
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 03 April 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1103813
Background: The impact of the degree of worsening renal function (WRF) and B-type natriuretic peptide (BNP) on the prognosis of patients with acute heart failure (AHF) is still debatable. The present study investigated the influence of different degrees of WRF and BNP levels at discharge on 1-year all-cause mortality in AHF.
Methods: Hospitalized AHF patients diagnosed with acute new-onset/worsening of chronic heart failure (HF) between January 2015 and December 2019 were included in this study. Patients were assigned into high and low BNP groups based on the median BNP level at discharge (464 pg/ml). According to serum creatinine (Scr) levels, WRF was divided into non-severe WRF (nsWRF) (Scr increased ≥0.3 mg/dl and <0.5 mg/dl) and severe WRF (sWRF) (Scr increased ≥0.5 mg/dl); non-WRF (nWRF) was defined as Scr increased of <0.3 mg/dl). Multivariable cox regression was used to evaluate the association of low BNP value and different degrees of WRF with a all-cause death, as well as testing for an interaction between the two.
Results: Among 440 patients in the high BNP group, there was a significant difference in WRF on mortality (nWRF vs. nsWRF vs. sWRF: 22% vs. 23.8% vs. 58.8%, P < 0.001). Yet, mortality did not significantly differ across the WRF subgroups in the low BNP group (nWRF vs. nsWRF vs. sWRF: 9.1% vs. 6.1% vs. 15.2%, P = 0.489). In multivariate Cox regression analysis, low BNP group at discharge (HR, 0.265; 95%CI, 0.162–0.434; P < 0.001) and sWRF (HR, 2.838; 95%CI, 1.756–4.589; P < 0.001) were independent predictors of 1-year mortality in AHF.There was a significant interaction between low BNP group and sWRF(HR, 0.225; 95%CI, 0.055–0.918; P < 0.05).
Conclusions: nsWRF does not increase the 1-year mortality in AHF patients, whereas sWRF does. A low BNP value at discharge is associated with better long-term outcomes and mitigates the adverse effects of sWRF on prognosis.
Acute renal impairment is a common complication in patients with acute heart failure (AHF), also known as type I cardiorenal syndrome (1). In patients with type I cardiorenal syndrome, acute renal impairment is assessed as worsening renal function (WRF) and is often defined as a 0.3 mg/dl increase in serum creatinine (Scr) level compared to admission (2). The estimated incidence of WRF in AHF is 25%–33% (3). WRF has traditionally been associated with worse long-term mortality; however, recent studies have challenged this association (4–6). The pathogenic mechanism of WRF in AHF is complex, and these opposing conclusions may stem from different pathophysiologic processes. Decreased renal perfusion, i.e., impaired renal “preload” was previously thought to be the main etiology of WRF (7, 8). However, renal congestion from volume overload may also contribute to WRF (9). Studies have shown that elevated central venous pressure is associated with the occurrence of WRF and that fluid volume status is inseparable from the occurrence of WRF (10, 11). More recently, several studies suggested that AHF patients with successful decongestion at discharge have favorable prognosis and that fluid volume status affects the relationship between WRF and prognosis (12, 13). Thus, adequately evaluating a patient's volume status in the setting of WRF can help understand its prognosis in AHF.
B-type natriuretic peptide (BNP) is a well-established biomarker in AHF, whose secretion is mainly dependent on ventricular volume expansion (14). Therefore, BNP levels may help physicians interpret the patients' volume status. Yet, the effect of different degrees of WRF on AHF prognosis under different BNP at discharge has not been studied. In this study, we divided WRF into two levels, nsWRF and sWRF, according to different degrees of Scr elevation during hospitalization to evaluate the effect of BNP levels at discharge and different degrees of WRF, as well as testing for an interaction between the two on mortality in AHF.
Patients diagnosed with acute new-onset/worsening of chronic heart failure (HF) in Shanghai General Hospital between January 2015 and December 2019 were included in this study. Demographic characteristics, medical history, physical examination results, laboratory indicators, etc., were collected at admission, during hospitalization, and at discharge. AHF was diagnosed based on the 2021 European Society of Cardiology guidelines for the diagnosis of AHF (15): plasma natriuretic peptide level (BNP ≥ 100 pg/ml) (Class I, Level A), a 12-lead electrocardiogram (Class I, Level C), laboratory measurements [troponins, blood urea nitrogen (BUN), creatinine, sodium, potassium, glucose, liver function, and complete blood counts] (Class I, Level C), and echocardiography (Class I, Level C). In addition, patients who presented with new or exacerbated symptoms and signs of HF, and met the Framingham Criteria (16), were also eligible. In brief, the Framingham criteria require two major criteria, or one major and two minor criteria: the major criteria include orthopnoea or paroxysmal nocturnal dyspnoea, neck vein distention, rales, cardiomegaly, acute pulmonary edema, S3 gallop, increased venous pressure, prolongation of circulation time, and hepatojugular reflux; the minor criteria include ankle edema, night cough, dyspnoea on exertion, hepatomegaly, tachycardia, and weight loss. An experienced physician assessed all patients at the emergency department within 30 min of admission.
Exclusion criteria were: (I) pulmonary embolism; (II) death during the hospital stay; (III) acute coronary syndrome; (IV) bradycardia requiring pacemaker implantation; (V) estimated glomerular filtration rate (eGFR) < 15 ml/(min·1.73 m2); (VI) planned dialysis or has entered maintenance dialysis; (VII) had a history of large organ transplantation; (VIII) participated in a drug treatment study within the past 30 days or before; (IX) pregnant and lactating women.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study protocol was approved by the Ethics Review Committee of Shanghai General Hospital (2022KY031). The Institutional Review Board waived the requirement for written informed consent because this study was a retrospective observational study.
WRF was divided into two grades according to the different degrees of Scr changes during hospitalization: non-severe WRF (nsWRF) and severe WRF (sWRF). Based on the commonly used definition of WRF (Scr increase ≥0.3 mg/dl from admission) (6), nsWRF was defined as the increase of Scr during hospitalization by ≥0.3 mg/dl and <0.5 mg/dl compared with that on admission; sWRF was defined as the Scr continued to increase by ≥0.5 mg/dl compared with admission. Non-WRF (nWRF) was defined as an increase in Scr of <0.3 mg/dl during hospitalization compared with admission. We divided patients into high and low BNP groups based on the median BNP at discharge to evaluate the effect of different degrees of WRF on 1-year death in AHF under different BNPs at discharge. The low BNP group were those with BNP levels lower than the median at discharge, while the high BNP group included those with BNP levels higher than the median BNP at discharge.
For numerical variables with normal distribution, the mean ± standard deviation (SD) was used to describe between-group differences; these data were assessed by t-test or one-way ANOVA. When the data of numerical variables were skewed, the median was used, and interquartile range [M(QR)] descriptions and comparisons between groups were performed using the Mann-Whitney U rank-sum test or the Kruskal-Wallis rank-sum test. Categorical variable data were described by the number of cases and frequency [n (%)], and comparisons between groups were performed using the Chi-square test or the exact probability method. Kaplan-Meier survival analysis was used to analyze the effects of discharge plasma BNP level and different degrees of WRF on the 1-year survival rate, and the log-rank test was performed. The same method was used to analyze the effect of different degrees of WRF on 1-year mortality under different BNP levels at discharge. Univariate and multivariate COX proportional hazards regression models were used to analyze whether low BNP value at discharge or different degrees of WRF were associated with mortality, as well as testing for an interaction between the two. In multivariate analysis, differences in age, sex, pulmonary crackles, peripheral edema, systolic blood pressure and hemoglobin on admission, serum sodium, BUN, and Scr at discharge, and history of admission for ischemic heart disease, atrial fibrillation, hypertension, diabetes, chronic kidney disease were adjusted based on prior studies (17–19). Two-sided P < 0.05 was considered statistically significant. Data were analyzed by IBM SPSS Statistics 19.
Table 1 shows the basic characteristics of patients at admission and discharge. Among 445 patients who initially met the inclusion requirements, 5 patients who could not be reached by telephone were excluded. Ultimately, we analyzed the survival status of 440 patients within 1 year after discharge. The median age was 74 years; there were 263 males (59.8%). In addition, 252 patients had a history of ischemic heart disease (57.3%), 161 had a history of atrial fibrillation (36.6%), 184 patients a history of diabetes (41.8%), 356 patients a history of hypertension (80.9%), and 106 patients had a history of chronic kidney disease (23.9%).
The median BNP at discharge was 464 pg/ml. WRF occurred in 159 patients (36.1%), including 75 cases of nsWRF and 84 cases of sWRF. Within 1 year after discharge, 89 patients died, resulting in a mortality rate of 20.2%. Patients in the sWRF group spent more time (days) in the hospital and had higher rates of crackles, a history of CKD, hypertension, and higher potassium at admission (P < 0.05). Intravenous loop diuretics were more frequently used in the sWRF group (P = 0.178). Patients in the nWRF group had higher hemoglobin on admission but lower BUN, Scr, and BNP at admission and discharge (P < 0.05). Patients with low BNP levels were older with higher SBP (P < 0.05). The incidence of CKD and edema was higher in patients with high BNP levels (P < 0.05). They had higher BUN, Scr, and BNP at admission and discharge (P < 0.05).
The one-year mortality (30.9%) of the high BNP group was higher than that of the low BNP group (9.5%) (P < 0.001). The Kaplan-Meier survival curves of the two groups are shown in Figure 1, and the low BNP group was associated with better outcomes (P < 0.001).
The mortality of the sWRF group (41.7%) was higher than that of the nWRF (14.9%) group and the nsWRF group (16.0%) (P < 0.001), while there was no difference in mortality between the nWRF group and nsWRF group (P = 0.821) (Table 2). The Kaplan-Meier survival curves of the three groups are shown in Figure 2, and sWRF was associated with worse outcomes (P < 0.001).
In the low BNP group, the 1-year morality of different degrees of WRF is shown in Table 3. There was no significant difference among the nWRF, nsWRF, and sWRF (P = 0.489). The Kaplan-Meier survival curves of the three groups are shown in Figure 3A, and the 1-year survival showed no significant difference between the three groups (P = 0.446). In the high BNP group, the differences among the three groups in morality were statistically significant (P < 0.001). The mortality of sWRF was significantly different from the other two groups (P < 0.01), but the mortality of nsWRF was not significantly different from that of nWRF (P = 0.813) in the high BNP group, as shown in Table 3. The Kaplan-Meier survival curves of different degrees of WRF are shown in Figure 3B, and sWRF in the high BNP group had the worst clinical outcomes.
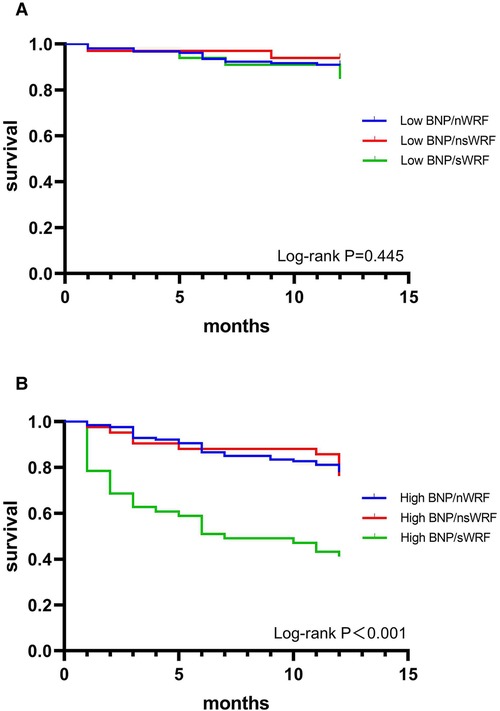
Figure 3. 1-year survival curves of nWRF, nsWRF, and sWRF in patients with low BNP (A) and high BNP (B).
According to nWRF, nsWRF, and sWRF, three groups were set to compare the 1-year morality of high BNP and low BNP groups. In each WRF group, mortality was higher in the high BNP group than in the low BNP group (Table 3). The Kaplan-Meier survival curves in Figures 4A–C showed that in each WRF group, the high BNP group always had the worse outcome in contrast to the low BNP group.
Results from univariate and multivariate Cox proportional hazards models for 1-year morality are shown in Table 4. In a multivariate COX proportional hazards model, the low BNP group was associated with decreased mortality [hazard ratio(HR), 0.265; 95% confidence interval (CI), 0.162%0.434; P < 0.001]. sWRF resulted as an independent predictor of mortality (HR, 2.838; 95% CI, 1.756–4.589; P < 0.001). Also, there was no increased 1-year risk of death in nsWRF compared to nWRF(HR, 0.886; 95% CI, 0.461%1.703; P = 0.717). There was a significant interaction between the low BNP group and sWRF (HR, 0.225; 95%CI, 0.055%0.918; P = 0.038).
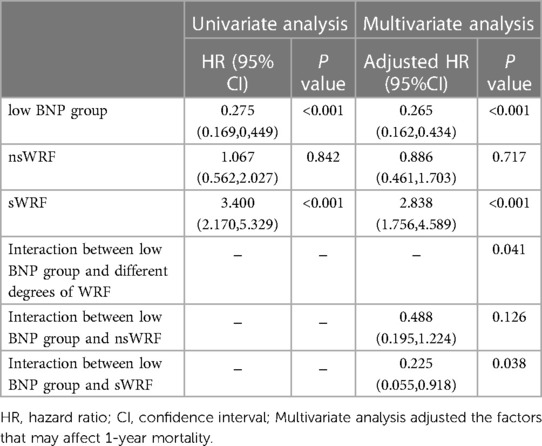
Table 4. Univariate and multivariate Cox proportional hazards regression analysis of 1-year mortality in patients with AHF.
The present study analyzed the effect of the degree of WRF on the long-term prognosis of AHF patients under different BNP levels at discharge. The key findings of this study are: (I) in AHF patients, nsWRF was not associated with 1-year all-cause mortality, while sWRF was associated with mortality and resulted as an independent predictor of AHF prognosis; (II) low-level BNP at discharge, regardless of the occurrence of WRF, was associated with lower mortality and resulted as an independent predictor of long-term prognosis in AHF; (III) low-level BNP at discharge could reduce the adverse effects of sWRF on prognosis.
In the present study, we divided the severity of WRF to assess whether the degrees of WRF have different effects on AHF's prognosis. Since the definition of WRF has not yet been unified, the increase of Scr concentration was utilized as the basis for grading the severity of WRF. COX multivariate regression analysis showed that compared to the nWRF, sWRF could increase the mortality risk of AHF patients. It also resulted as an independent predictor for mortality, which is consistent with previous studies (17, 20). In addition, nsWRF did not increase the risk of mortality in AHF compared to the nWRF. Previous studies have used Scr increase ≥0.3 mg/dl as the definition of WRF to study prognosis, but the results for the long-term prognosis of AHF were conflicting (17, 20, 21). The contradiction may be due to the different degrees of increased Scr in these studies. The proportion of patients with Scr may increase ≥0.3 mg/dl and <0.5 mg/dl was different in each study. This group is the nsWRF group that we studied. We speculate that this could be caused by neurohormonal or hemodynamic abnormalities, which lead to reversible renal dysfunction and does not cause real kidney injury (22, 23). Considering the prognosis of renal function in patients with nsWRF and sWRF after discharge remains unclear, as well as the eventual existence of differences in the long-term effects on kidney function, future studies should determine the long-term prognosis of nsWRF and sWRF based on the changes in sensitive renal function markers such as Scr, cystatin C, and neutrophil gelatinase-associated lipocalin (NGAL) after discharge.
Although sWRF resulted as an independent predictor of mortality in AHF, sWRF was only associated with mortality in the group with high BNP levels and interacted with a low BNP level, showing that BNP value at discharge assessed with creatinine can differentiate WRF with relevant prognosis from non-relevant WRF in AHF. Thus, the study results imply that sWRF occurring in hospitals should be interpreted in conjunction with BNP at discharge in patients with AHF. Metra et al. (13) used data from the PROTECT study (Placebo-Controlled Randomized Study of the Selective A1 Adenosine Receptor Antagonist Rolofylline for Patients Hospitalized With Acute Decompensated Heart Failure and Volume Overload to Assess Treatment Effect on Congestion and Renal Function) to investigate whether the degree of congestion affects the prognostic significance of WRF in AHF and found that among patients with an increase in Scr of 26.5 μmol/L during their hospital admission, only those with congestion had a higher risk of death. Similarly, McCallum et al. (24) demonstrated that when kidney function declines in a hospital, acute decompensated heart failure appears benign as long as decongestion is achieved. Our study reinforces the importance of congestion in terms of the prognostic relevance of sWRF.
Correct assessment of the extent of decongestion is critical in recognizing the prognostic meaning of sWRF, but it is also challenging. BNP has been used to assess patients' volume status because of its objective and convenient measurement and has been associated with prognosis in HF patients (25, 26). A previous study suggested that a percent BNP reduction from admission to discharge independently predicts worse outcomes in AHF (27). Nevertheless, Hamatani et al. (28) demonstrated that the change in the ratio of BNP at discharge to admission is often used to predict mortality in AHF but is not as accurate as the value of BNP at discharge. However, the exact effect of BNP reduction at discharge on the risk of death is clinically unclear for AHF patients. Studies have shown that the threshold of BNP at discharge ≤ 250 pg/ml can significantly reduce AHF patients' mortality and readmission rate (25). However, achieving this threshold for patients with high BNP values at admission with decongestion therapy such as diuretics is also difficult. Intermediate threshold BNP may be more clinically useful (29). Also, there is no clear intermediate threshold BNP to judge AHF patient prognosis. This study used the median BNP value at discharge (464 pg/ml) to group patients and confirmed results of previous studies; regardless of whether WRF occurred, low BNP value(<464 pg/ml) at discharge was associated with a lower 1-year risk of death.
The reason why sWRF has a different effect on prognosis under different fluid volume loads may be because pathogeneses leading to sWRF are different under different fluid volume loads. Renal vascular congestion may lead to sWRF due to increased renal interstitial pressure, renal tubule damage, and hypoxic damage to the renal cortex, affecting prognosis (30). Although aggressive decongestion therapy increases the incidence of sWRF, it is mainly transient kidney damage, which has no adverse effect on prognosis. A study using NGAL to assess tubular damage showed that WRF under decongestion appears to be associated with hemodynamic changes independent of tubular damage (31). Clinically, when renal function worsens in AHF patients, cardiologists and nephrologists often have different opinions on the next decongestion therapy plan. Cardiologists worry that insufficient decongestion increases the risk of death, while nephrologists are concerned that excessive decongestion might increase the risk of death by exacerbating kidney damage. Our study showed that in the absence of WRF, it is better to reduce the body volume load of patients at discharge as much as possible for long-term prognosis, and the occurrence of nsWRF should not be particularly emphasized to ensure renal perfusion and reduce decongestion therapy. However, only when sWRF occurs, it is necessary to pay attention not to excessively reduce body volume whilst focusing on renal function in order to timely detect and treat kidney damage. Reducing body volume load should not be regarded as the only goal of improving the prognosis.
Previous studies found that WRF with higher BNP levels at discharge had a worse prognosis (32, 33). This study further found that regardless of the degree of WRF, 1-year mortality was higher in patients with higher BNP levels at discharge. BNP had a better prognostic value than WRF. Therefore, WRF may be a prognostic parameter in certain subgroups of patients (e.g., with high BNP). When WRF occurs, the determination of BNP helps to assess the congestion status and prognosis of patients at this time and can further stratify such risk groups, which is helpful for clinicians to perform different treatment measures promptly. Our study showed that when BNP is controlled below 464 pg/ml at discharge while allowing an increase in Scr within 0.5 mg/dl during hospitalization, the prognosis of AHF patients may improve.
First, this is a single-center retrospective study. Unmeasured variables and missing data may affect the results. Second, this study did not explore other variables influencing BNP reduction and the relationship between WRF. Third, our study only analyzed changes in renal function during hospitalization, and data on subsequent changes in renal function after discharge are lacking. As these data may be more helpful in identifying whether there is actual renal impairment, they should also be included in further analyses. Fourth, we classified patients using the median BNP at discharge as a cutoff value. Although plasma BNP is a reliable marker for measuring congestion in HF patients, our classification may not fully identify congestion patients.
nsWRF does not increase the 1-year risk of all-cause mortality in AHF patients, whereas sWRF does. In addition, a low BNP value at discharge is associated with a lower 1-year risk of all-cause mortality in AHF patients, while a low BNP value at discharge could mitigate the adverse effects of sWRF on the prognosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Shanghai General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
DZ and SR: carried out the studies, participated in collecting data, and drafted the manuscript. LG: revised the manuscript, added content, and participated in the acquisition and analysis. WW, MY, and DP: performed the statistical analysis and participated in its design. WY: participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
This work was supported by the Natural Science Foundation of China [serial number 81970636].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zannad F, Rossignol P. Cardiorenal syndrome revisited. Circulation. (2018) 138(9):929–44. doi: 10.1161/circulationaha.117.028814
2. Seliger S. The cardiorenal syndrome: mechanistic insights and prognostication with soluble biomarkers. Curr Cardiol Rep. (2020) 22(10):114. doi: 10.1007/s11886-020-01360-8
3. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol. (2012) 60(12):1031–42. doi: 10.1016/j.jacc.2012.01.077
4. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. (2012) 5(1):54–62. doi: 10.1161/circheartfailure.111.963413
5. Breidthardt T, Weidmann ZM, Twerenbold R, Gantenbein C, Stallone F, Rentsch K, et al. Impact of haemoconcentration during acute heart failure therapy on mortality and its relationship with worsening renal function. Eur J Heart Fail. (2017) 19(2):226–36. doi: 10.1002/ejhf.667
6. Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. (2014) 35(7):455–69. doi: 10.1093/eurheartj/eht386
7. Fu K, Hu Y, Zhang H, Wang C, Lin Z, Lu H, et al. Insights of worsening renal function in type 1 cardiorenal syndrome: from the pathogenesis, biomarkers to treatment. Front Cardiovasc Med. (2021) 8:760152. doi: 10.3389/fcvm.2021.760152
8. Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. (2012) 59(24):2145–53. doi: 10.1016/j.jacc.2011.10.910
9. McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, et al. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail. (2020) 8(7):537–47. doi: 10.1016/j.jchf.2020.03.009
10. Uthoff H, Breidthardt T, Klima T, Aschwanden M, Arenja N, Socrates T, et al. Central venous pressure and impaired renal function in patients with acute heart failure. Eur J Heart Fail. (2011) 13(4):432–9. doi: 10.1093/eurjhf/hfq195
11. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. (2009) 53(7):589–96. doi: 10.1016/j.jacc.2008.05.068
12. Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. (2010) 122(3):265–72. doi: 10.1161/circulationaha.109.933275
13. Metra M, Cotter G, Senger S, Edwards C, Cleland JG, Ponikowski P, et al. Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: a post hoc analysis of the protect data. Circ Heart Fail. (2018) 11(5):e004644. doi: 10.1161/circheartfailure.117.004644
14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, et al. 2017 Acc/aha/hfsa focused update of the 2013 accf/aha guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Am Coll Cardiol. (2017) 70(6):776–803. doi: 10.1016/j.jacc.2017.04.025
15. Authors/Task Force Members. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail. (2022);24(1):4–131. doi: 10.1002/ejhf.2333
16. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the framingham study. N Engl J Med. (1971) 285(26):1441–6. doi: 10.1056/NEJM197112232852601
17. Wettersten N, Horiuchi Y, van Veldhuisen DJ, Mueller C, Filippatos G, Nowak R, et al. B-Type natriuretic peptide trend predicts clinical significance of worsening renal function in acute heart failure. Eur J Heart Fail. (2019) 21(12):1553–60. doi: 10.1002/ejhf.1627
18. Kadoglou NPE, Parissis J, Karavidas A, Kanonidis I, Trivella M. Assessment of acute heart failure prognosis: the promising role of prognostic models and biomarkers. Heart Fail Rev. (2022) 27(2):655–63. doi: 10.1007/s10741-021-10122-9
19. Tromp J, Ouwerkerk W, Cleland JGF, Angermann CE, Dahlstrom U, Tiew-Hwa Teng K, et al. Global differences in burden and treatment of ischemic heart disease in acute heart failure: rEPORT-HF. JACC Heart Fail. (2021) 9(5):349–59. doi: 10.1016/j.jchf.2020.12.015
20. Salah K, Kok WE, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, et al. Competing risk of cardiac Status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail. (2015) 3(10):751–61. doi: 10.1016/j.jchf.2015.05.009
21. Okabe T, Yakushiji T, Kido T, Kimura T, Asukai Y, Shimazu S, et al. Poor prognosis of heart failure patients with in-hospital worsening renal function and elevated bnp at discharge. ESC Heart Fail. (2020) 7(5):2912–21. doi: 10.1002/ehf2.12901
22. Yamada T, Ueyama H, Chopra N, Yamaji T, Azushima K, Kobayashi R, et al. Systematic review of the association between worsening renal function and mortality in patients with acute decompensated heart failure. Kidney Int Rep. (2020) 5(9):1486–94. doi: 10.1016/j.ekir.2020.06.031
23. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. (2011) 364(9):797–805. doi: 10.1056/NEJMoa1005419
24. McCallum W, Tighiouart H, Kiernan MS, Huggins GS, Sarnak MJ. Relation of kidney function decline and NT-proBNP with risk of mortality and readmission in acute decompensated heart failure. Am J Med. (2020) 133(1):115–122.e2. doi: 10.1016/j.amjmed.2019.05.047
25. McQuade CN, Mizus M, Wald JW, Goldberg L, Jessup M, Umscheid CA. Brain-type natriuretic peptide and amino-terminal pro-brain-type natriuretic peptide discharge thresholds for acute decompensated heart failure: a systematic review. Ann Intern Med. (2017) 166(3):180–90. doi: 10.7326/m16-1468
26. Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of Re-admission after decompensated heart failure. J Am Coll Cardiol. (2004) 43(4):635–41. doi: 10.1016/j.jacc.2003.09.044
27. Kagiyama N, Kitai T, Hayashida A, Yamaguchi T, Okumura T, Kida K, et al. Prognostic value of BNP reduction during hospitalization in patients with acute heart failure. J Card Fail. (2019) 25(9):712–21. doi: 10.1016/j.cardfail.2019.04.004
28. Hamatani Y, Nagai T, Shiraishi Y, Kohsaka S, Nakai M, Nishimura K, et al. Long-term prognostic significance of plasma B-type natriuretic peptide level in patients with acute heart failure with reduced, mid-range, and preserved ejection fractions. Am J Cardiol. (2018) 121(6):731–8. doi: 10.1016/j.amjcard.2017.12.012
29. Stienen S, Salah K, Eurlings LW, Bettencourt P, Pimenta JM, Metra M, et al. Challenging the two concepts in determining the appropriate Pre-discharge N-terminal pro-brain natriuretic peptide treatment target in acute decompensated heart failure patients: absolute or relative discharge levels? Eur J Heart Fail. (2015) 17(9):936–44. doi: 10.1002/ejhf.320
30. Tang WH, Mullens W. Cardiorenal syndrome in decompensated heart failure. Heart (British Cardiac Society). (2010) 96(4):255–60. doi: 10.1136/hrt.2009.166256
31. Villacorta H, Villacorta AS, Villacorta LSC, Xavier AR, Kanaan S, Rohen FM, et al. Worsening renal function and congestion in patients with acute heart failure: a study with bioelectrical impedance vector analysis (biva) and neutrophil gelatinase-associated lipocalin (ngal). Arq Bras Cardiol. (2021) 116(4):715–24. doi: 10.36660/abc.20190465
32. Yoshioka K, Matsue Y, Okumura T, Kida K, Oishi S, Akiyama E, et al. Impact of brain natriuretic peptide reduction on the worsening renal function in patients with acute heart failure. PLoS One. (2020) 15(6):e0235493. doi: 10.1371/journal.pone.0235493
Keywords: acute heart failure, B-type natriuretic peptide, worsening renal function, mortality, prognosis
Citation: Zhao D, Gu L, Wei W, Peng D, Yang M, Yuan W and Rong S (2023) Impact of the degree of worsening renal function and B-type natriuretic peptide on the prognosis of patients with acute heart failure. Front. Cardiovasc. Med. 10:1103813. doi: 10.3389/fcvm.2023.1103813
Received: 21 November 2022; Accepted: 15 March 2023;
Published: 3 April 2023.
Edited by:
Erberto Carluccio, University of Perugia, ItalyReviewed by:
Nicholas Wettersten, VA San Diego Healthcare System, Veterans Health Administration, United States© 2023 Zhao, Gu, Wei, Peng, Yang, Yuan and Rong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Rong amluZ3dlaXN5QDEyNi5jb20=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.