 10.1378/chest.13-3002
11. Diebels I, Hendriks JMH, Durnez J, Paelinck BP, El Addouli H, Laga S, et al. Retrograde type A aortic dissection 48 hours after TEVAR in a patient with a delayed diagnosis of vascular ehlers-danlos syndrome. Aorta (Stamford). (2022) 10(3):141–4. doi: 10.1055/s-0042-1749171
12. Ong KT, Perdu J, De Backer J, Bozec E, Collignon P, Emmerich J, et al. Effect of celiprolol on prevention of cardiovascular events in vascular ehlers-danlos syndrome: a prospective randomised, open, blinded-endpoints trial [published correction appears in lancet. 2016 aug 6;388(10044):564. Dosage error in published abstract; MEDLINE/PubMed abstract corrected; dosage error in article text]. Lancet. (2010) 376(9751):1476–84. doi: 10.1016/S0140-6736(10)60960-9
Keywords: COL3A1, vascular ehlers danlos syndrome, aneurysm - dissecting, pneumothorax, atypical onset
Citation: Taurino J, Micaglio E, Russo Raucci A, Zanussi M, Chessa M, Udugampolage NS, Carrera P, Pappone C and Pini A (2023) Case report: Complex arterial findings in vascular ehlers-danlos syndrome with a novel COL3A1 variant and death at young age. Front. Cardiovasc. Med. 10:1110392. doi: 10.3389/fcvm.2023.1110392
Received: 28 November 2022; Accepted: 30 May 2023;
Published: 19 June 2023.
Edited by:
Neil Morgan, University of Birmingham, United KingdomReviewed by:
Paola Mandich, University of Genoa, Italy© 2023 Taurino, Micaglio, Russo Raucci, Zanussi, Chessa, Udugampolage, Carrera, Pappone and Pini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacopo Taurino amFjb3BvLnRhdXJpbm9AZ3J1cHBvc2FuZG9uYXRvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.